- Get new issue alerts Get alerts

Secondary Logo
Journal logo.
Colleague's E-mail is Invalid
Your message has been successfully sent to your colleague.
Save my selection
A Case of Plasmodium Falciparum Malaria Presentation
Editor(s): Naveed, Khan.
From the Lincoln Medical and Mental Health Center, New York, New York, USA.
Correspondence: Osman Nawazish Salaria, Lincoln Medical Center, New York, New York USA (email: [email protected] ).
Abbreviations: BPb = lood pressure, bpm = beats per minute, BUNb = lood urea nitrogen, CDC = Center for Disease Control, cm = centimeters, Creatc = reatinine, DOHMH = Department of Health and Mental Hygiene, ED = Emergency Department, Hb = hemoglobin, Hct = hematocrit, ICU = intensive care unit, IV = intravenous, IVP = intravenous push, Plt = platelet, WBC = white blood cell, WHO = World Health Organization, y/o = year old.
Methods: Ethical approval was not necessary for this study as the study was focused on the patient hospital course and did in no way alter or affect her treatment. Informed Consent was taken from the patient regarding the publishing of this case report and the patient accepted.
The authors have no conflicts of interest to disclose.
This is an open access article distributed under the Creative Commons Attribution License 4.0, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. http://creativecommons.org/licenses/by/4.0
Received April 20, 2015
Received in revised form July 11, 2015
Accepted July 27, 2015
New York City is a multicultural city where people of different ethnicities and backgrounds from all over the world live together. Of the different ethnicities, it is home to a large population of Western African immigrants. This case report is that of an elderly female of Western African descent presenting to Lincoln Hospitals Emergency Department with fevers and fatigue.
The patients travel history to Togo, along with her symptoms, resulted in a differential diagnosis which included Ebola as well as Malaria. New York City's Department of Health and Mental Hygiene was contacted for further clarification of presence of Ebola in Togo. The present case report is meant to educate about the presentation, hospital course, and differential diagnoses of a patient traveling from Western Africa with fever and chills.
INTRODUCTION
Malaria is a frequent parasitic infection prevalent in Africa. Around 300 million are infected annually in Africa by malaria and 1 to 2 million will die from the disease. 1 Of the 4 human parasitic species that have been identified, Plasmodium falciparum has been known to cause significant morbidity and mortality, particularly in children and pregnant women. 1 Strategies to counteract malaria incidence, such as community health workers outreach and insecticide treated nets have been instituted in recent years; however, their effect has not been of much significance. 2
Ebola virus disease has caused much concern with its global rise in incidence and prevalence recently. The current epidemic which has centered mainly in Western African nations of Guinea, Sierra Leone, and Liberia has now spread outside of borders of Africa to involve the United States. 3 Much of the presenting symptoms and signs of the disease mimic other diseases such as typhoid fever and malaria. 3,4
There is much overlap between presentations of both P. falciparum malaria and Ebola virus disease. Without confirmatory blood tests searching for malaria parasites or viral RNA and viral antibodies a diagnosis is very difficult to achieve.
CASE REPORT
A 67 y/o (year old) female from Western Africa initially presented to the Emergency Department (ED) complaining of fatigue and subjective fevers for the past 2 days. Patient complained that her fevers were associated with headaches, but not chills, rigors, or chest pain. Index of suspicion for malaria was high as patient had recently traveled from an endemic region. Patients travel history to Western Africa and the presenting symptoms also made us consider a possibility of Ebola virus disease.
Past medical history included diabetes, hypertension, and a history of recent travel to her home country of Togo for 5 months. Patient had returned 5 days ago from her travel and started to develop symptoms of fevers and fatigue. Patient denied any immunizations received before traveling. Past surgical history included a left breast mastectomy done back in France 1987. Medication history included Amlodipine, Aspirin, Calcium Carbonate, Synthroid, Pioglitazone, Humalog, Glucovance, Crestor, Januvia, and Lisinopril. After initial presentation to the ED for 2 days of fevers and fatigue, she was accepted by Medicine and transferred to the general medical floors. The patient had a blood pressure of 123/55, pulse of 86 beats per minute (bpm), Temperature of 98.5 °F, and respiratory rate of 16 at the time of admission. Physical examination did not disclose any specific abnormalities.
Labs including complete blood count, chemistry, liver function tests, malaria peripheral smears, and reitculocyte level were withdrawn from the patient. Patient had white blood cell (WBC) count of 12.6, Hb (hemoglobin) 10.7, Hct (hematocrit) 30.6, Plt (platelet) 80, BUN (blood urea nitrogen) 12, Creat (creatinine) 0.3, and blood glucose of 291 consistent with diabetes. Blood smears were positive for P. falciparum malaria at 9.6% and reticulocyte count was reported at 3.2%. New York City's Department of Health and Mental Hygiene (DOHMH), was contacted and Ebola was not considered to be in Togo, most likely diagnosis was malaria from chloroquine resistant region. Patient was started on quinine 648 mg and doxycycline 100 mg, intravenous (IV) fluids, Lantus 21 U, Lispro 7 U, and was monitored in telemetry unit of medicine (Figures 1–3).

Attention was drawn to the patient at 4:45 AM on her 3rd hospital course day after becoming suddenly dyspneic. Patient denied any chest pain but upon pulmonary examination bilateral coarse crackles were heard up to mid lung level. Patient received 60 mg intravenous push (IVP) Lasix and sublingual nitroglycerin. She continued to be dyspneic and was given additional 40 mg IV Lasix and 4 mg Morphine IV were given. Bi-continuous positive airway pressure was started but patient did not tolerate well and decision was made to intubate the patient for acute hypoxemic respiratory failure. Patient was transferred to the medical intensive care unit (ICU) for further care.
Chest X-ray in the medical ICU revealed bilateral alveolar infiltrates; patient was started on Cefepime 2 g IV. Presumption was made that patient had Acute Respiratory Distress Syndrome secondary to sepsis from an unknown source of infection, but possibly from Falciparum Malaria. Abdominal ultrasound showed tiny echogenic foci within the gallbladder, prominent liver measuring 18.7 cm, and a dilated common bile duct measuring 8.2 mm. Choledocholithiasis was questioned although not directly visualized. Decision was made to monitor liver enzymes and if worsening of abdominal status cholecystostomy tube could be placed.
Patient remained in the medical ICU where she was daily monitored. Vital signs monitoring showed daily fever spikes of 101 to 103 °F 2 to 3 times per day. Liver enzymes were down trending after week 1, repeat right upper quadrant ultrasound was negative most probably from passage of a gallstone. On day 9 of hospital course patient was extubated and transferred to medical floors for continuation of care.
Patients of Western African descent presenting with symptoms of fevers and fatigue must be approached with precaution in present day circumstances. The Ebola virus disease outbreak has currently heightened healthcare professional's fears of contracting the virus by exposure to their patients. Furthermore, the impact of the Ebola virus disease in West Africa has left the local population vulnerable to other deadly diseases such as malaria. Control efforts for disease transmission and treatment of malaria have come to a halt. Anti-malaria medication, preventive insecticide bed nets are lying in warehouses far from the people which could benefit from them. International agencies such as the World Health Organization (WHO), US Agency for International Development supported and funded programs malaria control initiatives have virtually been shut down. 5 The similarities of the symptoms and signs of presentation of both diseases intimidates people from seeking treatment for fear of being infected with Ebola.
In face of all these difficulties, Ebola control efforts including government education programs partnered with WHO, travel measures has reduced the incidence significantly. Early identification of symptoms, isolation of contacts, and early monitoring and treatment has played a major role in limiting spread of infection of Ebola. This case report illustrates an example of how a patient with recent travel history to West Africa presenting with typical fevers, myalgias, and fatigue could be considered to have either or both diseases.
- + Favorites
- View in Gallery
A Patient/Family Care Study on Malaria
Journal Title
Journal issn, volume title, description, collections.
- Fact sheets
- Facts in pictures
Publications
- Questions and answers
- Tools and toolkits
- Endometriosis
- Excessive heat
- Mental disorders
- Polycystic ovary syndrome
- All countries
- Eastern Mediterranean
- South-East Asia
- Western Pacific
- Data by country
- Country presence
- Country strengthening
- Country cooperation strategies
- News releases
Feature stories
- Press conferences
- Commentaries
- Photo library
- Afghanistan
- Cholera
- Coronavirus disease (COVID-19)
- Greater Horn of Africa
- Israel and occupied Palestinian territory
- Disease Outbreak News
- Situation reports
- Weekly Epidemiological Record
Surveillance
- Health emergency appeal
- International Health Regulations
- Independent Oversight and Advisory Committee
- Classifications
- Data collections
- Global Health Estimates
- Mortality Database
- Sustainable Development Goals
- Health Inequality Monitor
- Global Progress
- World Health Statistics
- Partnerships
- Committees and advisory groups
- Collaborating centres
- Technical teams
- Organizational structure
- Initiatives
- General Programme of Work
- WHO Academy
- Investment in WHO
- WHO Foundation
- External audit
- Financial statements
- Internal audit and investigations
- Programme Budget
- Results reports
- Governing bodies
- World Health Assembly
- Executive Board
- Member States Portal
- Fact sheets /
- Globally in 2022, there were an estimated 249 million malaria cases and 608 000 malaria deaths in 85 countries.
- The WHO African Region carries a disproportionately high share of the global malaria burden.
- In 2022, the Region was home to 94% of malaria cases (233 million) and 95% (580 000) of malaria deaths.
- Children under 5 accounted for about 80% of all malaria deaths in the Region.
Malaria is a life-threatening disease spread to humans by some types of mosquitoes. It is mostly found in tropical countries. It is preventable and curable.
The infection is caused by a parasite and does not spread from person to person.
Symptoms can be mild or life-threatening. Mild symptoms are fever, chills and headache. Severe symptoms include fatigue, confusion, seizures, and difficulty breathing.
Infants, children under 5 years, pregnant women, travellers and people with HIV or AIDS are at higher risk of severe infection.
Malaria can be prevented by avoiding mosquito bites and with medicines. Treatments can stop mild cases from getting worse.
Malaria mostly spreads to people through the bites of some infected female Anopheles mosquitoes. Blood transfusion and contaminated needles may also transmit malaria. The first symptoms may be mild, similar to many febrile illnesses, and difficulty to recognize as malaria. Left untreated, P. falciparum malaria can progress to severe illness and death within 24 hours.
There are 5 Plasmodium parasite species that cause malaria in humans and 2 of these species – P. falciparum and P. vivax – pose the greatest threat. P. falciparum is the deadliest malaria parasite and the most prevalent on the African continent. P. vivax is the dominant malaria parasite in most countries outside of sub-Saharan Africa. The other malaria species which can infect humans are P. malariae, P. ovale and P. knowlesi .
The most common early symptoms of malaria are fever, headache and chills.
Symptoms usually start within 10–15 days of getting bitten by an infected mosquito.
Symptoms may be mild for some people, especially for those who have had a malaria infection before. Because some malaria symptoms are not specific, getting tested early is important.
Some types of malaria can cause severe illness and death. Infants, children under 5 years, pregnant women, travellers and people with HIV or AIDS are at higher risk. Severe symptoms include:
- extreme tiredness and fatigue
- impaired consciousness
- multiple convulsions
- difficulty breathing
- dark or bloody urine
- jaundice (yellowing of the eyes and skin)
- abnormal bleeding.
People with severe symptoms should get emergency care right away. Getting treatment early for mild malaria can stop the infection from becoming severe.
Malaria infection during pregnancy can also cause premature delivery or delivery of a baby with low birth weight.
Disease burden
According to the latest World malaria report , there were 249 million cases of malaria in 2022 compared to 244 million cases in 2021. The estimated number of malaria deaths stood at 608 000 in 2022 compared to 610 000 in 2021.
The WHO African Region continues to carry a disproportionately high share of the global malaria burden. In 2022 the Region was home to about 94% of all malaria cases and 95% of deaths. Children under 5 years of age accounted for about 78% of all malaria deaths in the Region.
Malaria can be prevented by avoiding mosquito bites and by taking medicines. Talk to a doctor about taking medicines such as chemoprophylaxis before travelling to areas where malaria is common.
Lower the risk of getting malaria by avoiding mosquito bites:
- Use mosquito nets when sleeping in places where malaria is present
- Use mosquito repellents (containing DEET, IR3535 or Icaridin) after dusk
- Use coils and vaporizers.
- Wear protective clothing.
- Use window screens.
Vector control
Vector control is a vital component of malaria control and elimination strategies as it is highly effective in preventing infection and reducing disease transmission. The 2 core interventions are insecticide-treated nets (ITNs) and indoor residual spraying (IRS).
Progress in global malaria control is threatened by emerging resistance to insecticides among Anopheles mosquitoes. As described in the latest World malaria report , other threats to ITNs include insufficient access, loss of nets due to the stresses of day-to-day life outpacing replacement, and changing behaviour of mosquitoes, which appear to be biting early before people go to bed and resting outdoors, thereby evading exposure to insecticides.
Chemoprophylaxis
Travellers to malaria endemic areas should consult their doctor several weeks before departure. The medical professional will determine which chemoprophylaxis drugs are appropriate for the country of destination. In some cases, chemoprophylaxis drugs must be started 2–3 weeks before departure. All prophylactic drugs should be taken on schedule for the duration of the stay in the malaria risk area and should be continued for 4 weeks after the last possible exposure to infection since parasites may still emerge from the liver during this period.
Preventive chemotherapies
Preventive chemotherapy is the use of medicines, either alone or in combination, to prevent malaria infections and their consequences. It requires giving a full treatment course of an antimalarial medicine to vulnerable populations at designated time points during the period of greatest malarial risk, regardless of whether the recipients are infected with malaria.
Preventive chemotherapy includes perennial malaria chemoprevention (PMC), seasonal malaria chemoprevention (SMC), intermittent preventive treatment of malaria in pregnancy (IPTp) and school-aged children (IPTsc), post-discharge malaria chemoprevention (PDMC) and mass drug administration (MDA). These safe and cost-effective strategies are intended to complement ongoing malaria control activities, including vector control measures, prompt diagnosis of suspected malaria, and treatment of confirmed cases with antimalarial medicines.
Since October 2021, WHO has recommended broad use of the RTS,S/AS01 malaria vaccine among children living in regions with moderate to high P. falciparum malaria transmission. The vaccine has been shown to significantly reduce malaria, and deadly severe malaria, among young children. In October 2023, WHO recommended a second safe and effective malaria vaccine, R21/Matrix-M. The availability of two malaria vaccines is expected to make broad-scale deployment across Africa possible.
Questions and answers on the RTS,S vaccine .
Early diagnosis and treatment of malaria reduces disease, prevents deaths and contributes to reducing transmission. WHO recommends that all suspected cases of malaria be confirmed using parasite-based diagnostic testing (through either microscopy or a rapid diagnostic test).
Malaria is a serious infection and always requires treatment with medicine.
Multiple medicines are used to prevent and treat malaria. Doctors will choose one or more based on:
- the type of malaria
- whether a malaria parasite is resistant to a medicine
- the weight or age of the person infected with malaria
- whether the person is pregnant.
These are the most common medicines for malaria:
- Artemisinin-based combination therapy medicines are the most effective treatment for P. falciparum malaria.
- Chloroquine is recommended for treatment of infection with the P. vivax parasite only in places where it is still sensitive to this medicine.
- Primaquine should be added to the main treatment to prevent relapses of infection with the P. vivax and P. ovale parasites.
Most medicines used are in pill form. Some people may need to go to a health centre or hospital for injectable medicines.
Antimalarial drug resistance
Over the last decade, partial artemisinin resistance has emerged as a threat to global malaria control efforts in the Greater Mekong subregion. WHO is very concerned about reports of partial artemisinin resistance in Africa, confirmed in Eritrea, Rwanda, Uganda and, most recently, Tanzania. Regular monitoring of antimalarial drug efficacy is needed to inform treatment policies in malaria-endemic countries, and to ensure early detection of, and response to, drug resistance.
For more on WHO’s work on antimalarial drug resistance in the Greater Mekong subregion, visit the Mekong Malaria Elimination Programme webpage. WHO has also developed a strategy to address drug resistance in Africa .
Elimination
Malaria elimination is defined as the interruption of local transmission of a specified malaria parasite species in a defined geographical area as a result of deliberate activities. Continued measures to prevent re-establishment of transmission are required.
In 2022, 34 countries reported fewer than 1000 indigenous cases of the disease, up from just 13 countries in 2000. Countries that have achieved at least 3 consecutive years of zero indigenous cases of malaria are eligible to apply for the WHO certification of malaria elimination . Since 2015, 12 countries have been certified by the WHO Director-General as malaria-free, including Maldives (2015), Sri Lanka (2016), Kyrgyzstan (2016), Paraguay (2018), Uzbekistan (2018), Argentina (2019), Algeria (2019), China (2021), El Salvador (2021), Azerbaijan (2023), Tajikistan (2023) and Belize (2023).
Countries and territories certified malaria-free by WHO .
Malaria surveillance is the continuous and systematic collection, analysis and interpretation of malaria-related data, and the use of that data in the planning, implementation and evaluation of public health practice. Improved surveillance of malaria cases and deaths helps ministries of health determine which areas or population groups are most affected and enables countries to monitor changing disease patterns. Strong malaria surveillance systems also help countries design effective health interventions and evaluate the impact of their malaria control programmes.
WHO response
The WHO Global technical strategy for malaria 2016–2030 , updated in 2021, provides a technical framework for all malaria-endemic countries. It is intended to guide and support regional and country programmes as they work towards malaria control and elimination.
The strategy sets ambitious but achievable global targets, including:
- reducing malaria case incidence by at least 90% by 2030
- reducing malaria mortality rates by at least 90% by 2030
- eliminating malaria in at least 35 countries by 2030
- preventing a resurgence of malaria in all countries that are malaria-free.
Guided by this strategy, the Global Malaria Programme coordinates the WHO’s global efforts to control and eliminate malaria by:
- playing a leadership role in malaria, effectively supporting member states and rallying partners to reach Universal Health Coverage and achieve goals and targets of the Global Technical Strategy for Malaria;
- shaping the research agenda and promoting the generation of evidence to support global guidance for new tools and strategies to achieve impact;
- developing ethical and evidence based global guidance on malaria with effective dissemination to support adoption and implementation by national malaria programmes and other relevant stakeholders; and
- monitoring and responding to global malaria trends and threats.
- World malaria report 2023
- Global technical strategy for malaria 2016–2030, 2021 update
- A framework for malaria elimination
- WHO guidelines for malaria
- World Malaria Day 2024
- Malaria health topic page
- World Malaria Day (25 April)
- WHO Global Malaria Programme (GMP)
- Malaria Policy Advisory Group
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
- My Bibliography
- Collections
- Citation manager
Save citation to file
Email citation, add to collections.
- Create a new collection
- Add to an existing collection
Add to My Bibliography
Your saved search, create a file for external citation management software, your rss feed.
- Search in PubMed
- Search in NLM Catalog
- Add to Search
The effects of modern housing on malaria transmission in different endemic zones: a systematic review and meta-analysis
Affiliations.
- 1 Division of Epidemiology and Biostatistics, Stellenbosch University, Stellenbosch, Western Cape, South Africa. [email protected].
- 2 Department of Epidemiology and Biostatistics, Levy Mwanawasa Medical University, Lusaka, Zambia. [email protected].
- 3 Department of Epidemiology and Biostatistics, Levy Mwanawasa Medical University, Lusaka, Zambia.
- 4 Division of Epidemiology and Biostatistics, Stellenbosch University, Stellenbosch, Western Cape, South Africa.
- 5 DSI-NRF Centre of Excellence in Epidemiological Modelling and Analysis (SACEMA), Stellenbosch University, Stellenbosch, Western Cape, South Africa.
- PMID: 39113048
- PMCID: PMC11308589
- DOI: 10.1186/s12936-024-05059-x
Background: Modern housing has been shown to reduce the risk of malaria infections compared to traditional houses; however, it is unclear if the effects differ in different malaria transmission settings. This study evaluated the effects of modern housing on malaria among different endemic areas.
Methods: Electronic databases, clinical trial registries and grey literature were searched for randomized controlled trials, cohort studies, case-control studies, and cross-sectional surveys on housing done between 1987 and 2022. Forest plots were done, and the quality of evidence was assessed using the Grading of Recommendations, Assessments, Development and Evaluation Framework.
Results: Twenty-one studies were included; thirteen were cross-sectional, four were case-control and four were cohort studies. Cohort studies showed an adjusted risk ratio of 0.68 (95% CI 0.48-0.96), and cross-sectional studies indicated an adjusted odds ratio (aOR) of 0.79 (95%CI 0.75-0.83). By endemic transmission regions, the adjusted odds ratio in the high endemic settings was 0.80 (95%CI 0.76-085); in the moderate transmission regions, aOR = 0.76 (95%CI 0.67-0.85) and in the low transmission settings, aOR = 0.67 (95%CI 0.48-0.85).
Conclusions: The evidence from observational studies suggests that there are no differences in the protective effects of modern houses compared to traditional houses on malaria by endemicity level. This implies that good quality modern housing protects against malaria regardless of the malaria transmission settings.
Keywords: Malaria-endemic zones; Modern; Modern housing; Traditional.
© 2024. The Author(s).
PubMed Disclaimer
Conflict of interest statement
The authors declare no competing interests.
Inclusion flow chart
Similar articles
- Folic acid supplementation and malaria susceptibility and severity among people taking antifolate antimalarial drugs in endemic areas. Crider K, Williams J, Qi YP, Gutman J, Yeung L, Mai C, Finkelstain J, Mehta S, Pons-Duran C, Menéndez C, Moraleda C, Rogers L, Daniels K, Green P. Crider K, et al. Cochrane Database Syst Rev. 2022 Feb 1;2(2022):CD014217. doi: 10.1002/14651858.CD014217. Cochrane Database Syst Rev. 2022. PMID: 36321557 Free PMC article.
- The evidence for improving housing to reduce malaria: a systematic review and meta-analysis. Tusting LS, Ippolito MM, Willey BA, Kleinschmidt I, Dorsey G, Gosling RD, Lindsay SW. Tusting LS, et al. Malar J. 2015 Jun 9;14:209. doi: 10.1186/s12936-015-0724-1. Malar J. 2015. PMID: 26055986 Free PMC article. Review.
- Housing Improvements and Malaria Risk in Sub-Saharan Africa: A Multi-Country Analysis of Survey Data. Tusting LS, Bottomley C, Gibson H, Kleinschmidt I, Tatem AJ, Lindsay SW, Gething PW. Tusting LS, et al. PLoS Med. 2017 Feb 21;14(2):e1002234. doi: 10.1371/journal.pmed.1002234. eCollection 2017 Feb. PLoS Med. 2017. PMID: 28222094 Free PMC article.
- Randomized trials of housing interventions to prevent malaria and Aedes-transmitted diseases: A systematic review and meta-analysis. Kua KP, Lee SWH. Kua KP, et al. PLoS One. 2021 Jan 8;16(1):e0244284. doi: 10.1371/journal.pone.0244284. eCollection 2021. PLoS One. 2021. PMID: 33417600 Free PMC article.
- Intermittent preventive treatment for malaria in children living in areas with seasonal transmission. Meremikwu MM, Donegan S, Sinclair D, Esu E, Oringanje C. Meremikwu MM, et al. Cochrane Database Syst Rev. 2012 Feb 15;2012(2):CD003756. doi: 10.1002/14651858.CD003756.pub4. Cochrane Database Syst Rev. 2012. PMID: 22336792 Free PMC article. Review.
- Alonso PL. Malaria: a problem to be solved and a time to be bold. Nat Med. 2021;27:1506–9. 10.1038/s41591-021-01492-6 - DOI - PubMed
- Sinnis P, Fidock DA. The RTS, S vaccine—a chance to regain the upper hand against malaria? Cell. 2022;185:750–4. 10.1016/j.cell.2022.01.028 - DOI - PubMed
- Parums DV. Current status of two adjuvanted malaria vaccines and the World Health Organization (WHO) strategy to eradicate malaria by 2030. Med Sci Monit. 2023;29: e939357. 10.12659/MSM.939357 - DOI - PMC - PubMed
- Monroe A, Moore S, Olapeju B, Merritt AP, Okumu F. Unlocking the human factor to increase effectiveness and sustainability of malaria vector control. Malar J. 2021;20:404. 10.1186/s12936-021-03943-4 - DOI - PMC - PubMed
- Nawa M, Halwindi H, Hangoma P. Modelling malaria reduction in a highly endemic country: evidence from household survey, climate, and program data in Zambia. J Public Health Afr. 2020;11:1096. 10.4081/jphia.2020.1096 - DOI - PMC - PubMed
Publication types
- Search in MeSH
LinkOut - more resources
Full text sources.
- BioMed Central
- PubMed Central
- Genetic Alliance
- MedlinePlus Health Information

- Citation Manager
NCBI Literature Resources
MeSH PMC Bookshelf Disclaimer
The PubMed wordmark and PubMed logo are registered trademarks of the U.S. Department of Health and Human Services (HHS). Unauthorized use of these marks is strictly prohibited.
- Open access
- Published: 07 August 2024
The effects of modern housing on malaria transmission in different endemic zones: a systematic review and meta-analysis
- Mukumbuta Nawa 1 , 2 ,
- Catherine Mupeyo-Mudala 2 ,
- Sylvia Banda-Tembo 2 &
- Olatunji Adetokunboh 1 , 3
Malaria Journal volume 23 , Article number: 235 ( 2024 ) Cite this article
189 Accesses
Metrics details
Modern housing has been shown to reduce the risk of malaria infections compared to traditional houses; however, it is unclear if the effects differ in different malaria transmission settings. This study evaluated the effects of modern housing on malaria among different endemic areas.
Electronic databases, clinical trial registries and grey literature were searched for randomized controlled trials, cohort studies, case–control studies, and cross-sectional surveys on housing done between 1987 and 2022. Forest plots were done, and the quality of evidence was assessed using the Grading of Recommendations, Assessments, Development and Evaluation Framework.
Twenty-one studies were included; thirteen were cross-sectional, four were case–control and four were cohort studies. Cohort studies showed an adjusted risk ratio of 0.68 (95% CI 0.48–0.96), and cross-sectional studies indicated an adjusted odds ratio (aOR) of 0.79 (95%CI 0.75–0.83). By endemic transmission regions, the adjusted odds ratio in the high endemic settings was 0.80 (95%CI 0.76–085); in the moderate transmission regions, aOR = 0.76 (95%CI 0.67–0.85) and in the low transmission settings, aOR = 0.67 (95%CI 0.48–0.85).
Conclusions
The evidence from observational studies suggests that there are no differences in the protective effects of modern houses compared to traditional houses on malaria by endemicity level. This implies that good quality modern housing protects against malaria regardless of the malaria transmission settings.
The fight against malaria has stalled in recent years partly due to the emergence of resistance to insecticides used in long-lasting insecticidal nets (LLINs) and indoor residual spraying (IRS), reduced investments and disruptions in interventions during the Coronavirus Disease of 2019 (COVID-19) pandemic [ 1 ]. Researchers have called for novel tools to enhance the fight against malaria for global elimination to be performed in line with the World Health Organization (WHO) Global Technical Strategy of eliminating 90% of incident cases and deaths by 2030 [ 1 ]. Two malaria vaccines have been approved so far, a malaria vaccine called RTS, S/AS01 (Mosquirix®) and another one called R21 (Matrix-M™), which have been added to the available tools to fight malaria [ 2 , 3 ]. Others have called for community and human-centred approaches [ 4 ].
While the disease is raging on, researchers and policymakers are looking for solutions to emerging challenges. The case for housing infrastructure improvements in the fight against malaria, which was superseded by the discovery of chemical agents, has emerged [ 5 ]. Studies have shown that the risks and odds of malaria infection can be reduced by about 47% for those who dwell in modern houses compared to those who dwell in traditional houses [ 6 ]. Another study, a secondary analysis which analysed data from 15 Demographic and Health Surveys and 21 Malaria Indicators Surveys in sub-Saharan Africa found that modern houses were associated with reduced odds of malaria infection by about 9% (aOR 0.91 95%CI 0.85 -0.97) when compared to traditional houses [ 7 ]. Further, another study, a systematic review and meta-analysis which included 18 randomized controlled trials (RCTs) on the prevention of malaria and Aedes -transmitted diseases, found a reduced odds ratio of malaria in all settings of 0.63 (95%CI 0.39–1.01) [ 8 ]. Another study, a Cochrane review of RCTs, found that house modifications can reduce malaria prevalence at RR 0.68 (95%CI 0.57–0.82) [ 9 ]. The two systematic reviews focused on house modifications or improvements, such as the effects of fitments of screening or ceilings and closing of eaves, compared to controls that did not have those interventions [ 8 ].
While there is sympatry or co-existence of primary vectors, the primary Anopheles mosquito vectors that predominantly transmit malaria in highly endemic areas differ from those that predominantly transmit malaria in low endemic areas in terms of their feeding host preferences, resting behaviour, Entomological Inoculation Rates (EIR) and Sporozoite Infection Rates (SIR) [ 10 , 11 ]. The effect of housing structures is likely to differ for high-endemic areas compared to low-endemic areas. This study, therefore, addressed this knowledge gap and can help government agencies target effective policies and interventions relevant to local settings.
The study used the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) guidelines to prepare a systematic review and meta-analysis [ 12 ]. The protocol was registered with the Prospective Register of Systematic Reviews (PROSPERO–ID 357186).
Study settings
This review included studies from sub-Saharan Africa, South America, and Middle and East Asia and was stratified according to malaria-endemic zones.
Inclusion and exclusion criteria
Types of studies.
Studies included were RCT designs and observational studies such as cross-sectional surveys, case–control and cohort studies published between 1987 and June 2022 in line with the establishment of the Roll Back Malaria Initiative in 1987. All studies with clear effect measures (such as Odds Ratios, Incidence Rate Ratios, Prevalence Ratios and Indoor Vector Density Ratios, Entomological Inoculation Rate Ratios) were included, whilst those that were qualitative or without effect measures were excluded. Those without clear geographical areas where the studies were conducted were also excluded. Studies that meet the criteria for modern houses versus traditional houses but only compared components of house improvements such as iron roofs versus thatched roofs or brick walls versus mud walls were also excluded.
Type of participants
The study included studies that compared malaria occurrence in all types of residents, whether children under five years or adults or specific subsections of adults such as pregnant women.
Interventions
Studies had to be clear that they compared modern housing structures against traditional or non-standard housing structures. Modern houses have finished wall and roofing materials; finished wall materials include cement, stone with lime or cement, bricks, cement blocks, covered adobe, and wood planks or shingles, while finished roofing materials include metal, wood, calamine or cement fibre, ceramic tiles, cement, and roofing shingles [ 7 ]. All other houses that do not have finished walls and roofing materials are considered traditional houses [ 7 , 13 ]. This study did not include floor materials because they do not play a role in mosquito house entry. This definition of finished materials is not arbitrarily defined by the authors of this study but is in line with the demographic and health surveys as well as malaria indicator survey methodology guidelines [ 14 ].
Type of outcome measures
Different studies measure malaria outcomes in different ways. Cross-sectional studies measure malaria prevalence diagnosed by blood slides using light microscopy regardless of symptoms. We did not include studies that measured malaria infection using rapid diagnostic tests (RDTs) due to their lack of specificity in detecting malaria. Cohort studies and RCTs measure malaria incidence. This study included prevalence and incidence as primary outcomes and analysed them separately by different endemic areas. Further, studies that compared entomological measures such as vector densities, human biting rates and entomological inoculation rates between modern houses and traditional houses were also included as secondary outcomes.
Information sources
Major databases were searched for peer-reviewed journal articles on the subject, including Cochrane, MEDLINE (PubMed), Scopus, The Global Index Medicus and Web of Science. Peer-reviewed scientific conference proceedings, such as the American Society of Tropical Medicine and Hygiene, and The International Congress for Tropical Medicine and Malaria, were searched. Further, the study also searched clinical trial registries, including the WHO clinical trials registry and the American clinicaltrials.gov and grey literature.
Search strategy
A literature search strategy was developed in Medline using Mesh subject headings combined with free text. The search strategy developed in Medline was adapted to other databases in collaboration with the University of Stellenbosch librarian and has been attached as supplementary material.
Study records
The identified articles were imported into a citation reference manager called Endnote; Endnote was used to de-duplicate articles.
Screening for eligibility
Rayyan QCRI Software was used to screen the articles for eligibility [ 15 ]. Three reviewers (NM, CMM and SBT) independently screened the titles and abstracts in Rayyan. Disagreements were resolved by discussion between the team members. (CMM and SBT), then read the full text for the selected articles and finalise the screening process with NM. OA supervised the screening process.
Data extraction
Two reviewers (CMM and SBT) extracted data from selected studies into a pre-piloted data extraction form. The consensus was established between the two, and arbitration by the third reviewer (NM) when needed. The data points included authors, year of publication, sample size, study design, effect measures with 95% confidence intervals, type of participants, and geographical coverage.
Assessment of risk of bias in included studies
Three reviewers (NM, CMM and SBT) assessed the risk of bias in the studies in duplicate. The risk of bias for observational studies was assessed using the Risk of Bias for Non-Randomised Studies for Exposure (RoBINS-E) [ 16 ]. The risk of bias in the papers was reported as low risk, moderate risk, serious or critical risk based on the algorithm.
Measures of treatment effects and associations
The outcome of this study was to establish the effects and measures of the association of modern houses on malaria cases (incidence and prevalence) stratified by low, moderate, and high endemic settings. The malaria endemicity settings of low, moderate and high were based on the WHO classification of the prevalence of Plasmodium falciparum/Plasmodium vivax of below 10% as low transmission, between 10 and 35% as moderate and above 35% prevalence as high transmission [ 17 ]. Clinical trials and cohort studies that report risk ratios were analysed and reported separately. At the same time, prevalence and case–control studies that report odds ratios were also analysed and reported separately.
Unit of analysis issues
For follow-up studies such as RCTs and Cohort studies, Incidence Risk Ratios (IRR), Rate Ratios or Absolute Risk Differences were used to compare malaria incidence in modern houses versus traditional houses in different endemic settings. Where events occur below 10% in the samples, odds ratios were used as they are better estimates in rare events. In cross-sectional and case–control studies, the analysis unit used was odds ratios.
Assessment of heterogeneity
In line with the Cochrane guidelines, heterogeneity in the studies was assessed using the I 2 statistics in the meta-analysis, which is calculated by:
where Q is the Chi 2 and df is the degree of freedom.
An I 2 of 75–100% would be interpreted as considerable heterogeneity, 50–90% as substantial heterogeneity, 30–60% as moderate, and below 40% as unimportant [ 18 ].
Assessment of reporting biases
Reporting bias was assessed using funnel plots where there were at least ten studies included in the meta-analysis.
Data synthesis
A summary of how many articles were identified during the literature search, how many were excluded at what stage of the process, why they were excluded, and how many were finally included are presented in a flow diagram [ 19 ]. A descriptive table of included articles, where, when, authors, and effect sizes are presented. Forest plots were done of the analysis displaying pooled effect measures, 95% confidence intervals, p values, Chi-square, and I 2 values. Meta-analyses were conducted among similar studies to find the pooled effect measures by endemic zone using RevMan for Windows (version 5.4) [ 20 ].
Similar study designs that reported the same measures of association and effect measures were used to create separate forest plots. Separate forest plots were run for each study design using the reported effect measure, whether risk ratio, rate ratio, absolute risk difference, or odds ratio low, moderate, and high endemic settings.
Certainty of the evidence
The certainty of the evidence was assessed using the Grading of Recommendations, Assessment, Development and Evaluations (GRADE) framework [ 21 ]. Evidence was categorised as very low, low, moderate, and high quality. The certainty of evidence has been included in the GRADE table.
Ethical considerations
The Department of Global Health research protocol panel at the University of Stellenbosch reviewed and approved this study. An exemption for review was obtained from the Health Research Ethical Committee at the University of Stellenbosch as it does not involve human subjects (HREC Reference number: X22/08/020).
A total of 3,167 articles were collected from the database search, and an additional three were collected from grey literature, totalling 3,170. Following screening, 2,923 articles were excluded, and a full-text screening was done on 247 articles. A total of 141 were excluded on full-text screening, and 84 were excluded because they compared components and not comprehensive houses. Figure 1 shows the inclusion flow chart.

Inclusion flow chart
Included studies
The majority of the studies included were cross-sectional study designs 13 (62%), case–control studies 4 (19%) and cohort studies 4 (19%). More than three-quarters of the studies were done in Africa 18 (86%), less than a fifth in Asia 3 (14%) and none in Latin America. Over half of the studies were cross-sectional surveys.
The majority of the studies done in Africa were done in high endemic settings 10/17 (59%), a third 4/17 (24%) from moderately endemic settings and 18% (3/17) in low endemic settings. Those from Asia were from moderate endemic settings in India and Pakistan.
Characteristics of study participants
Among the studies included those that assessed malaria parasites among participants were 21 studies, and altogether, there were 234 262 participants. Table 1 gives a summary of the characteristics of the included studies.
Housing characteristics
All 21 studies included in this review compared modern houses against traditional houses. Traditional houses were different in Africa and Asia but followed the DHS classification while modern houses were made of brick walls and iron/tiled ceramic roofs.
Primary outcomes
The primary outcomes reported in the included studies were malaria prevalence and incidence depending on the study design. A total of 17 outcomes of the interventions reported the prevalence of malaria parasites in the respondents. A total of four outcomes were reported on malaria incidence. Some studies reported more than one outcome.
Secondary outcomes
Two entomological studies reported indoor resting vector densities, while two other studies reported human biting rates. No entomological study among the included studies linked entomological inoculation rate and housing structures.
Excluded studies
A total of 84 studies were excluded on the basis that though they had effect measures on housing structures comparing malaria in traditional versus modern structures, they only compared components of houses such as thatched roof versus iron/tiled roof, ceiling versus no ceiling, closed eaves versus open eaves or mud walls versus brick walls.
Risk of bias assessment
Twenty of the included studies were observational and assessed for risk of bias using the Risk of Bias in Non-Randomised Studies of Exposure (RoBINS-E) Tool [ 16 ]. Five of the studies had a serious risk of bias arising from recall bias due to prolonged periods assessed [ 22 , 23 ], risk of selection bias [ 24 ] and confounding due to the use of unadjusted odds ratios in the studies [ 25 , 26 ]. Ten included studies had moderate concerns, mainly arising from residual confounding in cross-sectional and case–control studies, even after multivariate regression adjustment. Four had a low risk of bias mainly because they were cohort studies [ 13 , 27 , 28 , 29 ]. A summary of the risk of bias assessment for the observational studies is shown in Table 2 .
Effects and associations of interventions/ exposures on outcomes
The overall association of modern houses on the risk of malaria parasitaemia compared to traditional housing among cross-sectional surveys using the adjusted odds ratios reported in the individual studies was a reduction in the adjusted odds ratio of 0.79 (95%CI 0.75–0.83). The overall heterogeneity was high at I 2 = 66.2% and was statistically significant (P value < 0.001), implying that there were significant differences in the association of modern housing in different individual studies. Table 3 summarises the pooled measures of associations.
When the effect of modern housing was stratified by endemicity, the effect in the high endemic zones was at an odds ratio of 0.80 (95%CI 0.76–0.85) and was statistically significant. The heterogeneity in the high endemic zone was high at I 2 = 0.72.5% and statistically significant. The association of modern housing in the moderate malaria endemic zones compared to traditional housing was found to be statistically significant at odds ratio 0.76 (95%CI 0.67–0.85) with high heterogeneity at 74.4%. There was only one study done in India [ 22 ] while the rest were from Africa. In the low endemic zone, the association of modern housing compared to traditional housing also showed a significant reduction in malaria infections with an odds ratio of 0.67 (95%CI 0.45–0.85). There was an overlap in the confidence intervals of the odds ratios across the high, moderate and low endemic transmission areas indicating no statistical differences in the effects of modern houses on malaria compared to traditional houses.
Further, the study conducted a meta-analysis of cross-sectional studies using unadjusted odds ratios from reported actual numbers of infections and total participants included in studies. The pooled measure of association was an odds ratio of 0.28 (95%CI 0.27–0.29, I 2 = 94.5%) This association was more than the one calculated from adjusted odds ratios, probably because of confounding from other factors that were not adjusted for in the analysis using unadjusted odds ratios. Similarly, the effects of modern housing compared to traditional housing in the low transmission settings was more uOR 0.20 (95%CI 0.16 – 0.24) compared to the moderate and high transmission settings (uOR of 0.14, 95%CI 0.12 – 0.17 and 0.34, 95%CI 0.33–0.36, respectively).
The study further assessed the associations of modern housing compared to traditional housing using case–control studies that reported adjusted odds ratios by endemic zones. There were only two studies in the meta-analysis, one done in Zambia and the other in northern Namibia. The overall effect of modern housing compared to traditional housing was an odds ratio of 0.52 (95%CI 0.38–0.70, I 2 = 0%, P value = 0.40), which shows that modern housing had a statistically significant effect in reducing the risk of malaria compared to traditional housing. In terms of endemicity, only one case–control study was included in the high endemic region, and the effect measure was an adjusted odds ratio of 0.33 (95%CI 0.11–0.99). No studies were included that were done in the moderately endemic regions. In contrast, one study was included in the low malaria endemic region, and the effect measure was an odds ratio of 0.54 (95%CI 0.39–0.74). Due to the few studies included in the meta-analysis, the heterogeneity was low.
This review further analysed case–control studies that reported an unadjusted number of malaria infection events against totals and conducted a meta-analysis. Only two studies were included, one from Egypt and another from Zimbabwe, which were both in low transmission settings. The pooled measure of association was an odds ratio of 0.33 (95%CI 0.06–1.75, I 2 = 71% and P value = 0.19). This association was not statistically significant because of a wide confidence interval, few studies, and likely confounding from the unadjusted odds ratios used.
Further analysis of observational studies was done using cohort studies that compared adjusted incidence (risk) ratios among residents of modern houses against traditional houses. Two cohort studies compared the adjusted risk ratios in modern houses to traditional houses, both done in Uganda (13, 28). There was a reduced Incidence Risk Ratio (IRR) of 0.68 (95%CI 0.48–0.96, I = 71%, P value = 0.06). The risk reduction was statistically significant based on the confidence intervals. Still, the heterogeneity was not significant, probably because of the few studies and that they were done in the same country.
A meta-analysis of cohort studies that reported unadjusted Incidence Risk Ratios found only two studies from Uganda and pooled risk ratios of 0.89 (95%CI 0.70–1.14) [ 13 , 28 ]. This effect was not statistically significant, unlike the ones done from the same country, Uganda, in similar settings that reported adjusted Risk Ratios.
The same two cohort studies done in Uganda that reported non-significant risk ratios also reported unadjusted odds ratios, which were statistically significant (Odds Ratio 0.63 (95%CI 0.41–0.97) when pooled in a meta-analysis [ 27 , 30 ].
In cohort studies, the study further explored the association of modern housing compared to traditional housing using mosquito vectors’ Human Biting Rate (HBR). The same two studies from Uganda reported the unadjusted risk ratio using HBR (RR 0.53 (95%CI 0.43–0.65) [ 27 , 30 ].
Quality of evidence (GRADE)
The studies included in this review provide low-quality evidence from cohort studies and low to very low evidence from cross-sectional and case–control studies [ 31 ]. Table 4 shows the summary for the certainty of evidence using the GRADE Approach.
This systematic review and meta-analysis sought to find the effects and measures of association between modern housing and malaria infections in different malaria endemic zones. Previous meta-analyses, particularly non-Cochrane studies that included sufficient observational studies, found high heterogeneity in the measures of associations between housing structures and malaria parasitaemia [ 32 ]. The high heterogeneity may arise from differences from not only study designs but also in endemic settings. This study’s findings indicate that modern housing provides reduced risks of malaria infection as measured in different study designs including cohort (IRR 0.68, 95%CI 0.48–0.96), case–control (aOR 0.52, 95%CI 38–0.70) and cross-sectional studies (aOR 0.79, 95%CI 0.75–0.83). These findings are in agreement with other systematic reviews and meta-analyses on modern housing compared to traditional housing which showed that modern housing reduced the risk of malaria infections [ 7 , 32 , 33 ]. This study did not find RCTs that met the inclusion criteria, therefore it did not include any RCTs; existing RCTs and meta-analyses of RCTs consisted of studies that compared house improvements such as iron roofs versus traditional roofs, brick walls versus traditional walls and other interventions such as window and door screens, and eave closure versus no intervention [ 8 , 9 ].
This study, therefore, only included observational studies, such as cohort, case–control and cross-sectional studies; the results show that modern houses that include both iron roofs and brick walls reduce malaria risk and indoor vector densities with very few showing that the measures of association are not statistically significant [ 25 , 27 , 30 , 34 ]. However, socio-economic factors such as wealth, education, nutritional status and health status are also associated with living in modern houses compared to living in traditional houses [ 35 ]. Therefore, even when some socio-economic factors were adjusted for in the observational studies included in the review and meta-analysis, residual confounding was still an important factor. As such, the results were considered to have low to very low certainty of evidence using the GRADE system. They must be interpreted with caution [ 21 ].
From the meta-analysis, four cohort studies were all done in Uganda and were categorized as high-endemic settings [ 13 , 27 , 28 , 29 ]. Of the four case–control studies included, three were in low transmission settings [ 25 , 36 , 37 ], and only one was in high endemic settings [ 6 ]. So, the comparisons could only be made using cross-sectional studies where there were studies in all endemic settings, which allowed us to do comparisons using the same measures of association. The risk reduction of malaria in modern housing was not statistically different in high, moderate and low malaria transmission settings when comparing confidence intervals of the pooled odds ratios using adjusted odds ratios. Similarly, cross-sectional studies that reported unadjusted odds ratios also showed that the association between modern housing and malaria infection compared to traditional housing was not statistically different across high, moderate and low transmission settings, however, the effect measure was significantly lower when assessed using unadjusted odds ratios compared to adjusted odds ratios. This study based its conclusion on adjusted odds ratios because unadjusted odds ratios have statistical noise and confounding which were not adjusted for. It is possible that factors such as wealth status and residing in rural areas among others could have contributed to the overestimation of the effects of modern housing in unadjusted odds ratios i.e., more poor people in Africa tend to live in poor housing structures in rural areas, so not adjusting for these factors (wealth and residence location) may overestimate the effects of modern housing. One study from India showed a very minimal risk reduction, which was not statistically significant, probably because it only measured malaria in the adult population aged 45 years and above, which is different from children aged below five years and the general population, which most cross-sectional studies in Africa measure malaria in [ 22 ]. The authors did not find any systematic reviews or meta-analyses that compared malaria risk of infection in different endemic areas to compare with this study.
Modern housing has a biological plausibility of being more effective compared to traditional housing; from an entomological perspective, high transmission settings have higher entomological inoculation rates (EIRs) [ 38 ], so people get bitten many times by infected mosquitoes, and you would expect residents of traditional houses that do not impede mosquito entry to have a higher probability of infections compared to people in modern houses [ 39 ]. Conversely, as the EIRs reduce in moderate and low endemic transmission settings, you would expect a dose–response-like effect of reduced measures of association in moderate and low endemic settings [ 38 ]. So, based on the dose–response-like associations using higher EIRs in higher endemic settings and lower EIRs in moderate and low malaria endemic settings, it would be expected that measures of association of modern housing and risk of malaria would be higher in high endemic settings compared to low endemic settings. However, the effect was similar in all endemic transmission areas.
From an immunological perspective, those who get bitten more times in the higher transmission settings develop acquired immunity and can fend off infections and clinical disease even when bitten by infected mosquitoes multiple times [ 40 ]. Conversely, those in low transmission settings may not have had frequent bites enough to confer acquired immunity; for example, the EIR in some places in Uganda may be as high as 310 infective bites per person per year, whilst, in low transmission settings, such as Botswana, Namibia and the Southern parts of Zambia, the EIRs are below 1.6 infective bites per person per year [ 38 ]. A person bitten by an infective mosquito less than twice a year is less likely to develop acquired immunity than another who gets bitten by infective mosquitoes 310 times a year.
Elsewhere, policymakers and managers of malaria programmes have noted the reduced effects and associations of other interventions, such as LLINs and IRS [ 41 ]. Despite the low to very low quality of evidence available, the findings of this study may, therefore, be of interest in providing evidence for improved housing in fighting malaria in different endemic settings. Improving housing to modern standards to prevent malaria can be an addition to the tools available in the fight against malaria, especially now as the fight against malaria garner towards its elimination by 2030.
Limitations
The main limitation of this study was that there were no high-quality evidence studies such as RCTs. Moderate-quality evidence from cohort studies was also not available in all endemic settings, so it mainly relied on low to very low-quality evidence from cross-sectional studies which are prone to bias and confounding. Further, malaria was measured in different populations, in under-five children in some studies such as Malaria Indicator Surveys, in the general populations in some surveys in low transmission areas such as Egypt and Zimbabwe and in people over 45 years in India. In addition, modern housing characteristics were not standardized as it included modern brick walls and iron or roof tiles; some variations in the designs can affect their effects on malaria such as ensuring that eaves are closed or doors are tightly fitted in a standardized way as would happen in randomized controlled trials.
The currently available evidence on measures of association and effects of modern houses compared to traditional houses on malaria transmission in different endemic transmission settings is limited to low and very low-quality evidence. The evidence suggests that the risk reduction associated with modern housing compared to traditional housing structures is not significantly different in low, moderate and high transmission settings. Further, evidence from cohort studies done in high-transmission settings shows that modern houses may have the benefit of reducing the risk of malaria transmission and indoor vector densities.
Implications for research
More research is needed to generate high-quality evidence in low and moderate endemic settings regarding the effects of house improvements in different endemic settings.
Implications for practice
In all malaria-endemic areas, house improvements may be one of the additional tools for policymakers and programme managers to consider implementing in malaria programmes.
Data availability
No datasets were generated or analysed during the current study.
Alonso PL. Malaria: a problem to be solved and a time to be bold. Nat Med. 2021;27:1506–9.
Article CAS PubMed Google Scholar
Sinnis P, Fidock DA. The RTS, S vaccine—a chance to regain the upper hand against malaria? Cell. 2022;185:750–4.
Parums DV. Current status of two adjuvanted malaria vaccines and the World Health Organization (WHO) strategy to eradicate malaria by 2030. Med Sci Monit. 2023;29: e939357.
Article PubMed Google Scholar
Monroe A, Moore S, Olapeju B, Merritt AP, Okumu F. Unlocking the human factor to increase effectiveness and sustainability of malaria vector control. Malar J. 2021;20:404.
Article PubMed PubMed Central Google Scholar
Nawa M, Halwindi H, Hangoma P. Modelling malaria reduction in a highly endemic country: evidence from household survey, climate, and program data in Zambia. J Public Health Afr. 2020;11:1096.
Nawa M. Investigating the effect of prompt treatment on malaria prevalence in children aged below five years in Zambia: a nested case-control study in a cross-sectional survey. Adv Public Health. 2020;2020:4289420.
Article Google Scholar
Tusting LS, Bottomley C, Gibson H, Kleinschmidt I, Tatem AJ, Lindsay SW, et al. Housing improvements and malaria risk in sub-Saharan Africa: a multi-country analysis of survey data. PLoS Med. 2017;14: e1002234.
Kua KP, Lee SWH. Randomized trials of housing interventions to prevent malaria and Aedes -transmitted diseases: a systematic review and meta-analysis. PLoS ONE. 2021;16: e0244284.
Article CAS PubMed PubMed Central Google Scholar
Furnival-Adams J, Olanga EA, Napier M, Garner P. House modifications for preventing malaria. Cochrane Database Syst Rev. 2021;2021:CD013398.
Nawa M. Influence of history, geography, and economics on the elimination of malaria: a perspective on disease persistence in rural areas of Zambia. Int J Travel Med Glob Health. 2019;7:113–7.
Stevenson JC, Pinchoff J, Muleba M, Lupiya J, Chilusu H, Mwelwa I, et al. Spatio-temporal heterogeneity of malaria vectors in northern Zambia: implications for vector control. Parasit Vectors. 2016;9:510.
Moher D, Shamseer L, Clarke M, Ghersi D, Liberati A, Petticrew M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev. 2015;4:1.
Okiring J, Olwoch P, Kakuru A, Okou J, Ochokoru H, Ochieng TA, et al. Household and maternal risk factors for malaria in pregnancy in a highly endemic area of Uganda: a prospective cohort study. Malar J. 2019;18:144.
ICF International. DHS methodology Rockview, Maryland2017 [Available from: http://dhsprogram.com/What-We-Do/Survey-Types/DHS-Methodology.cfm .
Ouzzani M, Hammady H, Fedorowicz Z, Elmagarmid A. Rayyan—a web and mobile app for systematic reviews. Syst Rev. 2016;5:210.
Sterne J, Hernán M, McAleenan A, Reeves B, Higgins J. Assessing risk of bias in a non-randomized study. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (eds.); Cochrane Handbook of Sustematic Reviews of Interventions. 2 nd Edn. Chapt. 25. London: Wiley Online Library. 2022.
WHO. A Framework for Malaria Elimination. Geneva, World Health Organization, 2017. Available from: https://www.who.int/publications/i/item/9789241511988 .
Deeks J, Higgins J, Altman D. Analysing data and undertaking meta-analyses. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (eds.); Cochrane Handbook of Sustematic Reviews of Interventions. 2 nd Edn. Chapt. 10. London: Wiley Online Library. 2022.
Stovold E, Beecher D, Foxlee R, Noel-Storr A. Study flow diagrams in Cochrane systematic review updates: an adapted PRISMA flow diagram. Syst Rev. 2014;3:54.
Cochrane Group. Review Manager 2023 [Available from: https://revman.cochrane.org/#/myReviews
Guyatt GH, Oxman AD, Schünemann HJ, Tugwell P, Knottnerus A. GRADE guidelines: a new series of articles in the Journal of Clinical Epidemiology. J Clin Epidemiol. 2011;64:380–2.
Mohan I, Kodali NK, Chellappan S, Karuppusamy B, Behera SK, Natarajan G, et al. Socio-economic and household determinants of malaria in adults aged 45 and above: analysis of longitudinal ageing survey in India, 2017–2018. Malar J. 2021;20:306.
Manyangadze T, Mavhura E, Mudavanhu C, Pedzisai E. An exploratory analysis of the spatial variation of malaria cases and associated household socio-economic factors in flood-prone areas of Mbire district. Zimbabwe GeoJournal. 2022;87:4439–54.
Wolff CG, Schroeder DG, Young MW. Effect of improved housing on illness in children under 5 years old in northern Malawi: cross-sectional study. BMJ. 2001;322:1209.
Dahesh SM, Bassiouny HK, El-Masry SA. Socioeconomic and environmental factors affecting malaria infection in Fayoum Governorate. Egypt J Egypt Soc Parasitol. 2009;39:511–23.
PubMed Google Scholar
Zaidi AA. Syed, Kokab F, Bukhari IA, Nasir JA. The quantitative evidence of malarial transmission and its Associates in Bahawalpur, Pakistan. J Ayub Med Coll Abbottabad. 2015;27:164–7.
Rek JC, Alegana V, Arinaitwe E, Cameron E, Kamya MR, Katureebe A, et al. Rapid improvements to rural Ugandan housing and their association with malaria from intense to reduced transmission: a cohort study. Lancet Planet Health. 2018;2:e83–94.
Snyman K, Mwangwa F, Bigira V, Kapisi J, Clark TD, Osterbauer B, et al. Poor housing construction is associated with increased malaria incidence in a cohort of young Ugandan children. Am J Trop Med Hyg. 2015;92:1207–13.
Tusting LS, Rek J, Arinaitwe E, Staedke SG, Kamya MR, Cano J, et al. Why is malaria associated with poverty? Findings from a cohort study in rural Uganda. Infect Dis Poverty. 2016;5:78.
Tusting LS, Willey B, Lines J. Building malaria out: improving health in the home. Malar J. 2016;15:320.
Pinder M, Bradley J, Jawara M, Affara M, Conteh L, Correa S, et al. Improved housing versus usual practice for additional protection against clinical malaria in The Gambia (RooPfs): a household-randomised controlled trial. Lancet Planet Health. 2021;5:e220–9.
Degarege A, Fennie K, Degarege D, Chennupati S, Madhivanan P. Improving socioeconomic status may reduce the burden of malaria in sub-Saharan Africa: a systematic review and meta-analysis. PLoS ONE. 2019;14: e0211205.
Tusting LS, Ippolito MM, Willey BA, Kleinschmidt I, Dorsey G, Gosling RD, et al. The evidence for improving housing to reduce malaria: a systematic review and meta-analysis. Malar J. 2015;14:209.
Salunkhe L, Gupta A, Hameed S. A household survey to assess the prevalence of malaria and risk factors under urban field practice area, Dakshin Kannada. Int J Commun Med Public Health. 2019;6:223–8.
Martens PJ, Chateau DG, Burland EMJ, Finlayson GS, Smith MJ, Taylor CR, et al. The effect of neighborhood socioeconomic status on education and health outcomes for children living in social housing. Am J Public Health. 2014;104:2103–13.
Smith JL, Mumbengegwi D, Haindongo E, Cueto C, Roberts KW, Gosling R, et al. Malaria risk factors in northern Namibia: the importance of occupation, age and mobility in characterizing high-risk populations. PLoS ONE. 2021;16: e0252690.
Mundagowa PT, Chimberengwa PT. Malaria outbreak investigation in a rural area south of Zimbabwe: a case-control study. Malar J. 2020;19:197.
Kilama M, Smith DL, Hutchinson R, Kigozi R, Yeka A, Lavoy G, et al. Estimating the annual entomological inoculation rate for Plasmodium falciparum transmitted by Anopheles gambiae s.l. using three sampling methods in three sites in Uganda. Malar J. 2014;13:111.
Griffin JT, Hollingsworth TD, Okell LC, Churcher TS, White M, Hinsley W, et al. Reducing Plasmodium falciparum malaria transmission in Africa: a model-based evaluation of intervention strategies. PLoS Med. 2010;7: e1000324.
Doolan DL, Dobaño C, Baird JK. Acquired immunity to malaria. Clin Microbiol Rev. 2009;22:13–36.
Fullman N, Burstein R, Lim SS, Medlin C, Gakidou E. Nets, spray or both? The effectiveness of insecticide-treated nets and indoor residual spraying in reducing malaria morbidity and child mortality in sub-Saharan Africa. Malar J. 2013;12:62.
Download references
Acknowledgements
The authors wish to acknowledge Prof Peter Nyasulu and Ms Vera Ngah of Stellenbosch University.
This research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors.
Author information
Authors and affiliations.
Division of Epidemiology and Biostatistics, Stellenbosch University, Stellenbosch, Western Cape, South Africa
Mukumbuta Nawa & Olatunji Adetokunboh
Department of Epidemiology and Biostatistics, Levy Mwanawasa Medical University, Lusaka, Zambia
Mukumbuta Nawa, Catherine Mupeyo-Mudala & Sylvia Banda-Tembo
DSI-NRF Centre of Excellence in Epidemiological Modelling and Analysis (SACEMA), Stellenbosch University, Stellenbosch, Western Cape, South Africa
Olatunji Adetokunboh
You can also search for this author in PubMed Google Scholar
Contributions
MN and OA discussed the concept, and CMM & SBT collected the data. MN drafted the manuscript, CMM, SBT and OA reviewed and provided inputs and guidance. All authors reviewed and approved the manuscript for submission.

Corresponding author
Correspondence to Mukumbuta Nawa .
Ethics declarations
Consent for publication.
No patients or public involvement.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Nawa, M., Mupeyo-Mudala, C., Banda-Tembo, S. et al. The effects of modern housing on malaria transmission in different endemic zones: a systematic review and meta-analysis. Malar J 23 , 235 (2024). https://doi.org/10.1186/s12936-024-05059-x
Download citation
Received : 17 December 2023
Accepted : 27 July 2024
Published : 07 August 2024
DOI : https://doi.org/10.1186/s12936-024-05059-x
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Modern housing
- Traditional
- Malaria-endemic zones
Malaria Journal
ISSN: 1475-2875
- Submission enquiries: [email protected]
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Microorganisms

Malaria: The Past and the Present
Jasminka talapko.
1 Faculty of Dental Medicine and Health, Josip Juraj Strossmayer University of Osijek, Crkvena 21, HR-31000 Osijek, Croatia; rh.zmdf@okpalatj (J.T.); rh.zmdf@vecva (A.V.)
Ivana Škrlec
Tamara alebić.
2 Faculty of Medicine, Josip Juraj Strossmayer University of Osijek, Josipa Huttlera 4, HR-31000 Osijek, Croatia; [email protected] (T.A.); moc.liamg@71ikujm (M.J.)
Melita Jukić
3 General Hospital Vukovar, Županijska 35, HR-32000 Vukovar, Croatia
Aleksandar Včev
Malaria is a severe disease caused by parasites of the genus Plasmodium , which is transmitted to humans by a bite of an infected female mosquito of the species Anopheles . Malaria remains the leading cause of mortality around the world, and early diagnosis and fast-acting treatment prevent unwanted outcomes. It is the most common disease in Africa and some countries of Asia, while in the developed world malaria occurs as imported from endemic areas. The sweet sagewort plant was used as early as the second century BC to treat malaria fever in China. Much later, quinine started being used as an antimalaria drug. A global battle against malaria started in 1955, and Croatia declared 1964 to be the year of eradication of malaria. The World Health Organization carries out a malaria control program on a global scale, focusing on local strengthening of primary health care, early diagnosis of the disease, timely treatment, and disease prevention. Globally, the burden of malaria is lower than ten years ago. However, in the last few years, there has been an increase in the number of malaria cases around the world. It is moving towards targets established by the WHO, but that progress has slowed down.
1. Introduction
Malaria affected an estimated 219 million people causing 435,000 deaths in 2017 globally. This burden of morbidity and mortality is a result of more than a century of global effort and research aimed at improving the prevention, diagnosis, and treatment of malaria [ 1 ]. Malaria is the most common disease in Africa and some countries in Asia with the highest number of indigenous cases. The malaria mortality rate globally ranges from 0.3–2.2%, and in cases of severe forms of malaria in regions with tropical climate from 11–30% [ 2 ]. Different studies showed that the prevalence of malaria parasite infection has increased since 2015 [ 3 , 4 ].
The causative agent of malaria is a small protozoon belonging to the group of Plasmodium species, and it consists of several subspecies. Some of the Plasmodium species cause disease in human [ 2 , 5 ]. The genus Plasmodium is an amoeboid intracellular parasite which accumulates malaria pigment (an insoluble metabolite of hemoglobin). Parasites on different vertebrates; some in red blood cells, and some in tissue. Of the 172 of Plasmodium species, five species can infect humans. These are P. malariae , P.falciparum , P.vivax , P.ovale , and P.knowlesi . In South-East Asia, the zoonotic malaria P.knowlesi is recorded. Other species rarely infect humans [ 5 , 6 , 7 , 8 ]. All the mentioned Plasmodium species cause the disease commonly known as malaria (Latin for Malus aer —bad air). Likewise, all species have similar morphology and biology [ 9 ].
The Plasmodium life cycle is very complex and takes place in two phases; sexual and asexual, the vector mosquitoes and the vertebrate hosts. In the vectors, mosquitoes, the sexual phase of the parasite’s life cycle occurs. The asexual phase of the life cycle occurs in humans, the intermediate host for malaria [ 9 , 10 ]. Human malaria is transmitted only by female mosquitoes of the genus Anopheles . The parasite, in the form of sporozoite, after a bite by an infected female mosquito, enters the human blood and after half an hour of blood circulation, enters the hepatocytes [ 11 ]. The first phase of Plasmodium asexual development occurs in the hepatocytes, and then in the erythrocytes. All Plasmodium species lead to the rupture of erythrocytes [ 7 , 9 , 12 , 13 ].
The most common species in the Americas and Europe are P.vivax and P.malariae , while in Africa it is P.falciparum [ 14 ].
2. Discovery of Malaria
It is believed that the history of malaria outbreaks goes back to the beginnings of civilization. It is the most widespread disease due to which many people have lost lives and is even thought to have been the cause of major military defeats, as well as the disappearance of some nations [ 15 ]. The first descriptions of malaria are found in ancient Chinese medical records of 2700 BC, and 1200 years later in the Ebers Papyrus [ 2 ]. The military leader Alexander the Great died from malaria [ 15 ]. The evidence that this disease was present within all layers of society is in the fact that Christopher Columbus, Albrecht Dürer, Cesare Borgia, and George Washington all suffered from it [ 16 , 17 ].
Although the ancient people frequently faced malaria and its symptoms, the fever that would occur in patients was attributed to various supernatural forces and angry divinities. It is, thus, stated that the Assyrian-Babylonian deity Nergal was portrayed as a stylized two-winged insect, as was the Canaan Zebub (‘Beelzebub, in translation: the master of the fly’) [ 17 ]. In the 4th century BC, Hippocrates described this disease in a way that completely rejected its demonic origins and linked it with evaporation from swamps which, when inhaled, caused the disease. That interpretation was maintained until 1880 and Laveran’s discovery of the cause of the disease [ 18 ]. Laveran, a French military surgeon, first observed parasites in the blood of malaria patients, and for that discovery he received the Nobel Prize in 1907 [ 19 ].
Cartwright and Biddis state that malaria is considered to be the most widespread African disease [ 14 ]. The causative agent of malaria is a small protozoon belonging to the group of Plasmodium species, and it consists of several subspecies [ 14 ].
3. The Development of Diagnostic Tests for Proving Malaria through History
Malaria can last for three and up to five years, if left untreated, and depending on the cause, may recrudesce. In P. vivax and ovale infections, the persistence of the merozoites in the blood or hypnozoites in hepatocytes can cause relapse months or years after the initial infection. Additionally, relapse of vivax malaria is common after P. falciparum infection in Southeast Asia. Relapse cases were observed in P. falciparum infections, which can lead to a rapid high parasitemia with subsequent destruction of erythrocytes [ 20 , 21 ]. Children, pregnant women, immunocompromised and splenectomized patients are especially vulnerable to malaria infection, as well as healthy people without prior contact with Plasmodium . A laboratory test for malaria should always confirm clinical findings. The proving of malaria is carried out by direct methods such as evidence of parasites or parts of parasites, and indirect methods that prove the antibodies to the causative agents ( Table 1 ) [ 2 , 5 , 22 ].
Diagnostic tests for proving malaria.
| Advantages | Disadvantages | |
|---|---|---|
| Microscopic analysis | Fast test, cheap | Required much experience as well as equipment |
| Rapid diagnostic tests | Quick and simple | Less sensitive and accurate, price |
| Molecular tests | Correct determination of type, highly sensitive and accurate | Price, long-term in a large number of cases |
| Indirect immunofluorescence | Specific, sensitive | Long time to perform, subjective evaluation of results |
| ELISA | Correct determination of type, specific, sensitive | Long time to perform, price |
The gold standard method for malaria diagnosis is light microscopy of stained blood films by Giemsa. Due to a lack of proper staining material and trained technicians, this method is not available in many parts of sub-Saharan Africa. The sensitivity of the method depends on the professional expertise, and it is possible to detect an infection with 10–100 parasites/μL of blood. A negative finding in patients with symptoms does not exclude malaria, but smears should be repeated three times in intervals of 12–24 h if the disease is still suspected [ 23 , 24 ]. Diagnosis of malaria using serologic testing has traditionally been done by immunofluorescence antibody testing (IFA). IFA is time-consuming and subjective. It is valuable in epidemiological studies, for screening possible blood donors. It also demands fluorescence microscopy and qualified technicians [ 23 , 25 , 26 ].
Rapid Diagnostic Tests (RDT) for the detection of antigens in the blood are immunochromatographic tests to prove the presence of parasite antigens. No electrical equipment, and no special experience or skills are required to perform these tests. The RDTs are now recommended by WHO as the first choice of test all across the world in all malaria-endemic areas. The sensitivity of the antigen test varies depending on the selected antigens represented in the test. For some RDTs is 50–100 parasites/μL (PfHRP2) to <100 parasites/μL [ 27 , 28 ]. The FDA approved the first RDT test in 2007. It is recommended that the results of all RDT tests should be confirmed by microscopic blood analysis [ 29 ]. It is known that antigens detected with RDT test remain in the blood after antimalarial treatment, but the existence of these antigens varies after treatment. The false-positive rates should be less than 10% [ 30 ]. Several RDT tests in the eight rounds of testing revealed malaria at a low-density parasite (200 parasites/μL), had low false-positive rates and could detect P. falciparum or P. vivax infections or both [ 30 ]. False-positive rates of P. vivax were typically small, between 5% and 15%. On the other hand, the false-positive rates of P. falciparum range from 3–32% [ 30 , 31 ]. Good RDTs might occasionally give false-negative results if the parasite density is low, or if variations in the production of parasite antigen reduce the ability of the RDT to detect the parasite. False negative results of the RDT test for P. falciparum ranged between 1% and 11% [ 31 , 32 , 33 , 34 ]. The overall sensitivity of RDTs is 82% (range 81–99%), and specificity is 89% (range 88–99%) [ 35 ].
Polymerase chain reaction (PCR) is another method in the detection of malaria. This method is more sensitive and more specific than all conventional methods in the detection of malaria. It can detect below one parasite/μL. PCR test confirms the presence of parasitic nucleic acid [ 23 , 36 ]. PCR results are often not available fast enough to be useful in malaria diagnosis in endemic areas. However, this method is most helpful in identifying Plasmodium species after diagnosis by microscopy or RDT test in laboratories that might not have microscopic experts. Additionally, PCR is useful for the monitoring of patients receiving antimalaria treatment [ 36 , 37 ].
Indirect methods are used to demonstrate antibodies to malaria-causing agents. Such methods are used in testing people who have been or might be at risk of malaria, such as blood donors and pregnant women. The method is based on an indirect immunofluorescence assay (IFA) or an ELISA test. The IFA is specific and sensitive but not suitable for a large number of samples, and the results are subjective evaluations. For serological testing, ELISA tests are more commonly used [ 26 ].
Rapid and accurate diagnosis of malaria is an integral part of appropriate treatment for affected person and the prevention of the further spread of the infection in the community.
4. Malaria Treatment through History
Already in the 2nd century BC, a sweet sagewort plant named Qinghai (Latin Artemisia annua ) was used for the treatment of malaria in China [ 38 ]. Much later, in the 16th century, the Spanish invaders in Peru took over the cinchona medication against malaria obtained from the bark of the Cinchona tree (Latin Cinchona succirubra ). From this plant in 1820 the French chemists, Pierre Joseph Pelletie, and Joseph Bienaimé Caventou isolated the active ingredient quinine, which had been used for many years in the chemoprophylaxis and treatment of malaria. In 1970, a group of Chinese scientists led by Dr. Youyou Tu isolated the active substance artemisinin from the plant Artemisia annua , an antimalarial that has proved to be very useful in treating malaria. For that discovery, Youyou Tu received the Nobel Prize for Physiology and Medicine in 2015 [ 39 , 40 , 41 ]. Most of the artemisinin-related drugs used today are prodrugs, which are activated by hydrolysis to the metabolite dihydroartemisinin. Artemisinin drugs exhibit its antimalarial activity by forming the radical via a peroxide linkage [ 42 ]. WHO recommends the use of artemisinin-based combination therapies (ACT) to ensure a high cure rate of P. falciparum malaria and reduce the spread of drug resistance. ACT therapies are used due to high resistance to chloroquine, sulfadoxine-pyrimethamine, and amodiaquine [ 1 ]. Due to the unique structure of artemisinins, there is much space for further research. Extensive efforts are devoted to clarification of drug targets and mechanisms of action, the improvement of pharmacokinetic properties, and identifying a new generation of artemisinins against resistant Plasmodium strains [ 42 ].
The German chemist Othmer Zeidler synthesized dichlorodiphenyltrichloroethane (DDT) in 1874 during his Ph.D. At that time, no uses of DDT was found, and it just became a useless chemical [ 43 ]. The insecticide property of DDT was discovered in 1939 by Paul Müller in Switzerland. DDT began to be used to control malaria at the end of the Second World War [ 40 ]. During the Second World War, the success of DDT quickly led to the introduction of other chlorinated hydrocarbons which were used in large amounts for the control of diseases transmitted by mosquito [ 43 ]. From the late Middle Ages until 1940, when DDT began to be applied, two-thirds of the world’s population had been exposed to malaria, a fact that represented a severe health, demographic, and economic problem [ 29 , 40 , 41 , 44 , 45 ]. DDT is an organochlorine pesticide which was applied in liquid and powder form against the insects. During the Second World War people were sprayed with DDT. After the war, DDT became a powerful way of fighting malaria by attacking the vector [ 43 ].
Five Nobel Prizes associated with malaria were awarded: Youyou Tu in 2015. Ronald Ross received the Nobel Prize in 1902 for the discovery and significance of mosquitoes in the biology of the causative agents in malaria. In 1907, the Nobel was awarded to the already-mentioned Charles Louis Alphonse Laveran for the discovery of the causative agent. Julius Wagner-Jauregg received it in 1927 for the induction of malaria as a pyrotherapy procedure in the treatment of paralytic dementia. In 1947 Paul Müller received it for the synthetic pesticide formula dichlorodiphenyltrichloroethane.
Attempts to produce an effective antimalarial vaccine and its clinical trials are underway. Over the past several decades’ numerous efforts have been made to develop effective and affordable preventive antimalaria vaccines. Numerous clinical trials are completed in the past few years. Nowadays are ongoing clinical trials for the development of next-generation malaria vaccines. The main issue is P. vivax vaccine, whose research requires further investigations to identify novel vaccine candidates [ 46 , 47 , 48 ]. Despite decades of research in vaccine development, an effective antimalaria vaccine has not yet been developed (i.e., with efficacy higher than 50%) [ 49 , 50 , 51 ]. The European Union Clinical Trials Register currently displays 48 clinical trials with a EudraCT protocol for malaria, of which 13 are still ongoing clinical trials [ 52 ]. The malaria parasite is a complex organism with a complex life cycle which can avoid the immune system, making it very difficult to create a vaccine. During the different stages of the Plasmodium life cycle, it undergoes morphological changes and exhibits antigenic variations. Plasmodium proteins are highly polymorphic, and its functions are redundant. Also, the development of malaria disease depends on the Plasmodium species. That way, a combination of different adjuvants type into antigen-specific formulations would achieve a higher efficacy [ 53 , 54 ]. Drugs that underwent clinical trials proved to be mostly ineffective [ 5 , 7 , 55 ]. However, many scientists around the world are working on the development of an effective vaccine [ 56 , 57 , 58 ]. Since other methods of suppressing malaria, including medication, insecticides, and bed nets treated with pesticides, have failed to eradicate the disease, and the search for a vaccine is considered to be one of the most important research projects in public health by World Health Organization (WHO).
The best way to fight malaria is to prevent insect bites. Malaria therapy is administered using antimalarial drugs that have evolved from quinine. According to its primary effect, malarial vaccines are divided into pre-erythrocytic (sporozoite and liver-stage), blood-stage, and transmission-blocking vaccines [ 9 , 54 ]. Most medications used in the treatment are active against parasitic forms in the blood (the type that causes disease) ( Table 2 ) [ 59 ]. The two crucial antimalarial medications currently used are derived from plants whose medical importance has been known for centuries: artemisinin from the plant Qinghao ( Artemisia annua L, China, 4th century) and quinine from Cinchona (South America, 17th century). Side-by-side with artemisinin, quinine is one of the most effective antimalarial drugs available today [ 13 , 39 , 40 ]. Doxycycline is indicated for malaria chemoprophylaxis for travel in endemic areas. It is also used in combination with the quinine or artesunate for malaria treatment when ACT is unavailable or when the treatment of severe malaria with artesunate fails. The disadvantage of doxycycline is that children and pregnant women cannot use it [ 29 ]. Due to the global resistance of P. falciparum to chloroquine, ACTs are recommended for the treatment of malaria, except in the first trimester of pregnancy. ACTs consist of a combination of an artemisinin derivative that fast decreases parasitemia and a partner drug that eliminates remaining parasites over a more extended period. The most common ACTs in use are artemether-lumefantrine, artesunate-amodiaquine, dihydroartemisinin-piperaquine, artesunate-mefloquine, and artesunate with sulfadoxine-pyrimethamine. The ACTs were very efficient against all P. falciparum until recently when numbers of treatment failures raised in parts of Southeast Asia. Atovaquone-proguanil is an option non-artemisinin-based treatment that is helpful for individual cases which have failed therapy with usual ACTs. Although, it is not approved for comprehensive implementation in endemic countries because of the ability for the rapid development of atovaquone resistance. Quinine remains efficient, although it needs a long course of treatment, is poorly tolerated, especially by children, and must be combined with another drug, such as doxycycline or clindamycin. Uncomplicated vivax, malariae, and ovale malaria are handled with chloroquine except in case of chloroquine-resistant P. vivax when an ACT is used [ 7 , 29 , 60 , 61 , 62 ].
Overview of the most commonly used antimalarials.
| Medication Name | Year of Discovery/Synthesis | Origin | Usage | Mechanism of Action | Side Effects | Advantages/Disadvantages |
|---|---|---|---|---|---|---|
| Quinine | 1600 | tree, South America | Resistance to chloroquine, prophylaxis and treatment of malaria | Inhibition of DNA and RNA synthesis | Headache, abortion, or congenital malformations if taken during pregnancy | Toxic, less effective than other medication |
| Chloroquine | 1934 | Synthesized by German scientist Hans Andersag | Most powerful remedy for the prophylaxis and treatment of malaria | Inhibition of DNA and RNA synthesis | Gastrointestinal disturbances, headache, skin irritation | Developed resistance of most strains to the medication |
| Primaquine | 1953 | The 8-aminoquinoline derivative | Infections with and , prophylaxis and treatment of malaria | Interferes in transport chain of electrons and destroys parasite mitochondria | Anorexia, nausea, anemia, headaches, contraindicated in pregnancy and children under 4 years of age | Prevent relapse in and infection |
| Doxycycline | 1960 | Pfizer Inc. New York | Prophylaxis in areas with chloroquine resistance and against mefloquine resistant | Inhibition of protein synthesis by binding to 30S ribosomal subunit | Gastrointestinal disorders, nausea, vomiting, photosensitivity | Effective and cheap, use for treatment and prophylaxis in all malarious areas |
| Mefloquine | 1971 | USA army and WHO | Multiresistant strains, prophylaxis and treatment of malaria | Damage to parasite membrane | Gastrointestinal disorders, CNS disorder, contraindicated in pregnancy and patients with epilepsy | Partial resistance, brain damage |
| Proguanil (chloroguanide) | 1953 | Biguanide derivate | Prophylaxis in infections with | Inhibition of DNA synthesis | Digestive problems only in large doses | The least toxic antimalarial drug |
| Pyrimethamine | 1953 | Pyrimidine derivatives | For tissue parasites, prophylaxis and treatment of malaria | Folic acid antagonist | Gastrointestinal disorders, neuropathy, in high doses also megaloblastic anemia | Rapid development of resistance |
| Atovaquone/proguanil | 2000 | Ubiquinone analog | For the prophylaxis and treatment of malaria | Inhibition of cytochrome bc1 in | Nausea, vomiting, diarrhea, headache, dizziness, anxiety, difficulty falling asleep, rash, fever | Most commonly used, fewer side effects and more expensive than mefloquine, resistance |
CNS—central nervous system.
4.1. Malaria in Europe
In Europe, malaria outbreaks occurred in the Roman Empire [ 63 , 64 ] and the 17th century. Up until the 17th century it was treated as any fever that people of the time encountered. The methods applied were not sufficient and included the release of blood, starvation, and body cleansing. As the first effective antimalarial drug, the medicinal bark of the Cinchona tree containing quinine was mentioned and was initially used by the Peruvian population [ 14 ]. It is believed that in the fourth decade of the 17th century it was transferred to Europe through the Spanish Jesuit missionaries who spread the treatment to Europe [ 65 ].
Contemporary knowledge of malaria treatment is the result of the work of a few researchers. Some of researchers are Alphonse Laveran, Ronald Ross, and Giovanni Battista Grassi. In November 1880, Laveran, who worked as a military doctor in Algeria, discovered the causative agents of malaria in the blood of mosquitoes and found that it was a kind of protozoa [ 66 ]. Laveran noticed that protozoa could, just like bacteria, live a parasitic way of life within humans and thus cause disease [ 66 ]. Nearly two decades later, more precisely in 1898, Ronald Ross, a military doctor in India, discovered the transmission of bird malaria in the saliva of infected mosquitos, while the Italian physician Giovanni Battista Grassi proved that malaria was transmitted from mosquitoes to humans, in the same year. He also proved that not all mosquitoes transmit malaria, but only a specific species ( Anopheles ) [ 17 ]. This discovery paved the way for further research.
The global battle against malaria started in 1955, and the program was based on the elimination of mosquitoes using DDT and included malarial areas of the United States, Southern Europe, the Caribbean, South Asia, but only three African countries (South Africa, Zimbabwe, and Swaziland). In 1975, the WHO announced that malaria had been eradicated in Europe and all recorded cases were introduced through migration [ 67 , 68 ].
4.2. Malaria in Croatia
In Croatia, the first written document that testifies to the prevention of malaria is the Statute of the town of Korčula from 1265. In 1874, the Law on Health Care of Croatia and Slavonia established the public health service that was directed towards treating malaria. There was no awareness nor proper medical knowledge about malaria, but the drainage was carried out to bring the ‘healthy air’ in the cities [ 69 , 70 ]. In 1798 physician Giuseppe Arduino notified the Austrian government about malaria in Istria. A government representative Vincenzo Benini accepted a proposed sanitary measure of the drainage of wetlands [ 71 ]. In 1864, the drainage of wetlands around Pula and on the coastal islands began, and since 1902 a program for the suppression of malaria by treatment of patients using quinine has been applied [ 72 ]. In 1922, the Institute for Malaria was founded in Trogir. In 1923, on the island of Krk, a project was started to eradicate malaria by the sanitation of water surfaces and the treatment of the patients with quinine, led by Dr. Otmar Trausmiller [ 66 ]. Since 1924, besides chemical treatment, biological control of mosquitoes has been established by introducing the fish Gambusia holbrooki to Istria and the coast [ 73 ]. In 1930 legislation was passed to enforce village sanitation, which included the construction of water infrastructure and safe wells, contributing to the prevention of malaria. Regular mosquito fogging with arsenic green (copper acetoarsenite) was introduced, and larvicidal disinfection of stagnant water was carried out.
Since malaria occurs near swamps, streams, ravines, and places where mosquitoes live near water, this disease has been present throughout history in Croatia, and it has often become an epidemic [ 74 ]. It was widespread in the area of Dalmatia, the Croatian Littoral region, Istria, and river flows. In the area of the Croatian Littoral, it was widespread on some islands, such as Krk, Rab, and Pag, while the mainland was left mainly clear of it [ 75 ]. The ethnographer Alberto Fortis (1741–1803) who traveled to Dalmatia, noted impressions recording details of malaria that was a problem in the Neretva River valley. Fortis wanted to visit that area, but the sailors on ship were afraid, probably because the were afraid to go to a place where there had been a disease outbreak known as the Neretva plague [ 76 ]. This Neretva plague was, in fact, malaria, and it is believed that due to it, the Neretva was nicknamed “Neretva—damned by God” [ 77 , 78 ]. Speaking of the Neretva region, Fortis states that the number of mosquitoes in that wetland area was so high that people had to sleep in stuffy canopy tents to defend themselves. Fortis also states that there were so many mosquitoes that he was affected by it. During the stay, Fortis met a priest who had a bump on the head claiming it had occurred after a mosquito bite and believed that the fever that infected the people of the Neretva Valley was also a consequence of the insect bites there [ 76 ]. In a manuscript, Dugački described some of the epidemics in Croatia. Thus, noted the small population of Nin in 1348, which was the result of the unhealthy air and high mortality of the population. Three centuries later, in 1646, the fever was mentioned in Novigrad, while the year 1717 was crucial for to the Istrian town of Dvigrad, which was utterly deserted due to malaria. At the beginning of the 20th century, more precisely in 1902, the daily press reported that the Provincial Hospital in Zadar was full of people affected by malaria. The extent to which this illness was widespread is proved by the fact that at the beginning of the 20th century about 180,000 people suffered from it in Dalmatia [ 18 ]. The volume and frequency of epidemics in Dalmatia resulted in the arrival of the Italian malariologist Grassi and the German parasitologist Schaudin. The procedures of quininization began to be applied, and in 1908 25 physicians and 423 pill distributors were to visit the villages and divide pills that had to be taken regularly to the people to eradicate malaria [ 75 ].
Likewise, in Slavonia, malaria had also a noticeable effect, and it was widespread in the 18th century due to a large number of swamps that covered the region. Such areas were extremely devastating for settlers who were more vulnerable to the disease than its domestic population [ 79 ]. Friedrich Wilhelm von Taube (1728–1778) recorded the disease and stated that the immigrant Germans were primarily affected by malaria and that the cities of Osijek and Petrovaradin can be nicknamed "German Cemeteries" [ 80 ]. According to Skenderović, the high mortality of German settlers from malaria was not limited only to the Slavonia region but also to the Danubian regions in which the Germans had settled in the 18th century, with Banat and Bačka [ 79 ] having the most significant number of malaria cases. The perception of Slavonia in the 18th century was not a positive one. Even Taube stated that Slavonia was not in good standing in the Habsburg Monarchy and that the nobility avoided living there. As some of the reasons for this avoidance, Taube mentioned the unhealthy air and the many swamps in the area around in which there was a multitude of insects. Taube noted that mosquitoes appear to be larger than in Germany and that its bite was much more painful. A change in the situation could only be brought about by drying the swamp, in his opinion [ 80 ]. Since malaria had led to the death of a large number of people, the solution had to be found to stop its further spread. Swamp drying was finally accepted by the Habsburg Monarchy and some European countries as a practical solution and, thus, its drainage began during the 18th century, resulting in cultivated fields [ 79 ].
Since epidemics of malaria continued to occur, there is one more significant record of the disease in the Medical Journal of 1877. In it, the physician A. Holzer cites his experiences from Lipik and Daruvar where he had been a spa physician for a long time. Holzer warns of the painful illness noticed at spa visitors suffering from the most in July and August. As a physician, Holzer could not remain indifferent to the fact that he did not see anyone looking healthy. It also pointed out that other parts of Croatia were not an exception. As an example, Holzer noted Virovitica County, where malaria was also widespread. He wanted to prevent the development and spread of the illness. Believing that preventing the toxic substances from rising into the air would stop the disease, the solution was to use charcoal that has the properties of absorbing various gases and, thus, prevents vapor rising from the ground [ 81 ].
Dr. Andrija Štampar (1888–1958) holds a prominent place in preventing the spread of malaria. Štampar founded the Department of Malaria, and numerous antimalaria stations, hygiene institutes, and homes of national health. Dr. Štampar devoted his life to educating the broader population about healthy habits and, thus, prevents the spread of infectious diseases. Many films were shown, including a film entitled ‘Malaria of Trogir’ in Osijek in 1927, with numerous health lectures on malaria [ 82 ]. After the end of the Second World War, a proposal for malaria eradication measures was drafted by Dr. Branko Richter. These measures, thanks to Dr. Andrija Štampar, are being used in many malaria-burdened countries. For the eradication of malaria in Croatia and throughout Yugoslavia, DDT has been used since 1947 [ 83 ].
Malaria is still one of the most infectious diseases that cause far more deaths than all parasitic diseases together. Malaria was eradicated in Europe in 1975. After that year, malaria cases in Europe are linked to travel and immigrants coming from endemic areas. Although the potential for malaria spreading in Europe is very low, especially in its western and northern parts, it is still necessary to raise awareness of this disease and keep public health at a high level in order to prevent the possibility of transmitting the disease to the most vulnerable parts of Europe [ 84 ].
Unofficial data show that malaria disappeared from Croatia in 1958, while the World Health Organization cites 1964 as the year when malaria was officially eradicated in Croatia [ 45 , 75 ]. Nonetheless, some cases of imported malaria have been reported in Croatia since 1964. The imported malaria is evident concerning Croatia’s orientation to maritime affairs, tourism, and business trips. Namely, malaria is introduced to Croatia by foreign and domestic sailors, and in rare cases by tourists, mainly from the countries of Africa and Asia [ 75 , 85 ]. According to the reports of the Croatian Institute of Public Health, since the eradication of this disease 423 malaria cases have been reported, all imported [ 86 ]. Figure 1 shows the number of imported malaria cases in Croatia from 1987–2017, and Figure 2 the causative Plasmodium species of those cases ( Figure 1 and Figure 2 ) [ 86 , 87 ].

Imported malaria cases in Croatia from 1987–2017.

The causative agents of imported malaria in Croatia.
There is also massive and uncontrollable migration from Africa and Asia (mostly due to climate change) of both humans and birds, from countries with confirmed epidemics. This issue is an insurmountable problem if measured by the traditional approach. Insecticides (DDT, malathion, etc.) synthetic pyrethroids, in addition to inefficiency, impact the environment (harm bees, fruits, vines, etc.). Consequently, scientists have patiently established a mosquito control strategy (University of Grenoble, Montpellier) which includes a meticulous solution to the mosquito vector effect (malaria, arbovirus infection, West Nile virus) by changes in agriculture, urbanism, public services hygiene [ 88 ].
Northeastern Slavonia is committed to applying methods that are consistent with such achievements, with varying success, as certain limitations apply to protected natural habitats (Kopački rit) [ 89 ].
There is a historical link between population movement and global public health. Due to its unique geostrategic position, in the past, Croatia has been the first to experience epidemics that came to Europe through land and sea routes from the east. Adriatic ports and international airports are still a potential entry for the import of individual cases of communicable diseases. Over the past few years, sailors, as well as soldiers who worked in countries with endemic malaria, played a significant role in importing malaria into Croatia. Successful malaria eradication has been carried out in Croatia. Despite that in Croatia are still many types of Anopheles , which means that the conditions of transmission of the imported malaria from the endemic areas still exist. The risk of malaria recrudesce is determined by the presence of the vector, but also by the number of infected people in the area. Due to climate change, it is necessary to monitor the vectors and people at risk of malaria. Naturally- and artificially-created catastrophes, such as wars and mass people migration from endemic areas, could favor recrudescing of malaria. Once achieved, eradication would be maintained if the vector capacities are low and prevention measures are implemented. The increased number of malaria cases worldwide, the recrudesce of indigenous malaria cases in the countries where the disease has been eradicated, the existence of mosquitoes that transmit malaria and the number of imported malaria cases in Croatia are alarming facts. Health surveillance, including obligatory and appropriate prophylaxis for travelers to endemic areas, remains a necessary public health care measure pointed at managing malaria in Croatia.
5. Malaria Trends in the World
The WHO report on malaria in 2017 shows that it is difficult to achieve two crucial goals of a Global Technical Strategy for Malaria. These are a reduction in mortality and morbidity by at least 40% by 2020. Since 2010, there has been a significant reduction in the burden of malaria, but analysis suggests a slowdown, and even an increase in the number of cases between 2015 and 2017. Thus, the number of malaria cases in 2017 has risen to 219 million, compared to 214 million cases in 2015 and 239 million cases in 2010. Figure 3 presents the reported number of malaria cases per WHO region from 1990–2017 [ 1 , 90 ]. The most critical step in the global eradication of malaria is to reduce the number of cases in countries with the highest burden (many in Africa). The number of deaths from disease is declining, thus, in 2017 there were 435,000 deaths from malaria globally, compared with 451,000 in 2016, and 607,000 deaths in 2010. Figure 4 presents the number of malaria deaths from 1990-2017 [ 1 , 90 ]. Despite the delay in global progress, there are countries with decreasing malaria cases during 2017. Thus, India in 2017, compared with 2016, recorded a 24% decline of malaria cases. The number of countries reporting less than 10,000 malaria cases is growing, from 37 countries in 2010, to 44 in 2016, and to 46 in 2017. Furthermore, the number of countries with fewer than 100 indigenous malaria cases growing from 15 in 2010, to 26 countries in 2017 [ 1 ].

Reported malaria cases per WHO region from 1990–2017.

Reported malaria deaths per WHO region from 1990–2017.
Funding in malaria has not changed much. During 2017, US$3.1 billion was invested in malaria control and elimination globally. That was 47% of the expected amount by 2020. The USA was the largest single international donor for malaria in 2017 [ 1 , 91 ].
The most common global method of preventing malaria is insecticide-treated bed nets (ITNs). The WHO report on insecticide resistance showed that mosquitoes became resistant to the four most frequently used classes of insecticides (pyrethroids, organochlorines, carbamates, and organophosphates), which are widespread in all malaria-endemic countries [ 1 , 7 , 92 ].
Drug resistance is a severe global problem, but the immediate threat is low, and ACT remains an effective therapy in most malaria-endemic countries [ 1 , 93 ].
According to the WHO, Africa still has the highest burden of malaria cases, with 200 million cases (92%) in 2017, then Southeast Asia (5%), and the Eastern Mediterranean region (2%). The WHO Global Technical Strategy for Malaria by 2020 is the eradication of malaria from at least ten countries that were malaria-endemic in 2015 [ 1 ].
The march towards malaria eradication is uneven. Indigenous cases in Europe, Central Asia, and some countries in Latin America are now sporadic. However, in many sub-Saharan African countries, elimination of malaria is more complicated, and there are indications that progress in this direction has delayed. Elimination of vivax and human knowlesi malaria infections are another challenge [ 7 ].
6. Conclusions
The campaign to eradicate malaria began in the 1950s but failed globally due to problems involving the resistance of mosquitoes to the insecticides used, the resistance of malaria parasites to medication used in the treatment, and administrative issues. Additionally, the first eradication campaigns never included most of Africa, where malaria is the most common. Although the majority of forms of malaria are successfully treated with the existing antimalarials, morbidity and mortality caused by malaria are continually increasing. This issue is the consequence of the ever-increasing development of parasite resistance to drugs, but also the increased mosquito resistance to insecticides, and has become one of the most critical problems in controlling malaria over recent years. Resistance has been reported to all antimalarial drugs. Therefore, research into finding and testing new antimalarials, as well as a potential vaccine, is still ongoing, mainly due to the sudden mass migration of humans (birds, parasite disease vector insects) from areas with a large and diverse infestation.
The process towards eradication in some countries confirms that current tools could be sufficient to eradicate malaria. The spread of insecticide resistance among the vectors and the rising ACT failures indicate that eradication of malaria by existing means might not be enough.
Thus, given the already complicated problem of overseeing and preventing the spread of the disease, it will be necessary to supplement and change the principles, strategic control, and treatment of malaria.
Abbreviations
| ACT | Artemisinin-based combination therapy |
| CNS | Central nervous system |
| DDT | Dichlorodiphenyltrichloroethane |
| ELISA | Enzyme-linked immunosorbent assay |
| FDA | Food and Drug Administration |
| IFA | Immunofluorescence test |
| ITN | Insecticide-treated bed nets |
| PCR | Polymerase chain reaction |
| PfHRP2 | Plasmodium falciparum histidine-rich protein 2 |
| RDT | Rapid diagnostic tests |
| WHO | World Health Organization |
Author Contributions
Writing the manuscript: J.T., I.Š., and T.A.; updating the text: J.T., I.Š., T.A., and A.V.; literature searches: J.T., I.Š., T.A., and M.J.; tables and figures drawing: I.Š. and M.J.; critical reviewing of the manuscript: A.V.; organization and editing of the manuscript: I.Š. and A.V.
This research received no external funding. The article processing charges (APC) was funded by Faculty of Dental Medicine and Health, Osijek, Croatia.
Conflicts of Interest
The authors declare no conflict of interest.
- Open access
- Published: 09 August 2024
The prevalence of low birth weight and its associated maternal factors among women of reproductive age who gave birth to live babies within five years preceding the survey in Tanzania: an analysis of data from the 2015-16 Tanzania Demographic and Health Survey and Malaria Indicators Survey
- Glorialoveness S. Lyimo 1 &
- Fabiola Vincent Moshi 2
BMC Pregnancy and Childbirth volume 24 , Article number: 523 ( 2024 ) Cite this article
48 Accesses
Metrics details
Infant survival is an important factor in any community’s health. Low birth weight affects babies not only during their infancy but also has long-term consequences for their health as adults. Unfortunately, Sub-Saharan Africa as a region is still dealing with the burden of Low birth weight (LBW), and Tanzania as a part of this region is no exception. So this study aimed to determine the Magnitude of Low Birth Weight and Its Associated Maternal Factors among Women of Reproductive Age who gave birth to live babies.
The study used analytical cross-sectional study design to analyze secondary data from the Tanzania Demographic and Health Survey and Malaria Indicators Survey 2015–2016. A total of 4,644 women of reproductive age who gave birth to live babies within five years preceding the survey were included in the study. Both bivariate and multivariable logistics regression analyses were used to assess maternal factors associated with low birth weight.
The prevalence of LBW was 262(6.2%). After adjusting for confounders, the maternal factors associated with LBW were Age group of a pregnant woman [Less than 20 years (aOR = 1.907 CI = 1.134–3.205) in reference to those aged more than 34years], Number of ANC visits made [Inadequate visits (aOR = 1.612 CI = 1.266–2.05)], parity [para 2–4 (aOR = 0.609 CI = 0.453–0.818), para 5+ (aOR = 0.612 CI = 0.397–0.944)] and area of residence [Unguja (aOR = 1.981 CI = 1.367–2.87).
The prevalence of low birth weight in Tanzania remains high. Women’s age, parity, number of Antenatal care visits (ANC), and area of residence were found to be maternal factors associated with LBW. Thus, early prenatal diagnosis of risk factors for low birth weight in high-risk pregnant women may help to reduce the LBW burden in Tanzania and its detrimental effects.
Peer Review reports
An infant ever-changing milestones, health, and survival depend on its birthweight among other factors. Defined by World Health Organization (WHO), low birth weight (LBW) refers to a birth weight of less than 2500 g (5.5 lb). LBW is a major public health issue around the world. It is a serious health concern for newborns. The policy brief on low birth weight envisions a 30% reduction in low birth weight by 2025 [ 1 ].
The proportion of total LBW in all live births worldwide is between 15% and 20%. This indicates that more than 20 million live births that occur yearly are LBW. It is staggering to note that nearly half of all births associated with LBW occur in South-Central Asia, while 15% occur in Sub-Saharan Africa [ 2 ]. LBW is responsible for 60–80% of all neonatal deaths worldwide [ 3 ]. Several studies have reported on the magnitude of LBW, indicating that it is a concern in many countries. In Bangladesh, the prevalence of LBW is as high as 23% [ 4 ]. A study of ten countries found that the average prevalence of LBW was about 15.9%, with Indonesia recording the highest LBW rate of 37.3% of all the countries studied [ 5 ]. A study which was done in selected facilities in Tanzania and Kenya indicated that the magnitude of LBW was 10% and 7.8% respectively [ 6 ].
LBW outcomes include, but are not limited to cognitive issues, Low Intelligence Quotient (IQ), hypoglycemia immunosuppression, and delayed milestones. The risk of dying from complications is 20 times higher in these babies in comparison to babies born within a normal weight range [ 7 ].
Different studies have linked the cause of LBW to a variety of factors being it fetal, maternal, infectious and other factors. Infant causes may include preterm birth or Intrauterine growth restriction and sometimes a combination of both [ 8 ].Maternal characteristics contributing to LBW involve nutrition status of a woman, birth interval, ANC follow-up, obesity, drug use, maternal height of less than 150, lack of folic acid intake during pregnancy and maternal age [ 7 , 8 , 9 , 10 ]. Infections also play a role in the cause of LBW and these include malaria infection in pregnancy, anemia and Human Immunodeficiency Virus (HIV) status of the mother. Other factors include environmental factors, and genetic factors like previous history of giving a small baby, history of a mother being born with LBW. All of these factors have been shown to have a significant impact on birth weight [ 8 , 11 , 12 ].
Neonatal mortality rates in Sub-Saharan Africa remain particularly alarming. Without a doubt, the Tanzanian government has taken enormous efforts to combat LBW, like improving maternal health through ensuring access to healthcare services even to marginalized communities, provision of quality antenatal care, family planning services, ensuring continuous improvement of clinical knowledge and skills of health providers so as to provide integrated care to high risk pregnant women [ 13 , 14 ]. Even with the effort put forward but the problem persists, and very few studies have been conducted to explore the maternal factors that are directly linked to LBW. Therefore, this study aimed to determine the Magnitude of Low Birth Weight and Its Associated Maternal Factors among Women of Reproductive Age who gave birth to live babies in Tanzania.
Study area and period
This study was based on secondary data sources from Tanzania Demographic and Health Survey and Malaria Indicators Survey 2015–2016. The survey was done in the United Republic of Tanzania from August 22nd, 2015 to February 14th, 2016. The country is located south of the equator and bordered by eight countries. To the north there are Kenya and Uganda, to the west there are Rwanda, Burundi, the Democratic Republic of Congo, and Zambia. Malawi and Mozambique are in the south.
Study design
The study used analytical cross-sectional study design to analyze the 2015-16 Tanzania Demographic and Health Survey and Malaria Indicator Survey (TDHS-MIS) data. The survey was led by the National Bureau of Statistics (NBS) and the Office of Chief Government Statistician (OCGS), Zanzibar, in collaboration with the Ministry of Health in Tanzania Mainland and the Ministry of Health of Zanzibar. The data collected were fertility levels, marriage, sexual activity, fertility preference, awareness and use of family planning methods, breastfeeding practices, nutrition, childhood and maternal mortality, maternal and child health, malaria, and other health-related issues.
The 2015-16 TDHS-MIS
The 2015-16 TDHS-MIS is a national based cross-sectional study utilizing the 2015-16 Tanzania Demographic and Health Survey and Malaria Indicator Survey (2015-16 TDHS-MIS) dataset. The primary objective of the 2015-16 TDHS-MIS was to provide up-to-date estimates of basic demographic and health indicators. The survey collected information on fertility levels, marriage, sexual activity, fertility preferences, awareness and use of family planning methods, breastfeeding practices, nutrition, childhood and maternal mortality, maternal and child health, malaria, and other health-related issues.
The sample design for the 2015-16 TDHS-MIS was done in two stages and was intended to provide estimates for the entire country, for urban and rural areas in Tanzania Mainland, and for Zanzibar. The first stage involved selecting sample points (clusters), consisting of enumeration areas (EAs) delineated for the 2012Tanzania Population and Housing Census. A total of 608 clusters were selected. In the second stage, a systematic selection of 13,360 households was done and out of those households selected, 12,767were occupied. Of the occupied households, 12,563 were successfully interviewed, yielding a response rate of 98%. In the interviewed households, 13,634 eligible women were identified for individual interviews; interviews were completed with 13,266 women, yielding a response rate of 97%.
Study population and data extraction
The subset of the original TDHS-MIS dataset was extracted using the criteria of women of reproductive age who gave birth to live babies within five years preceding the survey. All other outcome variables in the subset of the TDHS-MIS were dropped and a total of 6924 women were included. Furthermore, the outcome variable was assessed and all women of reproductive age who gave birth to live babies with no response to the birth weight of their neonates were removed. The final sample size used in this study was 4,644 women of reproductive age who gave birth to live babies. A sampling weight was utilized to ensure the representativeness of the study sample.
Data collection tool
The survey used household questionnaires and individual questionnaires. They are based on Measure Demographic and Health Survey (DHS) standards, Acquired Immunodeficiency Syndrome (AIDS) Indicator Survey and Malaria Indicator Survey questionnaires standards. The tool was translated into Kiswahili, the National language of Tanzania. The data used for this study were those collected using the individual questionnaire.
Figure 1 below shows the conceptual framework which was developed to guide the conceptualization. The framework had independent variables (socio-demographic characteristics, obstetric characteristics, and maternal services, ANC utilization, place of childbirth ever took malaria, iron supplement and deworming). The maternal age was categorized into three categories, those who were aged less than 20 years, those aged 20 to 34 years and those aged more than 34 years. Wealth index in this study was categorized into three categories, poor wealth index, middle wealth index and rich wealth index. From the original DHS data, the first two categories (poorest and poorer) were combined to form poor wealth index category, middle wealth category was maintained and the last two categories (richer and richest) were combined to form rich category. Two categories (adequate and inadequate) were used for ANC services utilization. For pregnant women who made at least four ANC visits were considered as had adequate ANC services utilization and for those who made less than or no visit were considered as had inadequate ANC visits. For the place of childbirth, there were two categories either in health facility or outside health facility (home or birth before arrival). For anemia preventive services (deworming, intermittent preventive treatment of malaria and iron supplement), these variables were assessed based on whether interviewed women took the service at least once. The dependent variable was the birth weight of neonates. The neonates assessed were the youngest child born within five years preceding the survey. The dependent variable had two categories, low birth weight (weight less than 2500 g) and this was coded as 1 and normal birth weight (weight of ≥ 2500 g).

Conceptual framework
Data analysis
Data were analyzed using Statistical Package for Social Sciences (SPSS) version 25. All variables were described using frequencies and percentages. The relationships between independent variables (maternal characteristics) and outcome variable (birth weight) were assessed using a chi-square test. All variables with p-value ≤ 0.05 were entered into a regression model. First bivariate analysis was done to establish the Crude Odds ratio (COR). The maternal factors associated with low birth weight were established using multivariable logistic regression. Adjusted Odds Ratios (AOR) with a 95% Confidence Interval (CI) and p-value of 0.05 was used to identify significant maternal factors associated with low birth weight.
The majority of study respondents were aged 20-34years, were living in rural areas, had a primary level of education, were rich, had birth in a health facility, never took malaria treatment, and had 2 to 4 children (Table 1 ).
Birth-weight
A total of 262 (6.2%) neonates were born with low birth weight while 3935(93.8%) were born with normal birth weight (Fig. 2 ).

Prevalence of birth weight
The relationship between pregnant women’s characteristics and neonatal birth weight
Variables that showed a significant relationship with neonatal birth weight were age group (X 2 = 35.442, p < 0.001), ever received intermittent treatment for malaria at least once (X 2 = 5.98, p = 0.014), number of antenatal visits the pregnant women made (X 2 = 12.432, p < 0.001), parity of the mother (X 2 = 27.478, p < 0.001) and area of residence (X 2 = 9.686, p = 0.02 ) Table 2 .
Factors associated with low birth weight
Factors associated with low birth weight were the age group of a pregnant woman [ Less than 20 years (aOR = 1.907 at 95%CI = 1.134–3.205, p = 0.015)], Number of ANC visits made [Inadequate visits (aOR = 1.612 at 95%CI = 1.266–2.052, p < 0.001)], parity [ para 2–4(aOR = 0.609at 95%CI = 0.453–0.818, p = 0.001), para 5+ (aOR = 0.612 at 95%CI = 0.397–0.944, p = 0.026) and area of residence [Unguja (aOR = 1.981 at95%CI = 1.367–2.87, p < 0.001) Table 3 .
LBW is an important indicator of neonatal mortality and morbidity, it is a priority issue in children’s health globally. LBW prevalence varies greatly. According to the findings of the current study, 6.2% of all births are LBW. These findings are consistent with the findings of studies conducted in selected African countries in which the prevalence of LBW in Uganda was 10% [ 15 ]. Also, another study conducted in Subsaharan Africa indicated that the prevalence of LBW in Guinea was 6.3% [ 2 ] This agreement can be explained by the fact that both studies were community surveys. However, the prevalence of LBW in this study is lower than the studies conducted in South Sudan 23.5% [ 16 ] and Kolkata 38% [ 17 ]. This discrepancy may be due to design and geographical area differences with the current study.
Pregnant women’s characteristics have increasingly been linked as an important element of a neonate’s birth weight. In this study, the age group of women, the number of antenatal visits made by pregnant women, the mother’s parity, and the area of residence were all significantly related to neonate birth weight.
Women of less than 20 years of age were more likely to deliver babies with low birth weight compared to those aged 34 and above. This result may be attributed to the fact that women aged 20 and under are likely to be pregnant for the first time at this age and may have little antenatal experience. As a result, this may negatively influence the weight of the baby as they can’t articulate what’s best for themselves and the baby in terms of nutrition and healthcare-seeking behavior [ 18 ]. This finding is consistent with a study conducted in India, which found a 50% prevalence of LBW among women aged 18 and under [ 19 ]. On the other hand, this finding is not in agreement with the study done in Malaysia where it was revealed that there was no statistically significant difference between maternal age and the chance of delivering low birth weight infants [ 20 ]. The difference between these two studies may be attributed to variations in study time and setup.
Furthermore, women who received inadequate antenatal care visits were more likely to have babies with LBW than their counterparts who received adequate ANC visits. Tanzania’s Ministry of Health adopted the Focused Antenatal Care model from WHO in year 2002 which recommended a minimum of four antenatal visits. Among other services, women are given health education about the risks of LBW and how to avoid them during these visits. That could explain why there is a difference in the overall health of a newborn baby between those who had an adequate visit and those who did not [ 21 ]. This finding is supported by the findings of a study done in Iraq which concluded that there was an association between ANC visits and LBW in which attending at least four visits is linked with a low prevalence of LBW [ 22 ]. On the other hand, this finding was not congruent with a study done in Northwest Ethiopia which displayed no significant association between low birth weight and ANC follow-up [ 23 ]. This disagreement can be explained by the socio-cultural difference between the participants.
Furthermore, the study findings revealed that parity is one of the determinants of LBW. Women with 2–4 children were 0.609 more likely to have babies with LBW, while those with 5 + parity were 0.612 more likely to have neonates with LBW than their counterparts with less than 2 children. This study supports the findings of an Ethiopian study. The study found that women with parity 5 or more had a higher chance of having LBW babies than multiparous women. In contrast, the same study found that primiparous women were less likely than multiparous women to have LBW babies [ 24 ]. Meanwhile, this study is not in agreement with a study done in five low and middle-income countries Democratic Republic of Congo (DRC), Guate mala, Belagavi, and Nagpur, India, and Pakistan whereby, nulliparous women had a lower chance of delivering an LBW as compared to those with parity of 4 and above p < 0.0001 [ 25 ]. The discrepancy between these studies may be attributed to the difference in the sample.
According to this study, a neonate’s weight is affected by where the woman lives. Women living in Unguja had a greater likelihood of having an LBW baby than those living in Mainland rural. It is reasonable to assume that people on the mainland and those on the island have different cultural norms. Besides that, the approach and attitude toward seeking healthcare differ, describing the disparity. The findings of this study contravene those of a study performed in the Amhara Region, Ethiopia, which found that women living in urban areas were less likely to give birth to an LBW baby than rural residents [ 26 ]. The disparity between the two studies is understandable given that women in rural areas are more likely to have limited access to healthcare, poorer nutrition, and higher rates of poverty, which would explain their high rate of LBW, as shown in the Ethiopian study.
Malaria being a common infection during pregnancy in some African regions is widely associated with low birth weight other than increasing neonatal mortality and morbidity rates. Surprisingly, this is not the case in this study as the present findings displayed no statistically significant relationship between receiving intermittent treatment for malaria and a neonate birth weight. These findings don’t relatively match the findings of a topical review done in East Africa which indicated that malaria has a huge part in play for LBW with this effect mostly in areas with a high prevalence of malaria transmission [ 27 ]. The discrepancy between these findings may be due to the fact that the current study assessed if a pregnant woman ever took malaria preventive treatment at least once during pregnancy. Taking anti-malaria treatment at least once could have not played a protective measure against low birth weight.
This study found that the prevalence of low birth weight in Tanzania is 6.5%. Maternal factors associated with LBW were identified as age, number of antenatal visits, mother’s parity, and place of residence. These findings necessitate measures to enhance child health and also highlights the importance of the government and policymakers focusing on preventing risk factors associated with LBW. Early identification of these risk factors, as well as high-quality ANC care, will help to reduce the burden of LBW.
Data availability
The datasets used during the current study are available from the corresponding author upon reasonable request.
Abbreviations
Acquired Immunodeficiency Syndrome
Antenatal Care
Adjusted Odds Ratio
Crude Odds Ratio
Confidence Interval
Demographic Health Survey
Human Immunodeficiency Virus
Low Birth Weight
National Institute for Medical Research
National Bureau of Statistics
Office of Chief Government Statistician
Tanzania Demographic and Health Survey and Malaria Indicator Survey
World Health Organization
Zanzibar Medical Ethics and Research Committee
WHO. Global Nutrition Targets 2025: Low birth weight policy brief. In 2014. pp. 1–8.
Tadesse Z, Id T, Tamirat KS, Teshale AB. Prevalence of low birth weight and its associated factor at birth in Sub-Saharan Africa: A generalized linear mixed model. PLoS One [Internet]. 2021;16(3):1–13. https://doi.org/10.1371/journal.pone.0248417
Seid S, Wondafrash B, Gali N, Ali A, Mohammed B, Kedir S. Determinants of low Birth Weight among newborns delivered in Silte Zone Public Health Facilities, Southern Ethiopia: a case-control study. Ethiop J Heal Sci. 2022;32(4):19–29.
Google Scholar
Khan JR, Islam MM, Awan N, Muurlink O. Analysis of low birth weight and its co-variants in Bangladesh based on a sub-sample from nationally representative survey. BMC Pediatr. 2018;18(1):1–9.
Article Google Scholar
Mahumud RA, Sultana M, Sarker AR. Distribution and determinants of low birth weight in developing countries. J Prev Med Public Heal. 2017;50(1):18–28.
K’Oloo A, Godfrey E, Koivu AM, Barsosio HC, Manji K, Ndesangia V et al. Improving birth weight measurement and recording practices in Kenya and Tanzania: a prospective intervention study with historical controls. Popul Health Metr [Internet]. 2023;21(1):1–9. https://doi.org/10.1186/s12963-023-00305-x
Hussen A, Mohammed A, Factors associated with low birth, weight among newborns in Ethiopia. Public Heal Indones. 2020;6(1):1–6.
Lemlem GA, Mezen MK, Atinafu A, Abitew ZA. Maternal factors associated with low birth weight in governmental hospitals of Wollo District, Northeast Ethiopia: a cross sectional study. PAMJ-OH. 2021;4:18.
Da Silva Lopes K, Ota E, Shakya P, Dagvadorj A, Balogun OO, Peña-Rosas JP, et al. Effects of nutrition interventions during pregnancy on low birth weight: an overview of systematic reviews. BMJ Glob Heal. 2017;2(3):1–11.
Desta SA, Damte A, Hailu T. Maternal factors associated with low birth weight in public hospitals of Mekelle city, Ethiopia: a case-control study. 2020;1–9.
Cetin I, Mandò C, Calabrese S. Maternal predictors of intrauterine growth restriction. Curr Opin Clin Nutr Metab Care. 2013;16(3):310–9.
Article CAS PubMed Google Scholar
Mvunta MH, Mboya IB, Msuya SE, John B, Obure J, Mahande MJ. Incidence and recurrence risk of low birth weight in Northern Tanzania: a registry based study. PLoS ONE. 2019;14(4):1–10.
USAID, TANZANIA PROFILE OF PRETERM AND LOW BIRTH WEIGHT PREVENTION, AND CARE. 2016;(January):10–2. https://reliefweb.int/…tanzania/tanzania-profile-preterm-and-low-birth-weight-prevent
MOHSW, Ministry of Health and Social Welfare. The National Road Map Strategic Plan to Improve Reproductive, Maternal, Newborn, Child & Adolescent Health in Tanzania (2016–2020) One Plan II, Tanzania. PrbOrg [Internet]. 2015;(March 2015):174. https://www.prb.org/wp-content/uploads/2018/05/National-Road-Map-Strategic-Plan-to-Accelerate-Reduction-of-Maternal-Newborn-and-Child-Deaths-in-Tanzania-2016-2020-One-Plan-II.pdf
He Z, Bishwajit G, Yaya S, Cheng Z, Zou D, Zhou Y. Prevalence of low birth weight and its association with maternal body weight status in selected countries in Africa: a cross-sectional study. BMJ Open. 2018;8(8):1–8.
Bosco AJ, Bosco AJ, Ronald K. Magnitude and Associated Risk factors for low Birth Weight in Bentiu State Hospital in Unity State, Republic of South Sudan Abstract. Open Sci Journa. 2021;6(September):1–32.
Sain S, Mukhopadhyay P, Saha KT, Chattopadhyay A, Dey I, Mandal NK. Effect of maternal factors on low Birth Weight BabyDelivered in A Medical College of Kolkata. J Compr Heal. 2014;2(2):54–64.
Gokhale D, Rao S. Compromised maternal nutritional status in early pregnancy and its relation to the birth size in young rural Indian mothers. BMC Nutr. 2021;7(1):4–11.
Gogoi N. Maternal and neonatal risk factors of low Birth Weight in Guwahati Metro, Assam, Northeast India. Acad J Ped Neonatol. 2018;6(5):90–5.
Ratnam S. Annals of Community Medicine & Public Health Maternal Risk Factors Associated with Term Low Birth Weight infants: a case-control study. Ann Community Med Public Heal. 2021;1(1).
MoHSW. THE UNITED REPUBLIC OF TANZANIA MINISTRY OF HEALTH AND SOCIAL WELFARE FOCUSED ANTENATAL CARE MALARIA, AND SYPHILIS IN PREGNANCY Learner’s Guide for ANC Service Providers and Supervisors. 2009;85. http://pdf.usaid.gov/pdf_docs/pnaea268.pdf
Rashash DS, Ali HAR, Jebur NAM, Rzaij IA. Determination of the effect of antenatal care on newborn birth weight. Rev Latinoam Hipertens. 2023;18(2):58–62.
Talie A, Taddele M, Alemayehu M. Magnitude of low Birth Weight and Associated factors among newborns delivered in Dangla Primary Hospital, Amhara Regional State, Northwest Ethiopia, 2017. J ofPregnancy Prim. 2019;2019:6.
Bekele A, Seyoum G, Tesfaye K, Fantahun Y. The effects of maternal age and parity on the birth weight of newborns among mothers with singleton pregnancies and at term deliveries. Ethiop J Heal. 2019;33(8):1–6.
Garces A, Perez W, Harrison MS, Hwang KS, Nolen TL, Goldenberg RL et al. Association of parity with birthweight and neonatal death in five sites: The Global Network ’ s Maternal Newborn Health Registry study. Reprod Health [Internet]. 2020;17(3):1–8. https://doi.org/10.1186/s12978-020-01025-3
Mekie M, Taklual W. Magnitude of low birth weight and maternal risk factors among women who delivered in Debre Tabor Hospital, Amhara Region, Ethiopia: a facility based cross- sectional study. Ital J Pediatr. 2019;1:1–6.
Bakken L, Iversen PO. The impact of malaria during pregnancy on low birth weight in East – Africa: a topical review. Malar J [Internet]. 2021;1–9. https://doi.org/10.1186/s12936-021-03883-z
Download references
Acknowledgements
Not applicable.
This study did not receive any funding.
Author information
Authors and affiliations.
Department of Clinical Nursing, The University of Dodoma, P. O Box 395, Dodoma, Tanzania
Glorialoveness S. Lyimo
Department of Nursing Management and Education, The University of Dodoma, P. O Box 395, Dodoma, Tanzania
Fabiola Vincent Moshi
You can also search for this author in PubMed Google Scholar
Contributions
G.L: Conceptualization and design, manuscript writing, editing, and revision. F.M: Drafted the manuscript, oversaw data cleaning and analysis, and provided additional information. All authors reviewed the final manuscript.
Corresponding author
Correspondence to Glorialoveness S. Lyimo .
Ethics declarations
Ethics approval and consent to participate.
This study involved the analysis of secondary data Demographic and Health Survey and Malaria Indicator Survey (DHS-MIS). As a result, no official ethical approval was needed. Permission to use the data for this particular research and publishing in peer reviewed journal was obtained from DHS measures. The procedures for collecting DHS-MIS data, however, were approved by the following organizations: Tanzania’s National Institute for Medical Research (NIMR), the Zanzibar Medical Ethics and Research Committee (ZAMREC), International’s Institutional Review Board, and the Centers for Disease Control and Prevention in Atlanta, United States of America (USA). The participants’ legal guardian/next of kin supplied written informed consent to participate in this study. All methods are carried out in accordance with relevant guidelines and regulation.
Consent for publication
Competing interests.
The authors declare no competing interests.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it.The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder.To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/ .
Reprints and permissions
About this article
Cite this article.
Lyimo, G.S., Moshi, F.V. The prevalence of low birth weight and its associated maternal factors among women of reproductive age who gave birth to live babies within five years preceding the survey in Tanzania: an analysis of data from the 2015-16 Tanzania Demographic and Health Survey and Malaria Indicators Survey. BMC Pregnancy Childbirth 24 , 523 (2024). https://doi.org/10.1186/s12884-024-06719-1
Download citation
Received : 05 April 2023
Accepted : 25 July 2024
Published : 09 August 2024
DOI : https://doi.org/10.1186/s12884-024-06719-1
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Low birth weight
- Maternal factors
BMC Pregnancy and Childbirth
ISSN: 1471-2393
- Submission enquiries: [email protected]
- General enquiries: [email protected]
6 of the World’s Oldest Diseases
These ailments have accompanied humanity for thousands, even millions, of years—and they’re not done with us yet.
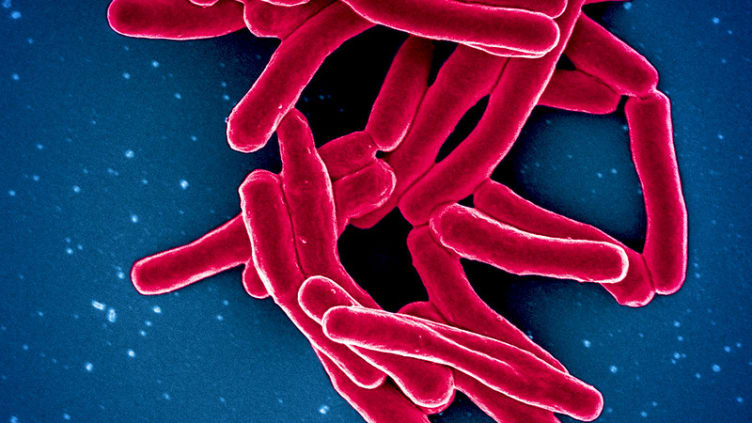
The emergence of the COVID-19 pandemic in late 2019 was pretty awful—but it also presented a rare moment when a completely new disease came to light. Other illnesses have been with us for hundreds or thousands of years, long before they were identified and treatments were discovered. Here are six that must have baffled ancient physicians—and aren’t done with us yet.
Cancer // At Least 1.7 Million Years Old
Tuberculosis // at least 70,000 years old, dental caries // at least 15,000 years old, malaria // at least 5500 years old, lyme disease // at least 5300 years old , leishmaniasis // at least 4000 years old.
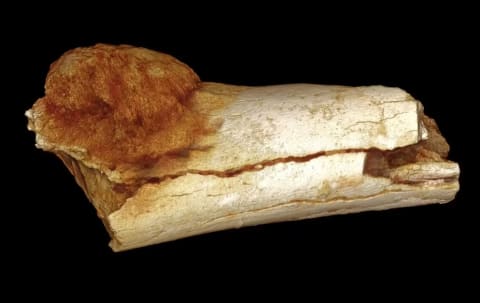
The true origins of cancer may always be murky, but recent research has shown that it’s likely one of the oldest diseases in the world.
In 2016, a paper in the South African Journal of Science reported the world’s oldest known human malignant tumor on a 1.7-million-year-old toe bone from a hominin unearthed in South Africa’s Swartkrans Cave . Using advanced 3-D imaging , the researchers’ analysis showed it to be osteosarcoma, a kind of cancer found in the cells that form new bone. Another study from the same research team reported the discovery of the earliest case of a benign tumor in a hominin: an osteoid osteoma on the vertebra of a 1.98-million-year-old Australopithecus sediba at the Malapa fossil site in South Africa.
In a more recent case, a 2250-year-old Egyptian mummy named M1 was diagnosed with prostate cancer via a CT scan. He showed signs of lesions in the spine and pelvis that indicated the cancer had metastasized in his bones. Fortunately, numerous options for the treatment of prostate cancer in its various stages is available today.
Tuberculosis , primarily a lung infection, is one of the world’s deadliest known diseases. It’s spread by tiny droplets carried through the air when an infected person speaks, sneezes, or otherwise exhales, and it may be the deadliest bacterium in human history .
Its origins are ancient. Paleomicrobiologists—scientists who study the microbes in prehistoric tissues —suggest that TB was present among Paleolithic humans in Africa 70,000 years ago . As people migrated around the world, the disease tagged along with them. In the 17th and 18th centuries it went by a variety of names, including consumption , white plague, or phthisis (pronounced “THI-suhss”).
By the time the bacterium that causes TB, Mycobacterium tuberculosis , was discovered by German physician Robert Koch in 1882, it was killing one out of every seven people in the U.S. and Europe.
Initial symptoms of a TB infection include cough, fever, and fatigue. The disease usually progresses to a latent infection in which people show no symptoms, followed by an active infection in which the person’s immune system can no longer control the spread of the bacterium, and the infected person may cough up blood, feel fatigued, and have no appetite. Active infections are now treated with antibiotics and latent TB can be treated before serious symptoms develop.
Thought it’s curable, tuberculosis still killed 1.3 million people in 2022 and is now the world’s second deadliest infectious disease after COVID-19. It’s also been recognized with a Guinness World Record as the oldest contagious disease. Congratulations?
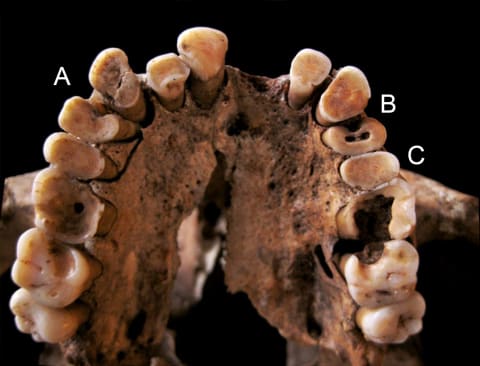
Dental caries, a.k.a tooth decay, is the most widespread disease in the United States—and humans have been suffering from it for thousands of years.
It was once thought that Paleolithic hunter-gatherers had sidestepped bad teeth thanks to their low-carb lifestyle , and that dental caries first showed up when people started farming grains and other starches. These foods begin breaking down into sugars in the mouth, and bacteria like Streptococcus mutans metabolize the sugars into tooth-decaying acids.
But a 2014 study from London’s Natural History Museum revealed an interesting twist at the site of a hunter-gatherer society in Morocco that lived as many as 15,000 years ago. Skeletal remains at the site showed serious tooth decay—so bad that many of the people would have been chewing food on the roots of their teeth. The likely culprits were the acorns and pine nuts that they frequently ate, which were full of fermented carbohydrates . Lead author Louise Humphrey told NPR that the prevalence of ancient dental caries depended on the foods that were available to forage.
Tooth decay can lead to cavities, pain, and tooth loss, and is preventable with good oral hygiene and dental care. Too bad for this group that the first sort-of toothbrush , the “ chew stick ,” didn’t come into use for teeth cleaning until about 3000 BCE.
In World War II, U.S. soldiers fighting in the Philippines and New Guinea were leveled by malaria , and many Americans still associate the disease with the war. Extensive public health efforts and other factors contributed to the eradication of malaria from the U.S. by 1951.
But malaria goes a great deal further back in time. In a 2024 study in the journal Nature , researchers looked at genetic material from slivers of ancient human remains from 16 countries and found malaria in specimens that were 5500 years old. The authors believe the disease may be even older than that.
Malaria is caused by a blood parasite in the genus Plasmodium , which is carried by the female Anopheles mosquito . Humans can contract malaria if they’re bitten by one of the infected insects. Initial symptoms are fever, chills and weakness, but if it’s left untreated, malaria can lead to organ failure and death. It’s a leading cause of illness in sub-Saharan Africa, where Anopheles mosquitoes bite humans 98 percent of the time and quickly transmit infections far and wide, and where the majority of the victims are children under 5 years old . The World Health Organization (WHO) recorded 249 million malaria cases and 608,000 fatalities worldwide in 2022.
There are several ways to diagnose and treat malaria depending on a patient’s physical condition and type of malarial symptoms. The WHO approved the first malaria vaccine in 2021.
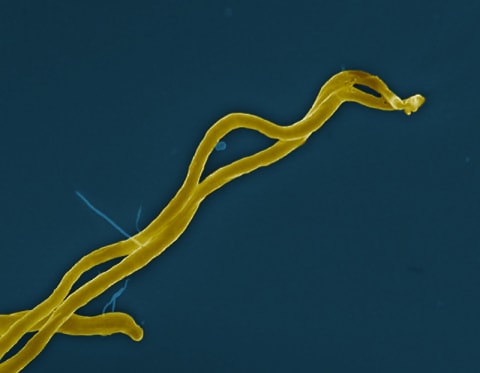
Lyme disease was first described in 1975, but evidence of it goes way back—to the Copper Age, roughly 5300 years ago.
The bacterium that causes Lyme’s disease, Borrelia burgdorferi , was found by mitochondrial DNA analysis in the bones of Ötzi the Iceman , the oldest known European mummy . Ötzi, named for the Ötzal Alps where he was found in 1991, died in 3300 BCE from a wound caused by an arrowhead lodged in his left shoulder. The injury would have caused his death, “most likely from blood loss, exposure, and shock,” according to a 2013 paper in the journal Inflammopharmacology . We may never know if Ötzi experienced symptoms of Lyme disease from his B. burgdorferi infection, but it’s unlikely that it contributed to his demise.
Lyme disease wasn’t identified for another 5275 years, and only when conditions were just right for it to emerge en masse. In 1975, a group of children in Lyme, Connecticut, developed symptoms that looked like rheumatoid arthritis. Soon more people in nearby towns came down with the same illness [ PDF ], and in 1981 scientists pointed to Borrelia burgdorferi, a pathogen carried by deer ticks, as the cause.
Why did it emerge so suddenly? According to a 1994 paper , reforestation in the Northeast U.S. beginning in the 1920s supported new habitats for deer . Then came the creation of suburbs, with patches of meadow framed by trees interspersed with woodland—a favorite environment of both deer and humans. The deer brought deer ticks, which carried the bacterium, into more frequent contact with people.
Signs of Lyme disease include fever, chills, muscle aches, and fatigue, progressing into arthritis-like symptoms, heart palpitations, and a red skin rash that looks like a target. If caught early, Lyme disease can be treated with antibiotics.
Leishmaniasis is another insect-borne disease that’s been with us for millennia. The infection comes from parasites in the genus Leishmania that live inside female sandflies belonging to the genus Phlebotomus . Humans contract Leishmaniasis from the sandflies’ bites, and cases are most common in tropical and subtropical areas around the world.
According to the WHO, up to 1 million cases are diagnosed each year, with the majority being cutaneous Leishmaniasis resulting in skin sores. The visceral and mucocutaneous forms of the disease are rare but more serious. Only a small percentage of those infected ever develops the disease, and there are several treatment options depending on the parasite species and other factors.
A 2006 study in the journal Emerging Infectious Diseases examined tissues from 42 ancient Egyptian and Nubian mummies dating from the Middle Kingdom (2050–1650 BCE). Researchers discovered the mitochondrial DNA of Leishmania in four of them, making Leishmaniasis at least 4000 years old, and Egyptian medical writings from 1500 BCE describe a Leishmaniasis-like skin condition called “ Nile pimple .” The pathogens in the Leishmania group emerged even earlier, likely during the Mesozoic Era (252 to 66 million years ago).
Discover More Stories About Medical History:
Disclaimer: Early release articles are not considered as final versions. Any changes will be reflected in the online version in the month the article is officially released.
Volume 30, Number 9—September 2024
Mpox Epidemiology and Risk Factors, Nigeria, 2022
Suggested citation for this article
To investigate epidemiology of and risk factors for laboratory-confirmed mpox during the 2022 outbreak in Nigeria, we enrolled 265 persons with suspected mpox. A total of 163 (61.5%) were confirmed to have mpox; 137 (84.0%) were adults, 112 (68.7%) male, 143 (87.7%) urban/semi-urban dwellers, 12 (7.4%) self-reported gay men, and 3 (1.8%) female sex workers. Significant risk factors for adults were sexual and nonsexual contact with persons who had mpox, as well as risky sexual behavior. For children, risk factors were close contact with an mpox-positive person and prior animal exposure. Odds of being mpox positive were higher for adults with HIV and lower for those co-infected with varicella zoster virus (VZV). No children were HIV-seropositive; odds of being mpox positive were higher for children with VZV infection. Our findings indicate mpox affects primarily adults in Nigeria, partially driven by sexual activity; childhood cases were driven by close contact, animal exposure, and VZV co-infection.
Human mpox is a zoonotic disease caused by 2 distinct clades (I and II) of the monkeypox virus (MPXV) ( 1 ). Clade I primarily affects children and adolescents in Central Africa, especially in the Democratic Republic of Congo (DRC) ( 1 , 2 ). Clade IIa was responsible for the 2003 human outbreak of mpox in the United States, and clade IIb caused the 2017–2019 mpox outbreak in Nigeria and the 2022 global outbreak ( 1 ); ≈92,000 confirmed cases and 171 deaths were reported in 116 countries as of December 22, 2023 ( 3 ).
The epidemiologic characteristics of mpox during the 2022 outbreak have been described ( 4 – 8 ). The evidence suggests that ≈96% of mpox cases during the 2022 outbreak were in men, mostly 20–41 years of age, and the predominant mode of transmission (≈80%) was sexual encounter. Furthermore, the most frequent independent predictors of laboratory-confirmed mpox during the 2022 mpox outbreak have been identified as being male; being gay, bisexual, and other men who have sex with men (GBMSM); being a person living with HIV (PLHIV); having multiple sex partners; and having lesions in the anogenital area ( 9 – 14 ).
The 2017–2019 mpox outbreak in Nigeria predominantly affected young urban adults; human-to-human and zoonotic-related transmissions were suspected ( 15 ). Confirmed cases were reported among prison inmates, household and sexual contacts, and persons exposed to wildlife ( 15 ). The 2017–2019 outbreak provided the first documented evidence of mpox transmission via sexual contact and of mpox being associated with having multiple sex partners or advanced HIV disease ( 16 – 18 ). However, in ≈60% of cases, the risk factors or sources of exposure for mpox were unknown, suggesting a substantial knowledge gap in the epidemiology of mpox in Nigeria ( 15 ).
During the 2022 mpox outbreak, ≈1,400 cases were reported in Africa, of which Nigeria accounted for 42% ( 3 ). However, only a few studies from Africa discuss the epidemiology of and risk factors for laboratory-confirmed mpox infections during that outbreak. A case series from Nigeria described the interplay of mpox with varicella zoster virus (VZV) but was limited to southern Nigeria ( 19 ), suggesting the need to explore the co-infection on a national scale. Since 2023, the DRC has reported increasing mpox cases, including those caused by the sexually transmitted clade I strain ( 20 , 21 ). That change in the epidemiology is concerning and calls for concerted action and more information about the epidemiology of and risk factors for mpox in countries in Africa where the disease was previously endemic.
To investigate the epidemiology of and risk factors for laboratory-confirmed mpox in Nigeria during the 2022 outbreak, we conducted an observational cross-sectional study to address existing knowledge gaps and provide insights that can be used to develop public health strategies and interventions to control future mpox outbreaks.
Ethics Statement
We obtained ethics approval for the study from the National Health Research Ethics Committee, Nigeria (NHREC/01/01/2007–25/10/2022). All participants gave informed consent to participate in the study.
Study Participants
Our cross-sectional study included persons with suspected mpox who attended mpox treatment centers and outpatient clinics across Nigeria during June 1–December 30, 2022. We defined a suspected case of mpox by using the Nigeria Centre for Disease Control and Prevention guidelines, as previously described ( 22 ). On the basis of an average of 12 suspected cases of mpox seen monthly during January–April 2022 in Nigeria, we estimated a minimum sample size of 158 participants, including a 10% dropout rate. We invited all mpox treatment centers and outpatient clinics across Nigeria to participate in the study and consecutively enrolled persons with suspected mpox who attended study sites and gave informed verbal or written consent. Suspected mpox was diagnosed by PCR at the National Reference Laboratory of the Nigeria Centre for Disease Control and Prevention as previously described ( 23 ). We defined an mpox-positive participant as an mpox-suspected participant for whom MPXV infection was confirmed by real-time PCR. Because of lack of laboratory diagnoses, we excluded probable cases of mpox ( Appendix ).
To document epidemiologic and clinical variables of all study participants, we used a structured case report form, which was developed from existing mpox literature review ( 4 , 15 , 18 , 23 – 25 ) and included variables such as patient age, sex, occupation, sexual orientation, and potential routes and risk factors for mpox transmission (e.g., animal exposure, close contact, and sexual behavior). Sexual history was not obtained for all children. We also documented comorbidities (e.g., HIV and VZV co-infections) ( Appendix ). All variables were documented at manifestation or at the time of participant recruitment.
We analyzed study data by using the SPSS Statistics 26 (IBM, https://www.ibm.com ). We summarized categorical variables as frequencies and percentages and summarized continuous variables by using median and interquartile ranges (IQRs) because of nonnormal distribution. We used χ 2 for categorical variables (or Fisher exact tests when assumptions for χ 2 were not met because of small sample size) and Mann-Whitney tests (comparing median values) to determine variables associated with being mpox positive. We determined independent predictors of mpox positivity separately by using logistic regression models that included significant epidemiologic variables on univariate analysis and other relevant variables known to be theoretically associated with mpox infection from prior literature. We deleted missing variables pairwise without replacements. We excluded educational level from the model because of strong correlation with age group. Because of missing data, we did not include HIV and VZV data in the logistic models. The logistic regression tables detail the variables included in each model. In view of differences in epidemiologic characteristics across age groups, we assessed the risk factors for mpox positivity for the entire study population and separately for children (<18 years of age) and adults. We reported results as crude odd ratios (ORs) and adjusted odds ratios (aORs) with 95% CIs. We considered p<0.05 (2-tailed) as statistically significant.
Study Population
Figure . Geographic distribution of sites in Nigeria participating in a study of epidemiology and risk factors for laboratory-confirmed mpox during the mpox outbreak, Nigeria, 2022. A total of 265 study participants...
We enrolled 280 persons with suspected cases of mpox during the study period, among whom we excluded 15 (5.4%) from the final analysis because of missing data related to sociodemographic and epidemiologic characteristics. We enrolled 265 study participants, 28 days–69 years of age (median 27 years, IQR 14–36 years) across 23 states and the Federal Capital Territory in Nigeria ( Figure ). Of the 265 participants, 163 (61.5%) were mpox positive and 102 (38.5%) were mpox negative ( Table 1 ). The mpox-positive participants (median age 30 years [IQR 22–37 years]) were older than the mpox-negative participants (median age 19 years [IQR 8–32 years]; p<0.0001).
Demographic and Epidemiologic Characteristics of Mpox-Positive Participants
Of the 163 mpox-positive participants, 137 (84.0%) were adults, 112 (68.7%) were male, 143 (87.7%) were urban/semi-urban dwellers, 12 (7.4%) were self-reported GBMSM, and 3 (1.8%) were female sex workers ( Appendix Table). Among the 163 mpox-positive participants, exposure was unknown for 87 (53.4%) and > 1 exposure was reported for 76 (46.6%). Specifically, 59 (36.2%) had contact with a person with a suspected case, 36 (22.1%) had close contact with a person with a confirmed case, 35 (21.5%) reported animal exposure, and 35 (21.5%) had sexual contact with a person with a suspected case. Of the 46 mpox-positive participants who provided information about possible places of exposure, 23 (50%) were exposed at home, 20 (43.5%) in the community, and 3 (6.5%) in the hospital. Among the mpox-positive participants tested for HIV and VZV co-infections, 35.9% (55/153) had VZV co-infection, 18.0% (24/133) had HIV co-infection, and 6.0% (8/133) had both HIV and VZV co-infections. Among the 102 mpox-negative participants, 31 (30.4%) were VZV positive, 52 (50.9%) were VZV negative, and 19 (18.6%) were missing data on VZV status. We did not investigate the causes of skin rash among participants who were VZV-negative and those for whom VZV status was missing.
Among the 23 study participants who reported a history of chickenpox ( Table 1 ), 7 (30.4%) were VZV positive; 2 were mpox-negative adults and 5 were mpox positive (a 16-year-old adolescent and 4 adults), all of whom were probably experiencing reactivated herpes zoster infection. Among the 2 mpox-negative adults with probable reactivated herpes zoster infection was a recently diagnosed 63-year-old man living with HIV whose CD4 cell count was unknown/missing.
Epidemiologic Risk Factors for Mpox Positivity
Univariate analysis indicated that the epidemiologic risk factors associated with mpox positivity among the 265 study participants were history of prior animal exposure, age group, close contact with a person with confirmed mpox, educational level, and local travel ( Table 1 ). In a logistic regression model that included age group, sex, animal exposure, close contact with confirmed case and local travel, the independent predictors of mpox positivity were age group and close contact with a person with confirmed mpox ( Table 1 ). The odds of being mpox positive were significantly higher among younger adults (18–35 years of age) (aOR 3.93, 95% CI 2.06–7.50) and older adults (>35 years of age) (aOR 4.75, 95% CI 2.23–10.13) than among children. Odds of being mpox positive were significantly higher among participants who had reported close contact with a person with confirmed mpox than among those who had not (aOR 2.96, 95% CI 1.26–6.96). Among 213 participants with known HIV status, odds of being mpox positive were greater among PLHIV than among those who were HIV negative (OR 8.59, 95% CI 1.97–37.40; p = 0.001).
Epidemiologic Risk Factors among Adults
Univariate analysis indicated that among the 192 adult participants, the variables associated with being mpox positive were prior sexual and nonsexual contact with a person with suspected mpox and recent history of risky sexual behavior ( Table 2 ). The independent predictors among adults for being mpox positive included recent history of risky sexual behavior (aOR 2.81, 95% CI 1.40–5.63), nonsexual contact with a person with a suspected case (aOR 5.50, 95% CI 1.12–27.14), and sexual contact with a person with a suspected case (aOR 2.81, 95% CI 1.01–7.79) ( Table 2 ). Among the 142 adults with known HIV status and the 172 with known VZV status, the odds of being mpox positive were significantly higher among PLHIV (OR 4.77, 95% CI 1.07–21.34) and significantly lower among those who were VZV positive (OR 0.43, 95% CI 0.21–0.87). History of having had multiple sex partners, having had sex recently, and having engaged in risky sexual behaviors were significantly associated with being mpox positive ( Table 3 ).
Epidemiologic Risk Factors Among Children
All 71 children with known HIV status tested negative for HIV. Multivariate analysis indicated that the predictors of mpox positivity among children were contact with animals (aOR 9.97, 95% CI 1.27–78.34) and close contact with a person with a confirmed case (aOR 4.76, 95% CI 1.14–19.87) ( Table 4 ). We did not include VZV in the model because of the high numbers of missing data. However, among the 64 children with known VZV status, the odds of being mpox positive were significantly higher among those who were VZV positive than among those who were VZV negative (OR 5.74, 95% CI 1.891–17.43).
Our study showed that laboratory-confirmed mpox was reported across various age groups and populations but was more common among persons who were young adult, male, and mostly urban or semi-urban dwellers. The demographic characteristics of the mpox-positive participants in our study are similar to those of the 2017–2019 mpox outbreak in Nigeria, which also predominantly affected young adult urban dwellers. Most cases of mpox during the 2022 outbreak in Europe and North America were among young adult urban dwellers, mostly GBMSM ( 6 , 26 ). In contrast, only 7.4% of the mpox participants in our study self-reported themselves as GBMSM; MPXV is probably not currently spreading within that particular social group in Nigeria. Another possibility is that cases of mpox in that group have either been overlooked or not accurately reported because GBMSM may avoid seeking clinical assessment because of laws in Nigeria that criminalize same-sex relationships.
The types of exposure settings reported in our study suggest human-to-human and zoonotic transmissions of MPXV during the 2022 outbreak in Nigeria. We identified independent epidemiologic risk factors for mpox positivity among study participants as having had close contact with a person with confirmed mpox and being in an adult age group. Specific risk factors for mpox among adults were > 1 markers of risky sexual behaviors (e.g., multiple sex partners and condomless casual sex), and both sexual and nonsexual close contact with a person with suspected mpox. Among children, independent risk factors for mpox positivity were close contact with a person with confirmed mpox and contact with wild/domestic animals. Besides nonsexual physical contact, it might be postulated that mpox in Nigeria is also partly transmitted via risky sexual behavior among adults who subsequently transmit it to children through close contact. Various studies conducted outside Africa during the 2022 outbreak also identified risk factors for being mpox positive as having had multiple sex partners and other markers of risky sexual behavior ( 9 , 11 , 12 , 14 ). Similarly, since 2023, a cluster of clade I strain mpox cases in the DRC was linked to sexual contact, including among GBMSM ( 21 ).
Studies conducted mainly outside Africa suggest that ≈80% of mpox patients during the 2022 outbreak had sexual encounters before their diagnosis ( 4 , 8 ), and other studies conducted outside Africa have shown prior sexual activity to be associated with mpox infection among GBMSM and among heterosexual adults ( 27 , 28 ). The role of sexual contact and sexual behavior in the transmission of mpox was first proposed during the 2017–2019 mpox outbreak in Nigeria ( 16 , 17 ). A single-center study conducted during the 2022 outbreak in Nigeria reported mpox among linked heterosexual partners, suggesting a relationship between prior sexual contact and mpox infection in Nigeria ( 29 ). Our study, which was conducted on a national scale in Nigeria, corroborates the prior observations and supports a role of sexual activity in transmission of the MPXV among adults during the 2022 mpox outbreak in Nigeria.
With regard to animal exposure being independently associated with mpox positivity among children and not adults, it is plausible but not confirmatory that zoonotic transmission of MPXV in Nigeria is more common among children than adults. However, the large confidence interval of the OR related to animal exposure suggests uncertainty of that finding.
In our study, 18% of participants with available HIV test results had positive results, and odds of being mpox positive were 5 times higher among PLHIV than among those without HIV. During the 2022 global outbreak, 30%–50% of mpox-positive persons were PLHIV ( 30 ); various studies, including reports from Nigeria, have shown that those with advanced HIV have more severe disease and higher death rates than their HIV-negative counterparts ( 31 ). A review of 86 confirmed mpox cases during the 2017–2019 mpox outbreak showed that persons with mpox were ≈7 times more likely to be living with HIV than were those without mpox ( 32 ). Because of missing HIV test data, we cannot make definitive conclusions regarding HIV as an independent risk factor for mpox in Nigeria during the 2022 mpox outbreak. Even so, HIV and mpox are both sexually transmitted infections, which makes it plausible that risky sexual behavior might be a common factor for acquisition and further transmission of mpox.
Approximately half of the mpox-negative participants in our study were VZV positive, and ≈36% of mpox-positive participants also had a VZV-positive test result. We previously reported VZV co-infection to be independently associated with severe mpox during the 2022 mpox in Nigeria ( 23 ). The high prevalence of VZV co-infection among mpox-positive and mpox-negative participants reflects the endemicity of chickenpox, herpes zoster infection, or both in Nigeria and underscores that those VZV-related conditions are the main differential diagnoses for mpox in Nigeria. Of note, VZV co-infection was associated with higher odds of mpox among children but lower odds among adults. The reasons for the contrasting findings are not obvious from our study data. Because we did not distinguish chickenpox from reactivated herpes zoster virus infection in all participants, we could not classify the prevalence of those VZV-related conditions in relation to age, if any. Furthermore, we did not include VZV in the multivariate analysis because of a substantial amount of missing data, and as such, we could not confirm whether our findings were truly reflective of an age-related difference in the associations between VZV and mpox infections or if they resulted from the effects of another confounder. On the basis of the high rates of VZV-mpox co-infections observed from prior studies of mainly the clade I virus ( 33 , 34 ), it has been proposed but not confirmed that a breach in the skin caused by VZV lesions could increase the likelihood of transmission of MPXV and that MPXV may directly trigger VZV reactivation, resulting in herpes zoster virus infection ( 34 , 35 ).
The major limitations of our study are associated with recruitment of hospital-associated cases only, which could have led to underascertainment of mild mpox-positive cases and mpox-negative suspected cases in the community and missing data related to VZV and HIV co-infections among some participants, which precluded inclusion of these variables for multivariate analysis. The predominance of moderate to severe cases could also bias our study toward HIV-positive participants, given that they are more likely to have severe illness and thus need to seek care at or get admitted into healthcare facilities. We did not determine virus clades in our study, but prior epidemiologic data suggest that the 2022 mpox outbreak in Nigeria probably resulted from the MPXV clade IIb strain ( 36 , 37 ).
In conclusion, our study reveals that mpox primarily affects adults in Nigeria, often associated with sexual transmission, and that among children affected by mpox, the prominent drivers are animal contact and VZV infection. Our findings emphasize the value of addressing both sexual and nonsexual transmission routes in public health efforts to control the spread of mpox in Nigeria.
Dr. Ogoina is an infectious diseases physician at the Niger Delta University Teaching Hospital and a professor of medicine and infectious diseases at the Niger Delta University, both in Bayelsa State, Nigeria, as well as president of the Nigerian Infectious Diseases Society. His research interests include HIV/AIDS and related opportunistic infections, healthcare-associated infections, antimicrobial use and resistance, infection prevention and control, and epidemic-prone infectious diseases.
Acknowledgments
We appreciate all healthcare workers in the various mpox treatment centers in Nigeria who participated in case management and the public health response during the 2022 mpox outbreak in Nigeria.
Additional members of the NIDS study group Nigeria who are co-authors and contributed data: Chiedozie James Maduka (Federal Medical Centre, Umahia, Abia State, Nigeria), Aliyu Mamman Na’uzo (Federal Medical Centre, Kebbi State, Nigeria), Sampson Omagbemi Owhin (Federal Medical Center Owo, Ondo, Nigeria), Mohammed Asara Abdullahi (Ahmadu Bello University Teaching Hospital Shika Zaria, Nigeria), Aisha Habiba Sadauki (Baze University Hospital, Abuja), Okonofua Martha (Irrua Specialist Teaching Hospital, Irrua, Edo State), Rosemary Audu and Ehimario Igumbor (Nigerian Institute of Medical Research, Lagos, Nigeria), Idotenyin Enyi (Ministry of Health, Delta State), Mohammed Yahaya (Usmanu Danfodiyo University Sokoto Nigeria), Chiemezie Amaku (Bingham University Teaching Hospital, Jos, Plateau State), Emeka Sampson (State Ministry of Health, Abakaliki, Ebonyi, Nigeria), Nathan Shehu (West African Center for Emerging Infectious Diseases, Jos University Teaching Hospital, Jos, Plateau State, Nigeria), Ogochukwu Chinedum Okoye and John Ohaju-Obodo (Delta State University, Abraka, Delta State, Nigeria), Olumuyiwa Elijah Ariyo (Federal Teaching Hospital Ido-Ekiti, Ekiti State, Nigeria), Eshan Henshaw (University of Calabar Teaching Hospital, Calabar, Cross Rivers State, Nigeria), Iorhen Ephraim Akase (College of Medicine, University of Lagos/ Lagos University Teaching Hospital, Lagos, Nigeria), Garba Iliyasu (College of Health Sciences. Bayero University Kano, Kano State, Nigeria), Adefolarin Opawoye (Lagos University Teaching Hospital, Lagos, Nigeria), Ubong Aniefio Udoh (Faculty of Medicine, University of Calabar, Calabar, Nigeria), Mahmoud Magaji Ado (Rasheed Shekoni Specialist Hospital and The Infectious Disease Centre, Dutse, Jigawa State, Nigeria), Ayanfe Omololu (King Fahad Specialist Hospital, Buraydah, Saudi Arabia), Ajayi David Bamidele (State Hospital Abeokuta Ogun state, Nigeria), and Adebola Olayinka (World Health Organization, Abuja, Nigeria).
- Happi C , Adetifa I , Mbala P , Njouom R , Nakoune E , Happi A , et al. Urgent need for a non-discriminatory and non-stigmatizing nomenclature for monkeypox virus. PLoS Biol . 2022 ; 20 : e3001769 .
- Mitjà O , Ogoina D , Titanji BK , Galvan C , Muyembe JJ , Marks M , et al. Monkeypox. Lancet . 2023 ; 401 : 60 – 74 .
- World Health Organization . 2022–24 Mpox (monkeypox) outbreak: global trends 2023 [ cited 2023 Apr 10 ]. https://worldhealthorg.shinyapps.io/mpx_global
- Sharma A , Prasad H , Kaeley N , Bondalapati A , Edara L , Kumar YA . Monkeypox epidemiology, clinical presentation, and transmission: a systematic review. Int J Emerg Med . 2023 ; 16 : 20 .
- Bragazzi NL , Kong JD , Mahroum N , Tsigalou C , Khamisy-Farah R , Converti M , et al. Epidemiological trends and clinical features of the ongoing monkeypox epidemic: a preliminary pooled data analysis and literature review. J Med Virol . 2023 ; 95 : e27931 .
- Thornhill JP , Barkati S , Walmsley S , Rockstroh J , Antinori A , Harrison LB , et al. ; SHARE-net Clinical Group . Monkeypox virus infection in humans across 16 countries—April–June 2022. N Engl J Med . 2022 ; 387 : 679 – 91 .
- Hennessee I , Shelus V , McArdle CE , Wolf M , Schatzman S , Carpenter A , et al. ; California Department of Public Health Monkeypox Pediatric Working Group . CDC Monkeypox Pediatric Working Group; CDC Monkeypox Pediatric Working Group. Epidemiologic and clinical features of children and adolescents aged <18 years with monkeypox—United States, May 17–September 24, 2022. MMWR Morb Mortal Wkly Rep . 2022 ; 71 : 1407 – 11 .
- Riser AP , Hanley A , Cima M , Lewis L , Saadeh K , Alarcón J , et al. Epidemiologic and clinical features of mpox-associated deaths—United States, May 10, 2022–March 7, 2023. MMWR Morb Mortal Wkly Rep . 2023 ; 72 : 404 – 10 .
- Moretti M , Heymans B , Yin N , Kaur S , Libois A , Quoilin S , et al. Diagnostic approach to monkeypox outbreak, a case-control study. Int J STD AIDS . 2023 ; 34 : 338 – 45 .
- De la Herrán-Arita AK , González-Galindo C , Inzunza-Leyva GK , Valdez-Flores MA , Norzagaray-Valenzuela CD , Camacho-Zamora A , et al. Clinical predictors of monkeypox diagnosis: a case-control study in a nonendemic region during the 2022 outbreak. Microorganisms . 2023 ; 11 : 2287 .
- Rimmer S , Barnacle J , Gibani MM , Wu MS , Dissanayake O , Mehta R , et al. The clinical presentation of monkeypox: a retrospective case-control study of patients with possible or probable monkeypox in a West London cohort. Int J Infect Dis . 2023 ; 126 : 48 – 53 .
- Núñez I , Ceballos-Liceaga SE , de la Torre A , García-Rodríguez G , López-Martínez I , Sierra-Madero J , et al. Predictors of laboratory-confirmed mpox in people with mpox-like illness. Clin Microbiol Infect . 2023 ; 29 : 1567 – 72 .
- Oeser P , Napierala H , Schuster A , Herrmann WJ . Risk factors for monkeypox infection—a cross-sectional study. Dtsch Arztebl Int . 2023 ; 120 : 65 – 6 .
- Zucker R , Lavie G , Wolff-Sagy Y , Gur-Arieh N , Markovits H , Abu-Ahmad W , et al. Risk assessment of human mpox infections: retrospective cohort study. Clin Microbiol Infect . 2023 ; 29 : 1070 – 4 .
- Yinka-Ogunleye A , Aruna O , Dalhat M , Ogoina D , McCollum A , Disu Y , et al. ; CDC Monkeypox Outbreak Team . Outbreak of human monkeypox in Nigeria in 2017-18: a clinical and epidemiological report. Lancet Infect Dis . 2019 ; 19 : 872 – 9 .
- Ogoina D , Yinka-Ogunleye A . Sexual history of human monkeypox patients seen at a tertiary hospital in Bayelsa, Nigeria. Int J STD AIDS . 2022 ; 33 : 928 – 32 .
- Ogoina D , Izibewule JH , Ogunleye A , Ederiane E , Anebonam U , Neni A , et al. The 2017 human monkeypox outbreak in Nigeria - report of outbreak experience and response in the Niger Delta University Teaching Hospital, Bayelsa State, Nigeria. PLoS One . 2019 ; 14 : e0214229 .
- Ogoina D , Iroezindu M , James HI , Oladokun R , Yinka-Ogunleye A , Wakama P , et al. Clinical course and outcome of human monkeypox in Nigeria. Clin Infect Dis . 2020 ; 71 : e210 – 4 .
- Mmerem JI , Umenzekwe CC , Johnson SM , Onukak AE , Chika-Igwenyi NM , Chukwu SK , et al. Mpox and chickenpox co-infection: case series from southern Nigeria. J Infect Dis . 2024 ; 229 ( Supplement_2 ): S260 – 4 .
- World Health Organization . Multi-country outbreak of mpox, external situation report #32–30 April 2024 [ cited 2024 May 4 ]. https://www.who.int/publications/m/item/multi-country-outbreak-of-mpox--external-situation-report-32--30-april-2024
- Kibungu EM , Vakaniaki EH , Kinganda-Lusamaki E , Kalonji-Mukendi T , Pukuta E , Hoff NA , et al. ; International Mpox Research Consortium . Clade I–associated mpox cases associated with sexual contact, the Democratic Republic of the Congo. Emerg Infect Dis . 2024 ; 30 : 172 – 6 .
- Nigeria Centre for Disease Control . National monkeypox public health response guidelines, Nigeria [ cited 2024 Aug 2 ]. https://ncdc.gov.ng/themes/common/docs/protocols/96_1577798337.pdf
- Ogoina D , Dalhat MM , Denue BA , Okowa M , Chika-Igwenyi NM , Yusuff HA , et al. ; Nigerian Infectious Diseases Society Mpox Study Group . Clinical characteristics and predictors of human mpox outcome during the 2022 outbreak in Nigeria: a cohort study. Lancet Infect Dis . 2023 ; 23 : 1418 – 28 .
- Ogoina D , Damon I , Nakoune E . Clinical review of human mpox. Clin Microbiol Infect . 2023 ; 29 : 1493 – 501 .
- Mitjà O , Alemany A , Marks M , Lezama Mora JI , Rodríguez-Aldama JC , Torres Silva MS , et al. SHARE-NET writing group. Mpox in people with advanced HIV infection: a global case series. Lancet . 2023 ; 401 : 939 – 49 .
- Vivancos R , Anderson C , Blomquist P , Balasegaram S , Bell A , Bishop L , et al. ; UKHSA Monkeypox Incident Management Team . Monkeypox Incident Management Team. Community transmission of monkeypox in the United Kingdom, April to May 2022. Euro Surveill . 2022 ; 27 : 2200422 .
- Sharpe JD , Charniga K , Byrd KM , Stefanos R , Lewis L , Watson J , et al. Possible exposures among mpox patients without reported male-to-male sexual contact—six U.S. jurisdictions, November 1–December 14, 2022. MMWR Morb Mortal Wkly Rep . 2023 ; 72 : 944 – 8 .
- Girometti N , Byrne R , Bracchi M , Heskin J , McOwan A , Tittle V , et al. Demographic and clinical characteristics of confirmed human monkeypox virus cases in individuals attending a sexual health centre in London, UK: an observational analysis. Lancet Infect Dis . 2022 ; 22 : 1321 – 8 .
- Ogoina D , James HI . Mpox among linked heterosexual casual partners in Bayelsa, Nigeria. N Engl J Med . 2023 ; 388 : 2101 – 4 .
- Ortiz-Saavedra B , Montes-Madariaga ES , Cabanillas-Ramirez C , Alva N , Ricardo-Martínez A , León-Figueroa DA , et al. Epidemiologic situation of HIV and monkeypox coinfection: a systematic review. Vaccines (Basel) . 2023 ; 11 : 246 .
- Girometti N , Ogoina D , Tan DHS , Pozniak A , Klein MB . Intersecting HIV and mpox epidemics: more questions than answers. J Int AIDS Soc . 2022 ; 25 : e26043 .
- Yinka-Ogunleye A , Dalhat M , Akinpelu A , Aruna O , Garba F , Ahmad A , et al. Mpox (monkeypox) risk and mortality associated with HIV infection: a national case-control study in Nigeria. BMJ Glob Health . 2023 ; 8 : e013126 .
- Hughes CM , Liu L , Davidson WB , Radford KW , Wilkins K , Monroe B , et al. A tale of two viruses: coinfections of monkeypox and varicella zoster virus in the Democratic Republic of Congo. Am J Trop Med Hyg . 2020 ; 104 : 604 – 11 .
- Hoff NA , Morier DS , Kisalu NK , Johnston SC , Doshi RH , Hensley LE , et al. Varicella coinfection in patients with active monkeypox in the Democratic Republic of the Congo. EcoHealth . 2017 ; 14 : 564 – 74 .
- MacNeil A , Reynolds MG , Carroll DS , Karem K , Braden Z , Lash R , et al. Monkeypox or varicella? Lessons from a rash outbreak investigation in the Republic of the Congo. Am J Trop Med Hyg . 2009 ; 80 : 503 – 7 .
- Faye O , Pratt CB , Faye M , Fall G , Chitty JA , Diagne MM , et al. Genomic characterisation of human monkeypox virus in Nigeria. Lancet Infect Dis . 2018 ; 18 : 246 .
- Ndodo N , Ashcroft J , Lewandowski K , Yinka-Ogunleye A , Chukwu C , Ahmad A , et al. Distinct monkeypox virus lineages co-circulating in humans before 2022. Nat Med . 2023 ; 29 : 2317 – 24 .
- Figure . Geographic distribution of sites in Nigeria participating in a study of epidemiology and risk factors for laboratory-confirmed mpox during the mpox outbreak, Nigeria, 2022. A total of 265 study...
- Table 1 . Demographic characteristics and epidemiologic risk factors for PCR-confirmed mpox among adults and children during mpox outbreak, Nigeria, 2022
- Table 2 . Demographic characteristics and epidemiologic risk factors for PCR-confirmed mpox among adults during the mpox outbreak, Nigeria, 2022
- Table 3 . Univariate analysis of associated between sexual histories of adults and mpox-PCR status during mpox outbreak, Nigeria, 2022
- Table 4 . Epidemiologic risk factors for PCR-confirmed mpox among children (<18 y) during mpox outbreak, Nigeria 2022
Suggested citation for this article : Ogoina D, Dalhat MM, Denue BA, Okowa M, Chika-Igwenyi NM, Oiwoh SO, et al. Mpox epidemiology and risk factors, Nigeria, 2022. Emerg Infect Dis. 2024 Aug [ date cited ]. https://doi.org/10.3201/eid3009.240135
DOI: 10.3201/eid3009.240135
Original Publication Date: August 06, 2024
1 Additional members of the NIDS study group Nigeria who are co-authors and contributed data to this work are listed at the end of this article.
Table of Contents – Volume 30, Number 9—September 2024
| EID Search Options |
|---|
| – Search articles by author and/or keyword. |
| – Search articles by the topic country. |
| – Search articles by article type and issue. |
Please use the form below to submit correspondence to the authors or contact them at the following address:
Dimie Ogoina, Infectious Diseases Unit, Niger Delta University and Niger Delta University Teaching Hospital, PMB 100, Bayelsa, Nigeria
Comment submitted successfully, thank you for your feedback.
There was an unexpected error. Message not sent.
Metric Details
Article views: 522.
Data is collected weekly and does not include downloads and attachments. View data is from .
What is the Altmetric Attention Score?
The Altmetric Attention Score for a research output provides an indicator of the amount of attention that it has received. The score is derived from an automated algorithm, and represents a weighted count of the amount of attention Altmetric picked up for a research output.

IMAGES
COMMENTS
Background. Malaria is the infection caused by the mostly obligate intraerythrocytic protozoa of the genus Plasmodium, which is spread to people by the inoculation from infected female Anopheles mosquitoes.Plasmodium falciparum is the major cause of severe malaria, which manifests as multiple organ dysfunction with high parasitemia counts that is characterized by coma, stupor, and severe ...
INTRODUCTION. Malaria is a frequent parasitic infection prevalent in Africa. Around 300 million are infected annually in Africa by malaria and 1 to 2 million will die from the disease. 1 Of the 4 human parasitic species that have been identified, Plasmodium falciparum has been known to cause significant morbidity and mortality, particularly in children and pregnant women. 1 Strategies to ...
This case‑study is part of a series of malaria elimination case‑studies conducted by the WHO Global Malaria Programme and the Global Health Group at the University of California, San Francisco (UCSF/GHG). The two groups wish to acknowledge the financial support of the Bill & Melinda Gates Foundation in
Malaria is caused by single-cell parasites of genus Plasmodium (P.). Four types of P. parasites infect humans: Plasmodium falciparum, vivax, ovale. and malariae. Among these, P. falciparum is the most common and most deadly. Malaria parasites develop through various stages of the life cycle in two hosts—female Anopheles mosquitoes and humans.
The development of the malaria elimination case study series is made possible by a grant from the Bill & Melinda Gates Foundation. Gretchen Newby, Aprielle Brackery, Luz Escubil, Christine Candari, Jenny Liu and Cara Smith Gueye (UCSF) collected and analysed data for this report, including the financial data.
Since the year 2000, 20 malaria deaths have been recorded in Cape Verde, out of a total of 677 malaria cases over the same period. The reported case-fatality rate reached a peak of 10% in 2006 reflecting delays in adequate case managmenet for falciparum malaria in a non-immune population.
Eswatini was the first country in sub-Saharan Africa to pass a National Malaria Elimination Policy in 2011, and later set a target for elimination by the year 2020. This case study aimed to review the malaria surveillance data of Eswatini collected over 8 years between 2012 and 2019 to evaluate the country's efforts that targeted malaria elimination by 2020.
One study from East Timor in 2006 reported a co-infection with Plasmodium falciparum malaria and dengue in a 7-year-old girl who subsequently died. 6 Three further descriptive studies and one case control study were published from India 7 8 and French Guiana, 9 10 all patients in these studies being adult patients. Malaria and dengue co ...
For Ghana to achieve its Malaria Strategic Plan 2021-2025, of reducing malaria case incidence by 50%, malaria mortality by 90%, and achieving malaria elimination as envisaged by 2028, the first RACD strategy in Ghana, in a yet to be implemented area of Asutsuare based on a cross sectional study of preliminary findings, coupled with previous ...
3 Global trends in the burden of malaria Malaria case incidence (i.e. cases per 1000 population at risk) reduced from 80 in 2000 to 58 in 2015 and 57 in 2019 (Table 3.1, Fig. 3.2). Between 2000 and 2015, malaria case incidence declined by 27% and then by less than 2% in the period 2015-2019, indicating a
0 to 229 million in 2019. In this same period, the population in sub-Saharan Africa, which accounts for more than 90% of the global burden of malaria, increased from 665. llion to over 1 billion.The mortality incidence rate (deaths per 100 000 population at risk) was reduced from 25 in 2000 t.
The Patient/Family Care Study involves a record of nursing care, identifying the problems of a nursing patient and how they are dealt with by the nurse in the course of finding solution to the problems. It provides a systematic way of collecting data, analysing information, and reporting the results of nursing care.
Plasmodium falciparum malaria is an important cause of morbidity and mortality worldwide. 1 It is not endemic to Europe, and reported cases in Europe are almost exclusively in travelers returning from malaria-endemic areas. 2 Imported infections with P. falciparum (P. falciparum malaria) account for most malaria-related morbidity and mortality in Europe. 3 The Netherlands was declared malaria ...
Case‑study 7. Elimination of malaria on the island of Reunion: 40 years on. WHO Library Cataloguing-in-Publication Data Elimination of malaria on the island of Reunion: 40 years on. (Eliminating malaria case-study, 7) 1.Malaria - prevention and control. 2.Malaria - epidemiology. 3.National Health Programs. 4.Reunion.
The burden of malaria is more in the eastern part of Indonesia than the Western part as well as the endemicity. Some cases of malaria will develop to severe form. Usually, the manifestation of children and adult are different. We reported a severe case of malaria in a 14-year-old boy who develops several manifestations such as anemia,
Overview. Malaria is a life-threatening disease spread to humans by some types of mosquitoes. It is mostly found in tropical countries. It is preventable and curable. The infection is caused by a parasite and does not spread from person to person. Symptoms can be mild or life-threatening. Mild symptoms are fever, chills and headache.
The use of National Malaria Case Management Guidelines is key to achieving this objective. In addition, the aim is to attain uniform malaria case management in the country. The guiding principle of antimalarial drug policy is to promote safe, effective, good quality, affordable, accessible and acceptable malaria treatment.
This study evaluated the effects of modern housing on malaria among different endemic areas. Methods: Electronic databases, clinical trial registries and grey literature were searched for randomized controlled trials, cohort studies, case-control studies, and cross-sectional surveys on housing done between 1987 and 2022. Forest plots were done ...
This study evaluated the effects of modern housing on malaria among different endemic areas. Electronic databases, clinical trial registries and grey literature were searched for randomized controlled trials, cohort studies, case-control studies, and cross-sectional surveys on housing done between 1987 and 2022.
1. Introduction. Malaria affected an estimated 219 million people causing 435,000 deaths in 2017 globally. This burden of morbidity and mortality is a result of more than a century of global effort and research aimed at improving the prevention, diagnosis, and treatment of malaria [].Malaria is the most common disease in Africa and some countries in Asia with the highest number of indigenous ...
This case-study is part of a series of malaria elimination case-studies conducted by the WHO Global Malaria Programme and the University of California, San Francisco (UCSF), Global Health Group. The WHO Global Malaria Programme and the UCSF Global Health Group wish to acknowledge the financial support of the Bill & Melinda Gates Foundation in
The following fictional storyline is intended to provide an opportunity to gain a deeper understanding behind various determinants of malaria, explore the mechanisms of popular malaria tests, and attempt to address and solve barriers relating to malaria outbreaks within Sub-Saharan Africa. Through a discovery-oriented case study approach, you ...
The study used analytical cross-sectional study design to analyze secondary data from the Tanzania Demographic and Health Survey and Malaria Indicators Survey 2015-2016. A total of 4,644 women of reproductive age who gave birth to live babies within five years preceding the survey were included in the study.
This case-study is part of a series of malaria elimination case-studies conducted by the World Health Organization (WHO) Global Malaria Programme and the University of California, San Francisco (UCSF), Global Health Group. The two groups wish to acknowledge the financial support of the Bill & Melinda Gates Foundation in
But malaria goes a great deal further back in time. In a 2024 study in the journal Nature , researchers looked at genetic material from slivers of ancient human remains from 16 countries and found ...
Our cross-sectional study included persons with suspected mpox who attended mpox treatment centers and outpatient clinics across Nigeria during June 1-December 30, 2022. We defined a suspected case of mpox by using the Nigeria Centre for Disease Control and Prevention guidelines, as previously described . On the basis of an average of 12 ...
Haematological and biochemical findings in severe malaria. Anaemia is normocytic and may be 'severe' (haemoglobin < 5g/dl or erythrocyte volume fraction (haematocrit) < 15%. Thrombocytopenia (< 100 000 platelets/μl) is usual in malaria, and in some cases the platelet count may be extremely low (< 20 000/μl).