- Case report
- Open access
- Published: 03 May 2020

Case report: acute abdominal pain in a 37-year-old patient and the consequences for his family
- Elisabeth Niemeyer ORCID: orcid.org/0000-0002-1530-3720 1 ,
- Hamid Mofid 2 ,
- Carsten Zornig 3 ,
- Eike-Christian Burandt 4 ,
- Alexander Stein 1 ,
- Andreas Block 1 na1 &
- Alexander E. Volk 5 na1
BMC Gastroenterology volume 20 , Article number: 129 ( 2020 ) Cite this article
2445 Accesses
1 Citations
1 Altmetric
Metrics details
Hereditary diffuse gastric cancer is a rare condition that accounts for approximately 1–3% of all gastric cancer cases. Due to its rapid and invasive growth pattern, it is associated with a very poor prognosis. As a result, comprehensive genetic testing is imperative in patients who meet the current testing criteria in order to identify relatives at risk. This case report illustrates the substantial benefit of genetic testing in the family of a patient diagnosed with hereditary diffuse gastric cancer.
Case presentation
A 37-year-old patient was admitted to the emergency department with acute abdominal pain. Following explorative laparoscopy, locally advanced diffuse gastric cancer was diagnosed. The indication for genetic testing of CDH1 was given due to the patient’s young age. A germline mutation in CDH1 was identified in the index patient. As a result, several family members underwent genetic testing. The patient’s father, brother and one aunt were identified as carriers of the familial CDH1 mutation and subsequently received gastrectomy. In both the father and the aunt, histology of the surgical specimen revealed a diffuse growing adenocarcinoma after an unremarkable preoperative gastroscopy.
Awareness and recognition of a potential hereditary diffuse gastric cancer can provide a substantial health benefit not only for the patient but especially for affected family members.
Peer Review reports
Gastric cancer is the fifth most commonly diagnosed malignancy and the third most deadly cancer in males worldwide [ 1 ]. Gastric adenocarcinoma can be classified according to Lauren’s criteria, which define two major histological subtypes: intestinal and diffuse type adenocarcinoma [ 2 ]. These two subtypes have a variety of distinct clinical and molecular pattern. While the more common intestinal type of adenocarcinoma has a stronger association with Helicobacter pylori infection and dietary risk factors, diffuse gastric cancer more often has a genetic etiology [ 3 ]. Intestinal type of gastric cancer is frequently preceded by long-standing, precancerous, ulcerous lesions and is easily detectible by gastroscopy [ 4 ]. In contrast, diffuse gastric cancer typically develops within the submucosa with small foci of signet cells dispersed throughout the tissue, often not easily detected by routine upper endoscopy and can be missed in superficial biopsies [ 5 ]. Diffuse gastric cancer is typically associated with an aggressive growth pattern and poor prognosis [ 3 ].
Germline mutations in the CDH1 gene, encoding E-cadherin, are associated with an autosomal-dominant inherited susceptibility for diffuse gastric cancer (hereditary diffuse gastric cancer/HDGC [Online Mendelian Inheritance in Man®/OMIM entry #137215). Mutations in CDH1 can be identified in up to 54% of HDGC cases by sequence analysis and gene-targeted analysis for deletion and duplication [ 6 ] Although rare, other genetic causes of HDGC are still under investigation, including mutations in the CTNNA1 gene and mutations in other genes [ 7 ]. Invasion of carcinomas into surrounding tissues and their eventual metastasis requires the process of epithelial-mesenchymal transition (EMT). Downregulation or dysfunction of E-cadherin is the hallmark event in EMT, as E-cadherin deficient cells lose their ability to adhere to each other and gain individual cell motility [ 8 ]. In the case of HDGC, E-cadherin deficiency leads to a diffuse growth pattern of cancer cells throughout the submucosa. The gastric mucosa is intact and appears normal during gastric endoscopy even in late disease [ 5 ]. Loss of expression of E-cadherin in immunohistochemistry may be an indicator for a CDH1 -associated gastric cancer. The cumulative life time risk for carriers of a pathogenic variant in CDH1 to develop gastric cancer is 70% for men and 56% for women [ 5 , 7 ]. Women carrying a CDH1 mutation also have an increased risk for breast cancer, especially lobular breast cancer (42% lifetime risk with an average age of onset of 53 years [ 6 ]). It is still unresolved whether CDH1 -mutation carriers also have an increased risk of colon cancer [ 5 ].
A 37-year-old male (Fig. 1 , III.2) was referred to the emergency room with acute abdominal pain and fever. Physical examination showed signs of an acute peritonitis and lab tests revealed elevated inflammation markers (C-reactive protein). CT-scan showed a pneumoperitoneum and an emergency exploratory laparoscopy was performed. Since the site of the perforation could not be detected by laparoscopic approach, the procedure had to be converted to an open laparotomy. A gastric perforation (2 mm in diameter) was found, excised and sutured. Unexpectedly, the surgeons noted a rigid thickened stomach wall (consistent with linitis plastica) during operation. Furthermore, a tumor mass invading the minor omentum and the mesentery was found. Intra-surgical frozen section analysis confirmed the initial suspicion and revealed diffuse growing gastric cancer with signet cells. Finally, the patient was diagnosed with a diffuse gastric cancer (pT4bN3M1).
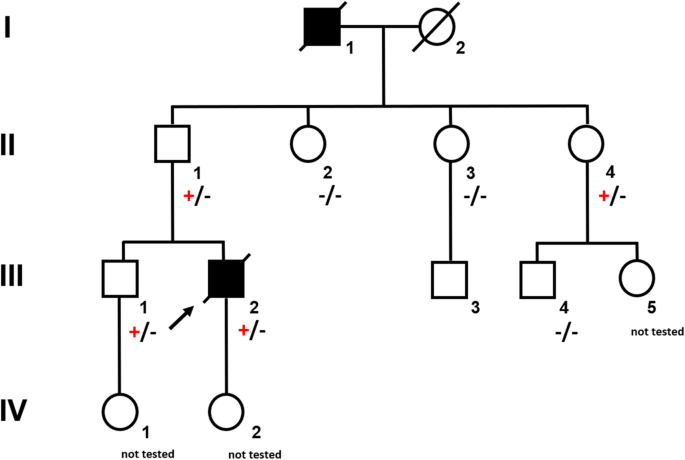
Pedigree of a large German family segregating autosomal dominant CDH1 -associated gastric cancer. Circles represent females and squares males. Filled symbols indicate clinically affected individuals and a plus sign carriers of the CDH1 mutation c.1137G > A
The young age at diagnosis and histological subtype prompted the surgeon to refer the patient and his family to genetic counselling. Evaluation of the family history revealed that the patient’s paternal grandfather (Fig. 1 , I.1) died of abdominal cancer at the age of 40. No other family members suffered from cancer, especially not the father (61 years old; Fig. 1 , II.1). Both the father and the healthy older brother (Fig. 1 III.1) underwent elective gastroscopies. In both patients, endoscopy showed an unremarkable mucosa (Fig. 2 c/d, data for brother not shown). Multiple random “button hole biopsies” were taken during gastroscopy and diffuse growing malignant cells could be detected in one out of three of the biopsies taken from the father. Genetic testing revealed the heterozygous germline mutation c.1137G > A of CDH1. This genetic alteration affects the last exonic nucleotide at the canonical splice donor site leading to impaired splicing and consequently to a dysfunctional E-cadherin molecule (Human Gene Mutation Database/HGMD® No. CS0060517). As this mutation has already been described in other HDGC patients, the diagnosis of HDGC in the father and also in the index patient carrying the same mutation was confirmed. Surprisingly, despite the presence of a CDH1 mutation, E-cadherin expression could still be immunohistochemically detected in tumor tissue with a monoclonal antibody raised against E-cadherin (Fig. 3 , Agilent Dako FLEX Monoclonal Mouse Anti-Human E-Cadherin, Clone NCH-38).
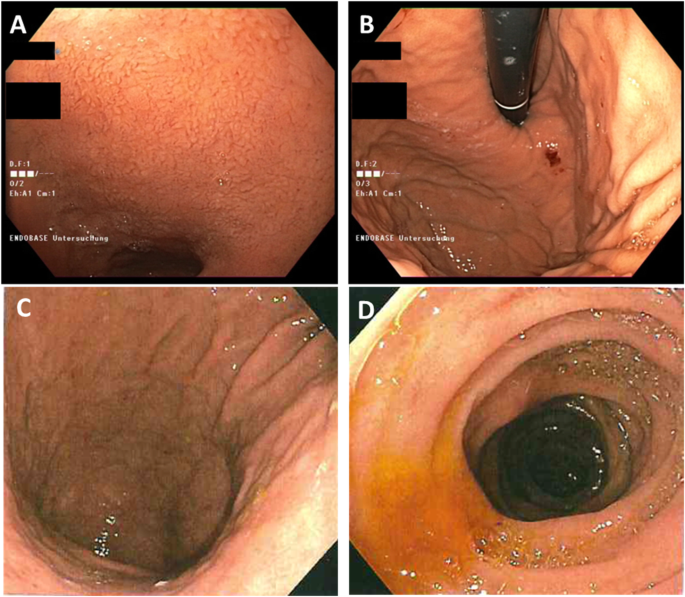
Endoscopic images of the paternal aunt ( a/b ) and the father ( c/d ) showing normal appearance of the gastric mucosa despite later confirmed signet cell adenocarcinoma in the submucosa
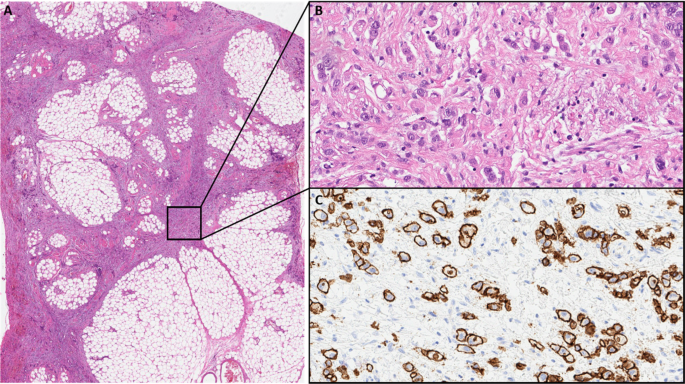
a Low power view of perigastric fat infiltrated by the gastric cancer (HE). b High power view showing diffuse growing isolated cancer cells (HE). c High power view of an E-cadherin immunohistochemistry stain with Antibody: Agilent Dako FLEX Monoclonal Mouse Anti-Human E-Cadherin, Clone NCH-38 showing strong membranous positivity of the cancer cells. With knowledge of the molecular data the E-cadherin antibody most probably detects a non-functional E-cadherin protein
With identification of a pathogenic CDH1 mutation, index patient’s healthy brother (38 years old; Fig. 1 III.1) as well as the three paternal aunts (53-, 56-, 58- years old, respectively, Fig. 1 , II.2, II.3, II.4) had an a-priori risk of 50% to also carry the CDH1 mutation. Ultimately, all four relatives underwent predictive testing after genetic counselling. While the mutation was detected in the brother and one aunt (Fig. 1 , II.4), two aunts did not inherit the mutation. As a result, his brother and his aunt underwent prophylactic gastrectomy. While no malignant cells were found in the histologic examination of the brother’s stomach, a diffuse growing adenocarcinoma was detected during histologic workup in the stomach of the aunt. Despite a macroscopically unremarkable appearance of the surgical specimen, two isolated cancer foci of signet cells located 7 mm from one another were detected in the upper third of the lamina propria of the cardiac region. Similar to the index patient’s father /herbrother, this diffuse gastric cancer was not macroscopically detectable during gastroscopy (Fig. 2 a/b) before gastrectomy was performed, not even after retrospectively analyzing the affected sites in the endoscopic images. Further staging showed no involvement of lymph nodes or distant metastasis in her case (pT1a pN0 (0/16) cM0).
The father, brother and aunt recovered well after their surgeries. Unfortunately, the condition of the index patient worsened quickly due to perioperative complications. Systemic chemotherapy could not be administered due to his poor general condition. He died 2.5 months after diagnosis.
Discussion and conclusions
Given that monogenic factors are rare and account only for 1–3% of gastric cancers, awareness among health care professionals is of utmost importance. Regardless of the family history, a personal history of a diffuse gastric cancer under the age of 40 qualifies for genetic testing of the CDH1 gene following the latest International Gastric Cancer Linkage Consortium (IGCLC) consensus guidelines. Furthermore, a CDH1 mutation should be suspected if two or more family members have been diagnosed with diffuse gastric cancer at any age or in a family if two family members were diagnosed with diffuse gastric cancer or lobular breast cancer with one diagnosis before the age of 50 years (Table 1 , [ 5 ]). In our family, the young age of the index patient and the family history with abdominal cancer of the paternal grandfather lead to genetic testing and the identification of the causal CDH1 mutation in the family. With the identification of the underlying genetic defect, all family members had the chance to learn about their exact individual risks. First degree relatives of a mutation carrier have a 50% chance to inherit the mutation. Extensive genetic counseling and discussion of the clinical management is especially important when detecting a mutation by predictive testing in healthy relatives. If a family member has not inherited the mutation, he and his progeny have no increased risk to develop gastric cancer. On the other hand, if the pathogenetic mutation is detected, the individual is faced with the cancer risks previously mentioned. As diffuse gastric cancer is difficult to detect at an early and treatable stage, mutation carriers may either choose prophylactic gastroscopy or endoscopic surveillance according to the Cambridge protocol [ 9 ]. This protocol recommends targeted biopsies of any suspicious lesion in the gastric mucosa as well as a minimum of 6 random biopsies taken from each anatomic area of the stomach (antrum, transitional zone, body, fundus, cardia) and should begin 5–10 years prior to the diagnosis of the youngest family member [ 9 , 10 ]. Current recommendations suggest prophylactic gastrectomy in healthy CDH1 mutation carriers rather than endoscopic surveillance [ 5 ]. The estimated risk for diffuse gastric cancer is 1% by the age of 20 years and around 4% by the age of 30 years [ 10 ]. Therefore, most authors recommend offering a gastrectomy to CDH1 mutation carriers between ages 20 and 30 [ 5 , 10 ]. The mortality rate of gastrectomy itself is less than 1% but it has a high morbidity for nutritional, metabolic and psychological well-being. Most patients report rapid intestinal transit, reflux, dumping syndrome and diarrhea after surgery. After gastrectomy patients need life-long vitamin supplementation. In our family, all mutation carriers chose to undergo gastrectomy, and gastric cancer was diagnosed at an early stage in two individuals. In both cases, preoperative endoscopy failed to detect the cancer. However, in random biopsies taken from endoscopically normal gastric mucosa, cancer cells were detected. Considering the overall small tumor size in both patients, the detection of tumor cells through random biopsies was a very fortunate incident for our family and underlines the need for taking a minimum of 30 deep biopsies as recommended in the Cambridge protocol. Despite advanced endoscopic techniques, the overall detection rate through preoperative endoscopic biopsies is low. In 87.9% of CDH1 -mutation carriers, foci of signet cells were initially detected after prophylactic gastrectomy [ 11 ].
In addition, women carrying a CDH1 mutation have an increased risk for lobular breast cancer. There is insufficient evidence for a risk-reducing mastectomy, although it might be discussed in individual cases based on the family history [ 12 ]. Compared to non-lobular breast cancers, there is a reduced sensitivity to detect lobular breast cancer by mammography [ 13 ]. Extrapolated from the data for high-risk hereditary breast cancer, surveillance programs for early detection include e.g. bilateral MRIs beginning at the age of 30 years [ 12 ]. Following the recommendation of the German Consortium for Hereditary Breast and Ovarian Cancer (GC-HBOC), our patient was offered annual MRIs and ultrasound of the breast until the age of 70 years. Although it is still unclear whether colon cancer is part of the CDH1- related tumor spectrum, we recommended colonoscopies more frequently and at a younger age for all mutation carriers (every 3 years starting at age 40) compared to our current national guidelines (every 5 years starting at age 50).
The diagnosis of diffuse gastric cancer with signet cells will likely prompt the pathologist to an immediate histopathological workup including immunohistochemistry for E-cadherin. However, these steps should be taken with great caution. As the CDH 1 gene is a tumor suppressor gene, the inactivation of both alleles is necessary for tumor initiation. The inactivation of the second allele in mutation carriers occurs mainly by hypermethylation of the CDH1 promotor [ 14 ]. While hypermethylation itself will result in a silencing of the respective allele and ultimately to a loss of its protein, some mutations give rise to a translated but non-functional protein. Currently, more than 180 pathogenic germline mutations in the CDH1 gene are listed in HGMD® Professional (2019.4). Most of these are truncating mutations which are predicted to elicit nonsense-mediated mRNA decay [ 15 ], which would again result in a loss of the protein. Around 20% of all the published pathogenic alleles are missense mutations which are more prone to result in non-functional, but translated and therefore potentially immunohistochemically detectable protein. The latter could result in an erroneous exclusion of a CDH1 -associated disease. The mutation identified in our family was shown before in another publication to escape nonsense-mediated mRNA decay and lead to aberrantly spliced RNA [ 16 ]. Indeed, E-cadherin expression was immunohistochemically detected in the histopathological workup in the surgical specimen of our index patient and could have led to the misinterpretation of a non- CDH1- associated diffuse gastric cancer. Given this assumption, no genetic testing would have been performed, and the underlying genetic cause would not have been unraveled. In the case of our family, this again would have hindered predictive testing for relatives at risk and the identification of two mutation carriers and three non-mutation carriers. Considering the poor prognosis of patients suffering from invasive diffuse gastric cancer, the patient’s father, brother and aunt (and potentially their progeny) had a major benefit from this genetic testing. The index patient’s child and the brother’s child (Fig. 1 , IV. 2, IV. 1) were infants at the time of genetic counseling. Predictive testing in individuals at risk younger than 18 years is a controversial issue. Rare cases of hereditary diffuse gastric cancer in individuals before the age of 18 have been reported [ 17 , 18 ]. It has therefore been suggested to offer predictive testing to individuals before the age of 18 on a case by case basis [ 5 ]. The IGCLC has agreed that genetic testing of minors at risk should consider the earliest age of cancer onset in the respective family as well as their physical and psychological resources.
In our case, we decided to evaluate future recommendations for clinical management for families affected by hereditary diffuse gastric cancer and offer genetic counseling to the minors involved in their teens. Because of an independent but severe medical illness, the aunt (III.) and her husband as the legal guardians, decided not to test their daughter. With regard to prenatal testing, there are a number of ethical issues as well as legal provisions to be addressed, especially if testing is being considered for the purpose of pregnancy termination rather than early detection and surveillance. This sensitive issue was thoroughly discussed with the brother of the index patient. The German national legal framework allows invasive prenatal testing in case of a potential treatment during pregnancy or childhood (not applicable in case of CDH1 -associated diffuse gastric cancer) or in case of an expected disease manifestation before the age of 18 years (subject of interpretation in case of CDH1 -associated diffuse gastric cancer, see above). During consultation the family ruled out invasive prenatal testing for further family planning due to ethical reasons, although preimplantation genetic diagnosis was an option the family would consider in the future.
In this case of a young patient with sudden death, awareness and recognition of a CDH1-associated hereditary diffuse gastric cancer facilitated the detection of one additional case of early gastric cancer in a family member and most likely prevented two more relatives (and potentially their progeny) from developing gastric cancer, which has a poor prognosis. Therefore, a family history leading to the suspicion of a hereditary cause of diffuse gastric cancer should prompt genetic counseling and low-threshold genetic testing of the CDH1 gene despite histological expression of E-cadherin.
Availability of data and materials
Abbreviations.
Computed tomography
Hereditary Diffuse Gastric Cancer
International Gastric Cancer Linkage Consortium
(messenger) Ribonucleic Acid
Rawla P, et al. Epidemiology of gastric cancer: global trends, risk factors and prevention. Prz Gastroenterol. 2019;14(1):26–38.
CAS PubMed Google Scholar
Lauren P. The two histological main types of gastric cancer: diffuse and so-called intestinal type carcinoma. Acta Pathol Microbiol Scand. 1965;64:331–49.
Article Google Scholar
Junli MA, et al. Lauren classification and individualized chemotherapy in gastric cancer. Oncol Lett. 2016;11(5):2959–64.
Lee JY, et al. The characteristics and prognosis of diffuse-type early gastric cancer diagnosed during health check-ups. Gut Liver. 2017;11(6):807–12.
van der Post RS, et al. Hereditary diffuse gastric cancer: updated clinical guidelines with an emphasis on germline CDH1 mutation carriers. J Med Genet. 2015;52:361–74.
Kaurah P, et al. Hereditary diffuse gastric cancer. Seattle: GeneReviews®, University of Washington; 2002. 1993-2020. [last updated 2018 Mar 22].
Google Scholar
Weren RDA, et al. Role of germline aberrations affecting CTNNA1, MAP3K6 and MYD88 in gastric cancer susceptibility. J Med Genet. 2018;55:669–74.
Article CAS Google Scholar
Sánchez-Tilló E, et al. ZEB1 represses E-cadherin and induces an EMT by recruiting the SWI/SNF chromatin-remodeling protein BRG1. Oncogene. 2010;2010(29):3490–500.
Fitzgerald RC, et al. Hereditary diffuse gastric cancer: updated consensus guidelines for clinical management and directions for future research. J Med Genet. 2010;47(7):436–44.
Shenoy S. CDH1 (E-cadherin) mutation and gastric cancer: genetics, molecular mechanisms and guidelines for management. Cancer Manag Res. 2019;11:10477–86.
Rocha JP, et al. Pathological features of total gastrectomy specimens from asymptomatic hereditary diffuse gastric cancer patients and implications for clinical management. Histopathology. 2018;73(6):878–86.
Daly MB, Pilarski R, Berry M, Buys SS, Farmer M, Friedman S, Garber JE, Kauff ND, Khan S, Klein C, Kohlmann W, Kurian A, Litton JK, Madlensky L, Merajver SD, Offit K, Pal T, Reiser G, Shannon K, Swisher E, Vinayak S, Voian NC, Weitzel JN, Wick MJ, Wiesner GL, Dwyer M, Darlow S. NCCN Guidelines Insights: Genetic/Familial High-Risk Assessment: Breast and Ovarian, Version 2.2017. J Natl Compr Canc Netw. 2017;15(1):9-20. Retrieved Apr 27, 2020, from http://www.jnccn.org/view/journals/jnccn/15/1/article-p9.xml .
Porter AJ, et al. Mammographic and ultrasound features of invasive lobular carcinoma of the breast. J Med Imaging Radiat Oncol. 2014;58:1–10.
Grady W, et al. Methylation of the CDH1 promoter as the second genetic hit in hereditary diffuse gastric cancer. Nat Genet. 2000;26:16–7.
Krempely K, et al. A novel de novo CDH1 germline variant aids in the classification of carboxy-terminal E-cadherin alterations predicted to escape nonsense-mediated mRNA decay. Cold Spring Harb Mol Case Stud. 2018;4(4):1–8.
Frebourg T, et al. Cleft lip/palate and CDH1/E-cadherin mutations in families with hereditary diffuse gastric cancer. J Med Genet. 2006;43(2):138–42.
Wickremeratne T, et al. Prophylactic gastrectomy in a 16-year-old. Eur J Gastroenterol Hepatol. 2014;26(3):353–6.
Guilford P, et al. E-cadherin germline mutations in familial gastric cancer. Nature. 1998;392:402–5.
Download references
Acknowledgements
We are indebted to all family members for the participation in this study.
Author information
Andreas Block and Alexander E. Volk contributed equally to this work.
Authors and Affiliations
Department of Medical Oncology and Hematology, University Cancer Center Hamburg, University Hamburg-Eppendorf, Hamburg, Germany
Elisabeth Niemeyer, Alexander Stein & Andreas Block
Department of Surgery, Regio Klinikum Pinneberg, Pinneberg, Germany
Hamid Mofid
Israelitisches Krankenhaus, Hamburg, Germany
Carsten Zornig
Institute of Pathology, University Medical Center Hamburg-Eppendorf, Hamburg, Germany
Eike-Christian Burandt
Institute of Human Genetics, University Medical Center Hamburg-Eppendorf, Hamburg, Germany
Alexander E. Volk
You can also search for this author in PubMed Google Scholar
Contributions
MH and CZ were the referring physicians and surgeons of the patients. EB performed pathologic workup. AEV performed genetic counseling and testing. AB, AS and EN performed genetic counseling and was involved in the oncological treatment. EN, AB and AEV wrote the manuscript. All authors read and approved the final manuscript.
Corresponding author
Correspondence to Elisabeth Niemeyer .
Ethics declarations
Ethics approval and consent to participate.
All participants were referred for routine diagnostic testing and genetic counseling. All participants gave their written informed consent for genetic testing. This study is in accordance with the Code of Ethics of the World Medical Association (Declaration of Helsinki). A copy of the consent form is available for review by the editor of this journal.
Consent for publication
All participants and their relatives gave written consent for publication of their personal and clinical details along with medical images.
Competing interests
Additional information, publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Niemeyer, E., Mofid, H., Zornig, C. et al. Case report: acute abdominal pain in a 37-year-old patient and the consequences for his family. BMC Gastroenterol 20 , 129 (2020). https://doi.org/10.1186/s12876-020-01283-2
Download citation
Received : 03 September 2019
Accepted : 22 April 2020
Published : 03 May 2020
DOI : https://doi.org/10.1186/s12876-020-01283-2
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Hereditary diffuse gastric cancer
- CDH1 germline mutation
- Prophylactic gastrectomy
BMC Gastroenterology
ISSN: 1471-230X
- Submission enquiries: [email protected]
- General enquiries: [email protected]
- Open access
- Published: 14 October 2022
The effectiveness of case management for cancer patients: an umbrella review
- Nina Wang 1 , 2 ,
- Jia Chen 3 ,
- Wenjun Chen ORCID: orcid.org/0000-0001-5398-8508 4 , 5 ,
- Zhengkun Shi 1 ,
- Huaping Yang 1 ,
- Peng Liu 6 ,
- Xiao Wei 7 ,
- Xiangling Dong 6 ,
- Chen Wang 3 ,
- Ling Mao 8 &
- Xianhong Li 3
BMC Health Services Research volume 22 , Article number: 1247 ( 2022 ) Cite this article
2945 Accesses
36 Citations
1 Altmetric
Metrics details
Case management (CM) is widely utilized to improve health outcomes of cancer patients, enhance their experience of health care, and reduce the cost of care. While numbers of systematic reviews are available on the effectiveness of CM for cancer patients, they often arrive at discordant conclusions that may confuse or mislead the future case management development for cancer patients and relevant policy making. We aimed to summarize the existing systematic reviews on the effectiveness of CM in health-related outcomes and health care utilization outcomes for cancer patient care, and highlight the consistent and contradictory findings.
An umbrella review was conducted followed the Joanna Briggs Institute (JBI) Umbrella Review methodology. We searched MEDLINE (Ovid), EMBASE (Ovid), PsycINFO, CINAHL, and Scopus for reviews published up to July 8th, 2022. Quality of each review was appraised with the JBI Critical Appraisal Checklist for Systematic Reviews and Research Syntheses. A narrative synthesis was performed, the corrected covered area was calculated as a measure of overlap for the primary studies in each review. The results were reported followed the Preferred reporting items for overviews of systematic reviews checklist.
Eight systematic reviews were included. Average quality of the reviews was high. Overall, primary studies had a slight overlap across the eight reviews (corrected covered area = 4.5%). No universal tools were used to measure the effect of CM on each outcome. Summarized results revealed that CM were more likely to improve symptom management, cognitive function, hospital (re)admission, treatment received compliance, and provision of timely treatment for cancer patients. Overall equivocal effect was reported on cancer patients’ quality of life, self-efficacy, survivor status, and satisfaction. Rare significant effect was reported on cost and length of stay.
Conclusions
CM showed mixed effects in cancer patient care. Future research should use standard guidelines to clearly describe details of CM intervention and its implementation. More primary studies are needed using high-quality well-powered designs to provide solid evidence on the effectiveness of CM. Case managers should consider applying validated and reliable tools to evaluate effect of CM in multifaced outcomes of cancer patient care.
Peer Review reports
Cancer ranks as one of the leading causes of premature death among population around 30–69 years old across 134 countries [ 1 ], and the global incidence of cancer is about to reach 30.2 million new cases and 25.7 million deaths by 2040 [ 2 ]. Earlier detection and diagnosis, and development of diverse cancer treatments have increased the survival rate of cancer patients. According to Quaresma et al. [ 3 ], the cancer survival in the UK has doubled over the last 40 years alongside the advancement in cancer diagnosis and treatment. However, number of challenges exist in the current cancer care all over the world. Many cancer patients oftentimes receive a series of long-running and exhausting multi-modal treatments and experience descent in psychological, physical and social functioning, which have a significant negative impact on their quality of life (QoL) [ 4 , 5 ]. In addition, the significant healthcare spending and productivity losses of cancer patients lead to a heavy patient economic burden, which is another substantial issue with cancer care [ 6 ]. A systematic approach is needed to mobilize and deliver appropriate resources, provide accessible, safe, and well-coordinated care for cancer patients received stressful treatments and shouldered heavy economic burden [ 7 ].
Case management (CM) is defined by the Case Management Society of America (CMSA) as “a collaborative process of assessment, planning, facilitation, care coordination, evaluation, and advocacy for options and services to meet an individual’s and family’s comprehensive health needs through communication and available resources to promote quality, cost-effective outcomes” (P. 11) [ 8 ]. According to the definition, CM is designed to use resources effectively to improve the quality of treatments, patient care services, and QoL of patients while reducing the relevant healthcare costs.
With the worldwide utilization of CM in cancer patient care, studies examining the effect of CM in improving patient-related outcomes or healthcare service use outcomes have been skyrocketing. Numbers of systematic reviews and meta-analyses have been published to synthesis the effectiveness of CM in recent years and often arrive at discordant conclusions. For example, Joo et al. [ 9 ] retrieved and synthesised results from nine experimental studies and found that CM effectively improved patients’ QoL and symptom management. While Aubin et al. [ 10 ] reported equivocal effect on both QoL and symptom management. Chan et al. [ 11 ] reported that four of the five randomized controlled trials showed insignificant impact of CM on patients’ QoL. The inconsistent evidence on the impact of CM may confuse or mislead the future case management development and relevant policy making. Considering the exist of several systematic reviews and research synthesis available to inform the application of case management for cancer patient care improvement, umbrella review could now be undertaken to compare and contrast published reviews and to highlight the consistent or contradictory findings around the effect of CM on manifold aspects of cancer patient care [ 12 ]. Thus, the current review was conducted to 1) synthesis systematic reviews that assess the effects of CM on cancer patient outcomes (e.g., QoL, functioning status, symptom management, satisfaction, etc.) and health care utilization outcomes (e.g., cost, hospital admissions, length of stay, treatment received compliance, etc.), 2) summarize measurement used in evaluating patient outcomes and health care utilization outcomes.
This umbrella review followed the Joanna Briggs Institute (JBI) Umbrella Review (UR) methodology [ 12 ] and adhered to the Preferred Reporting Items for Overviews of systematic reviews (PRIO) checklist (see Additional file 1 ) [ 13 ]. This review has been registered with the Open Science Framework ( https://doi.org/10.17605/OSF.IO/7YQAP ).
Study searching methods
We performed literature search in five databases including MEDLINE (Ovid), EMBASE (Ovid), PsycINFO, CINAHL, and Scopus from inception to July 2022. Ethical approval and patient consent were not necessary since all analyses were based on previously published articles. The searching strategies in all five databases were developed with the help of a health science librarian. See Additional file 2 for the searching strategy and results in MEDLINE (Ovid). The studies were selected using the following inclusion and exclusion criteria.
Inclusion and exclusion criteria
Individuals diagnosed with any type of cancer at any cancer stages (early to advanced). Reviews targeted on people with no specified cancer diagnose were excluded.
Intervention
Case management interventions targeted on cancer patients. Case management is defined as a “collaborative process of assessment, planning, facilitation, care coordination, evaluation, and advocacy for options and services to meet an individual’s and family’s comprehensive health needs through communication and available resources to promote quality, cost-effective outcomes” [ 8 ]. Only reviews in which the effectiveness of CM as defined above was analyzed separately from other interventions were considered.
Individuals in comparison groups received “treatment as usual” (TAU). TAU may include various interventions called “standard of care,” “usual care,” or “standard treatment,” but generally refers to treatment as it is commonly provided. Only studies that compared case management with “TAU” were selected.
Patient outcomes (e.g., quality of life, symptom management, functioning status), health care utilization outcomes (e.g., cost, hospital admissions, length of stay), etc.
Acute care hospitals and primary care settings (e.g., long-term care, nursing homes, community care services). Hospital was defined as any department of internal medicine or surgery as well as unspecified hospital settings.
Study design
Systematic review/meta-analysis that only included quantitative studies. We excluded studies full-texts unavailable online.
Study selection
All retrieved studies were imported into Covidence systematic review software [ 14 ] and the duplicates were removed. Then, titles and abstracts were independently assessed by two researchers (XW and XD) according to the inclusion criteria. After that, the full texts of the selected abstracts were obtained and reviewed by the same two researchers (XW and XD) independently. The reference list of included studies was reviewed and searched for additional studies. Any disagreement between the two researchers were resolved through consultation with a senior researcher (PL).
Quality appraisal for included reviews
Two reviewers (NW and LM) independently assessed the methodological quality of the individual studies using the JBI Critical Appraisal Checklist for Systematic Reviews and Research Syntheses [ 15 ]. The tool aims to determine the extent to which the review has addressed the possibility of bias in its design, conduct and analysis [ 15 ]. It consists of 11 criteria scored as yes, no, unclear, or not applicable. We adopted a scoring system used in previously published systematic reviews [ 16 , 17 ]. For each article, a rating score was derived by taking the number obtained in the quality rating and dividing it by the total number of possible points allowed, giving each manuscript a total quality rating between 0 and 1. Studies were then classified as low (0–0.25), low-moderate (0.26–0.50), moderate (0.51–0.75), or high (0.76–1.0).
Data extraction
We developed the data extraction form based on the research questions, and extracted following information: characteristics of included reviews such as publication year range, whether conducted meta-analysis or not, type of cancer patients, age of population, type and number of primary studies included; intervention names, components, and duration; outcomes and evaluation tools used; author’s conclusions and interpretations. Two researchers (NW and LM) extracted data independently from all included articles into an Excel spreadsheet and another researcher (XL) verified it for accuracy.
Data synthesis
We were unable to statistically pool outcomes due to the heterogeneity of outcomes of the included reviews. Therefore, we conducted a narrative synthesis [ 18 ] of the numerical data of individual studies outcomes. The studies were summarized and synthesised by two reviewers (NW and ZS) independently and double checked by a third author (HY). Following the JBI UR methodology [ 12 ], we used a summary table to present clear, specific, and structured results from the selected reviews, and then synthesised these results to identify broad conclusions. To summarized information about the interventions we coded data into features, components and delivery strategies, and inductively developed themes within each domain as they emerged from the studies. As suggested by Li and colleagues [ 19 ], we grouped outcomes into: global QoL of patients, functional status (i.e. physical, cognitive, emotional, role, social), symptom management, cost, hospital (re)admission, length of stay, treatment received compliance, provision of timely treatment.
For clarity the term ‘primary studies’ refers to the articles found within the included reviews. As several primary studies are included in more than one review, the overall results and conclusions of an overview can be biased. To assess this bias, the degree of overlap between reviews was calculated with the Corrected Covered Area (CCA) method. The details of the CCA calculation have been described by Pieper and colleagues [ 20 ] elsewhere. A CCA score of less than 5% is regarded as a slight overlap, 5–9.9% as moderate overlap, 10–14.9% as high overlap and over 15% as a very high level of overlap. This measure has been validated in which the number of overlapped primary publications has a strong correlation with the CCA [ 21 ].
Search outcome
As shown in Fig. 1 , our search strategy generated 804 potentially relevant records. Upon removing the duplicates, 582 studies screened by title and abstract, 16 were identified for full text screening. We excluded eight of the 16 studies for the following reasons: no independent analysis on the effect of case management ( n = 6), or conference abstract ( n = 2). The eight remaining systematic reviews were selected and assessed for methodological quality. In total, all the eight reviews included 57 primary studies, among which 12 were duplicated included in two or three reviews. Forty-one of the 57 primary studies were randomized controlled trials (see Additional file 3 for included primary studies).
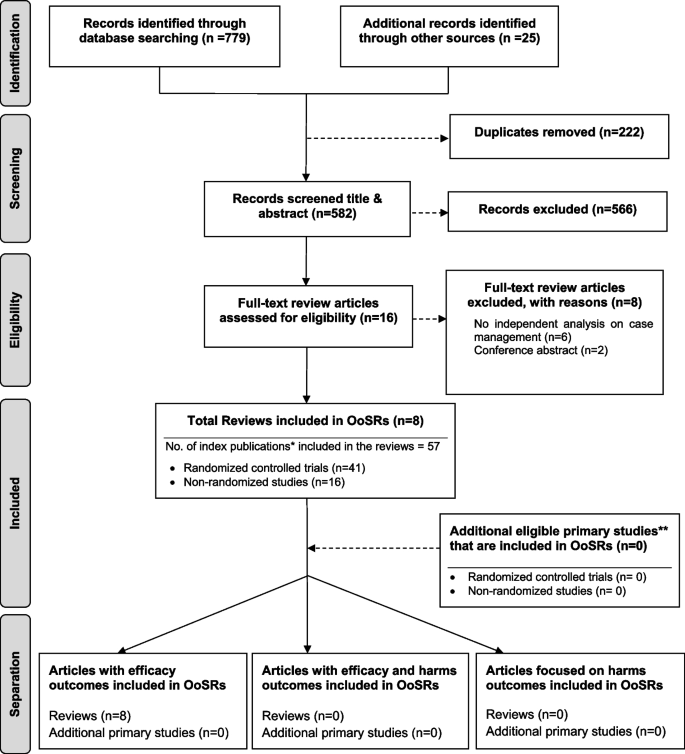
Flow chart for umbrella review. *Index publication is the first occurrence of a primary publication in the included reviews. **Additional eligible primary studies that had not been initially indentified by the search of the relevant reviews or obtained by updating the search of the included reviews
Methodological quality assessment
The quality assessment scores are presented in Table 1 . Only one review was rated as moderate because not clarify whether two or more reviewers independently assessed the quality of included primary studies, and did not report the methods to minimize errors in data extraction or publication bias. The other seven reviews were rated as high quality. Despite rated as strong, the seven reviews still companied with one or two issues on the assessment of heterogeneity, search strategy, and recommendations for policy and/or practice.
Characteristics of included studies
Table 2 presents a descriptive summary of characteristics of the eight systematic reviews [ 9 , 10 , 11 , 19 , 23 , 24 , 25 , 26 ]. The eight reviews aimed to identify evidence of the effectiveness of CM on cancer patients. Three of the studies were a systematic review with meta-analysis [ 10 , 25 , 26 ]. Five of the eight reviews adhered to the PRISMA statement [ 11 , 19 , 24 , 25 , 26 ], two adopted Cochrane systematic review methodology [ 9 , 10 ].
The eight reviews were published between 2008 and 2021, the primary studies in the reviews were published between 1983 and 2018. The number of primary studies regarding to CM included in each review ranged from three to 20. Five of the eight reviews included only randomized controlled trials (RCTs), the remaining reviews included a combination of study designs that involved RCTs, quasi-experimental and non-experimental studies (e.g., cohort study). The age of review participants ranged from 7 to 97 years and mean ages range from 48.63 to 66.31 years, which covers populations from children to elders. The total number of participants in each review ranged from 327 to 9601. Seven of the eight reviews included primary studies targeted on multiple types of cancer including breast, lung, colorectal, cervical, ovarian, prostate, gastric, hepatocellular, etc. Most of the primary studies included in the eight reviews were conducted in the United States, and there were also studies conducted in Canada, Australia, Europe (i.e., Germany, UK, Turkey, Switzerland, Denmark, Switzerland, Sweden, Norway, Netherlands) and East Asia (i.e., Hong Kong, Taiwan, South Korea, and Malaysia).
CM interventions
As shown in Table 2 , three studies reviewed trials of nurse-led CM interventions [ 9 , 25 , 26 ], two reviewed CM-like interventions that not termed as ‘CM’ while meet the CM definition by the CMSA [ 8 , 23 , 24 ]. Only one study reviewed CM focus solely on skill-training or symptom management [ 19 ]. All studies reviewed trials that facilitated the CM in a multidisciplinary collaboration approach. The duration of CM ranged from 4 days to 5 years. We presented the feature, components and delivery strategies of CM interventions for cancer patients in Fig. 2 by summarizing descriptions in each review. Congruent with the components defined by CMSA [ 8 ], all CM interventions included patient assessment, supportive services such as information and emotion support, care coordination by conducting education, consultation, and in-person, telephone or online coaching for regular follow-up. One critical component of CM interventions for cancer patients is the provision of palliative care. Control groups (CGs) of all studies reviewed in the reviews received usual treatment of care.
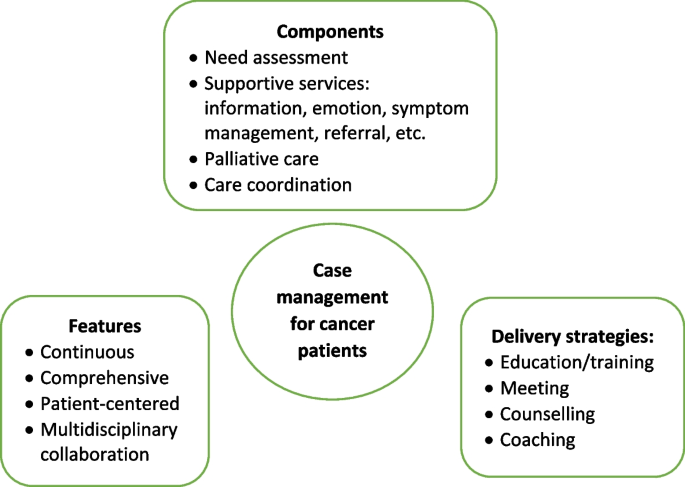
Features, components, and delivery strategies of case management for cancer patient care
Corrected Covered Area (CCA)
Table 3 presents the CCA for each outcome and as a whole. Overall, primary studies had a slight overlap across the eight reviews (CCA = 4.5%). In addition, no overlapping of primary studies was found for six of the 16 outcomes, including self-efficacy, psychological function, hospital (re)admissions, length of stay, and provision of timely treatment. Only one outcome (i.e., symptom management) showed slight overlap (0.7%). The CCA for other five outcomes (i.e., global QoL, physical function, role function, patient satisfaction, cost) evaluated by more than 2 reviews were between 5 to 9.9%, indicated a moderate overlap. The CCA for survivor status, cognitive function, emotional function, and treatment received compliance were over 10%.
Measurement used
Table 4 presents the quantitative measurement used in primary studies. As shown in Table 4 , studies investigated global QoL using different QoL-related scales, among which Functional Assessment of Cancer Therapy (FACT) (used in 15 primary studies) were most frequently applied, followed by the European Organisation for Research and Treatment of Cancer Core Quality of Life Questionnaire 30 (EORTC QLQ-C30) (used in 11 primary studies), and short form health survey (i.e., SF-8, SF-12, SF-36) (used in 10 primary studies). Different types of FACT tool were used according to the cancer types. For example, FACT-G was used for general cancer patients assessment, and FACT-B was used to evaluate breast cancer-related QoL. For the assessment of overall symptom management, SF-36 and Symptom Distress Scale (SDS) were used most frequently (used in four primary studies each). Different dimensions of SF-36 were also applied to evaluate other outcomes such as physical, emotional, and social function. Hospital Anxiety and Depression Scale (HADS) was the top employed tool in measuring the psychological function of patients. Patients’ sick leave days and the number of patients return to work were top employed metrics to evaluate the role function of patients. No unified tools were utilized to assess patient satisfaction towards the CM and majority of the primary studies used self-developed questionnaires.
Effect of CM on patient and health care utilization outcomes
The main outcomes from the seven systematic reviews are presented and summarized in Table 5 . Seven of the eight reviews reported the effects of case management on patients’ global QoL and showed mixed findings. Around half (49%, 19/39) of the primary studies included in the seven reviews reported significant positive impact of CM on global QoL. As for the functional status, there was a strong concordance among primary studies regarding the effectiveness of CM in improving cognitive function (e.g., uncertainty, health perceptions) (89%, 8/9); Equivocal effects were reported on psychological (e.g., patient anxiety, depression), physical (e.g., arm function), role function (e.g., sick leave days, patients returning to work), emotional (e.g., mood) and social function (e.g., social support) [ 9 , 11 , 26 ]. The findings regard to symptom management were more positive, with 75% (18/24) primary studies included in seven reviews revealed significant positive impact of CM on symptom severity and symptom distress decrease of pain, nausea, fatigue, discomfort, etc. Three of the four primary studies in two reviews [ 9 , 11 ] showed no significant influence of CM on patients’ self-efficacy. Wulff et al. [ 23 ] and Aubin et al. [ 10 ] reported mixed findings on the impact of CM on survivor status, with four of the six primary studies reported significant positive impact. The effect of CM on patient satisfaction was reported in five reviews and showed mixed results.
Of the eleven primary studies reported cost, only one controlled before-and-after study in Joo et al.’s [ 9 ] review reported significant impact on monthly cancer-related medical costs. The evidence concerning patients’ length of stay yielded no significant findings. Overall significant positive effect was reported on hospital (re)admission (e.g., inpatient and ICU admission rate), treatment received compliance (e.g., therapy acceptance or completion rate), and provision of timely treatment.
This umbrella review is the first to summarize the results of systematic reviews that synthesised the evidence on the effectiveness of CM on cancer patient outcomes and relevant health care utilization. Most reviews (7/8) showed a high methodological quality. Different tools were used to measure the effect of CM on the same outcome. The evidence regards to the effectiveness of CM is mixed. The summarized results revealed that CM was more likely to improve symptom management, cognitive function, hospital (re)admission, treatment received compliance, and provision of timely treatment for cancer patients. Overall equivocal effect was reported on cancer patients’ global QoL, psychological, physical, role, emotional and social function, self-efficacy, survivor status, and patient satisfaction.
No universal tools were used to measure improvement of each outcome in the CM group compared with the control group, making it challenging to conduct a meta-analysis of studies results [ 22 , 27 ]. This is a common issue faced the included reviews. Five of the eight reviews failed to conduct meta-analysis due to the heterogeneity [ 9 , 11 , 19 , 23 , 24 ]. Joo and Huber [ 22 ] conducted a review of reviews on the effect of CM on health care utilization outcome of chronic illness patients, they recognized the same problem and suggested using valid and standardized tools to minimize the differences in measurements. Despite various tools used, our review showed that FACT, EORTC QLQ-C30, and short form health survey (i.e., SF 36, SF 12, and SF 8) were most frequently applied to measure the effect of CM on the global QoL of cancer patients. These tools were also used in evaluating specific dimensions of QoL such as psychological, physical, emotional, and social function. This aligned with previous reviews [ 28 , 29 ] that found FACT and EORTC QLQ-C30 were the most common and well developed QoL instruments in cancer patients. FACT-G is considered appropriate for use with any types of cancer patients [ 30 ]. It is a 27-item tool that includes four primary QoL domains: physical well-being, social/family well-being, emotional well-being, and functional well-being [ 31 ]. Other versions of FACT (FACT-B [ 32 ], FACT-L [ 33 ] and FACT-E [ 34 ]) for specific type of cancer patients were developed by incorporating the four dimensions of FACT-G with additional cancer type-specific questions. EORTC QLQ-C30 was another type of QoL assessment tools for cancer patients specifically. It was developed by Aaronson et al. [ 35 ] and contains four domains: physical, emotional, cognitive and social functions, and a higher score indicates better QoL. The Short Form Health Survey is the most commonly used measure in evaluating QoL domains of patients suffering from a wide range of medical conditions [ 36 ]. Research found it provides reliable and valid indication of general health among cancer patients [ 37 , 38 ].
QoL is the most frequently evaluated outcome in our review with 39 primary studies in seven reviews reported the global QoL of cancer patients. Joo et al. [ 9 ] found that CM interventions improved QoL of cancer patients. Yin and colleagues [ 24 ] revealed that cancer patients achieved better physical and psychological condition through symptom management, needs assessment, direct referrals, and other services in CM. However, summarized results in our review show that the CM had equivocal effect on cancer patients’ global QoL and dimensions including psychological, physical, role, emotional and social function. Cognitive function is the only dimension showed positive change. Despite CM interventions share similar definitions and principles [ 8 ]. It is hard to foresee which aspect(s) of CM interventions contribute to certain effects due to their comprehensiveness [ 24 ]. Yin et al. [ 24 ] argued that the control group may receive a higher quality treatment than planned usual care since all the participants were not blinded and they have been informed about the aim of the study. Indicating a more rigorous design and evaluation is needed to avoid this information bias.
In the meantime, included reviews claimed that few primary studies reported enough details about CM interventions, including model used [ 10 , 11 ], dose and intensity [ 9 , 19 , 24 ], interventionist qualifications [ 11 ], protocol or manual used [ 9 , 23 ], and fidelity [ 23 ]. Particularly, the COVID-19 pandemic has considerable influence on the care delivery for cancer patients. For example, the more frequently utilization of remote patient monitoring technologies that incorporate community resources, primary care and allied health disciplines, as well as clinics to keep cancer patients away from acute care hospitals as much as possible [ 39 ]. Many of these changes have been integrated within routine case management for cancer care during the pandemic [ 39 ]. It is well-needed to report how those CM intervention were conducted follow standard reporting guidelines, in order to provide recommendation for future research.
Our review showed that CM is likely to improve the symptom management. Eighteen of the 24 included primary studies reported positive effect of CM on symptom management, including decrease symptom distress or severity of fatigue, pain, nausea, and vomiting. The same positive effect on symptom management was also revealed in other types of patients. Joo and colleagues [ 40 ] found that CM reduced substance use and significantly influenced abstinence rates among populations experienced substance disorders. Reviews by Stokes et al. [ 27 ] and Welch et al. [ 41 ] revealed positive effect on symptom release among people with long-term conditions and diabetes patients, respectively. The multidisciplinary collaboration approach adopted [ 10 ], and availability of professional support post-hospitalization [ 9 , 41 ] in CM might contribute to the improvement of symptom management. Specifically, multidisciplinary team involves physicians, nurses, and aligned healthcare professionals provides throughout and multifaced symptom assessment and management [ 10 ]. In addition, CM programs continuously follow up and advocate for patients’ concerns [ 8 ]. Specifically, case managers are available to patients 24 hours a day by phone call even after discharged, providing opportunity for immediate professional guidance on symptom management [ 9 ].
As for other patient outcomes, there is insufficient evidence of effect on self-efficacy and survivor status of cancer patients. Only three and four primary studies in total reported these two outcomes, respectively. Eleven primary studies in five reviews reported patient satisfaction and showed mixed results. Inconsistent results were found in a review of reviews by Buja et al. [ 7 ] which concluded strong evidence of CM improving satisfaction of patients with long term condition. In agreement with Joo and Huber’s [ 25 ] review, we found that CM favorably affect healthcare utilization outcomes such as treatment received compliance, hospital (re)admission, and provision of timely treatment. While the strength of the evidence was limited either by the high level of primary studies overlapping (CCA) (i.e., treatment received compliance, CCA = 13.3%) or the small number of studies reported certain outcomes (i.e., hospital admission, provision of timely treatment). Notably, the summarized results from included reviews conclude that despite theoretical benefits [ 8 ], in practice there is only slight evidence of benefits on reduction in the cost of care for cancer patients participated in CM interventions.
We provide some recommendations for future research based on the summarized results: 1) Future research should clearly describe details of CM intervention and its implementation, including theoretical underpinnings, dose and intensity, interventionist qualifications, protocol or manual used, fidelity, etc. In that way these details can be included in future systematic reviews, and effectiveness of individual elements of the intervention can be examined [ 27 ]. We recommend use standard guidelines to help organize the CM intervention reporting. For example, the Template for Intervention Description and Replication (TIDeiR) is one of the most popular guidelines that could be used to report the full breadth of CM interventions: from intervention rationale to assessments of treatment adherence and fidelity [ 42 ]. 2) More rigorous trials are needed to evaluate the effectiveness of CM. 3) Studies should also explore the barriers to and facilitators of CM implementation across various types of cancer patients at different stages, providing evidence for conducting successful CM implementation in the future.
Strengths and limitations
We conducted an umbrella review instead of a meta-analysis due to the heterogeneity of review outcomes. Although an umbrella review can only show the tendency or direction of the effect of CM rather than providing the magnitude or significance level of influence [ 12 ], the current evidence on the effect of CM in cancer patients was comprehensively summarized. There were some challenges when conducting the review. First, the quality of the umbrella reviews was greatly affected by the quality of the original reviews [ 12 ]. In this study, we confirmed that the quality of the original reviews were mostly high as assessed by the JBI Critical Appraisal Checklist [ 15 ]. Second, if the primary studies were included in several reviews, they may produce bias related to overlapping effects [ 20 ]. By calculating the CCA, we showed that 75% (12/16) of the individual outcomes had no to moderate overlapping of primary studies between included reviews, revealing that these results from each review were relatively independent. Cautious are needed on the summarized evidence regards to the effect of CM on survivor status, cognitive function, emotional function, and treatment received compliance because of the high overlapping (CCA > 10) between the reviews reported those outcomes.
There are limitations in our review. The first limitation concerns that the searching was limited to English-language articles and did not access unpublished papers. Second, as suggested by the JBI UR methodology [ 12 ], we did not assess the quality of evidence from included reviews, it increased the uncertainty of the review findings.
Effective CM aims to influence the health care delivery system in improving the health outcomes of cancer patients, enhancing their experience of health care, and reducing the cost of care. Our review found mixed effects of CM reported in cancer patient care. The summarized results revealed that CM was likely to improve symptom management for cancer patients. We also found CM has the tendency to enhance cancer patients’ experience of health care such as reducing hospital (re)admission rates, improving treatment received compliance and provision of timely treatment. Only slight evidence of benefits was reported on reducing the cost of care for cancer patients. Overall, more rigorous designed primary studies are needed to demonstrate the effects of CM on cancer patients and explore the elements of effective CM interventions.
Availability of data and materials
All data generated or analysed during this study are included in this published article and its supplementary information files.
Abbreviations
Corrected Covered Area
Control groups
- Case management
Case Management Society of America
European Organization for Research and Treatment of Cancer Core Quality of Life Questionnaire 30
Functional Assessment of Cancer Therapy - Breast Cancer
Functional Assessment of Cancer Therapy- Esophagus
Functional Assessment of Cancer Therapy- General
Functional Assessment of Cancer Therapy Scale-Lung
- Quality of life
Hospital Anxiety and Depression Scale
Joanna Briggs Institute
Preferred Reporting Items for Systematic Reviews and Meta-Analyses
Randomized controlled trials
Symptom Distress Scale
Medical Outcomes Study 8-item short form health survey
Medical Outcomes Study 12-item short form health survey
Medical Outcomes Study 36-item short form health survey
Treatment as usual
Ferlay J, Ervik M, Lam F, Colombet M, Mery L, Piñeros M, et al. Global cancer observatory: Cancer today: International Agency for Research on Cancer; 2020. https://gco.iarc.fr/today .
Ferlay J, Ervik M, Lam F, Colombet M, Mery L, Piñeros M, et al. Global Cancer observatory: Cancer tomorrow: International Agency for Research on Cancer; 2020. https://gco.iarc.fr/tomorrow .
Quaresma M, Coleman MP, Rachet B. 40-year trends in an index of survival for all cancers combined and survival adjusted for age and sex for each cancer in England and Wales, 1971-2011: a population-based study. Lancet. 2015;385:1206–18. https://doi.org/10.1016/S0140-6736(14)61396-9 .
Article PubMed Google Scholar
Yabroff KR, Dowling EC, Guy GP, Banegas MP, Davidoff A, Han X, et al. Financial hardship associated with cancer in the United States: findings from a population-based sample of adult cancer survivors. J Clin Oncol. 2016;34:259–67. https://doi.org/10.1200/JCO.2015.62.0468 .
Yan AF, Stevens P, Holt C, Walker A, Ng A, McManus P, et al. Culture, identity, strength and spirituality: a qualitative study to understand experiences of African American women breast cancer survivors and recommendations for intervention development. Eur J Cancer Care (Engl). 2019;28:e13013. https://doi.org/10.1111/ecc.13013 .
Article Google Scholar
Yabroff K, Mariotto A, Tangka F. Annual report to the nation on the status of Cancer, part II: patient economic burden associated with Cancer care. J Natl Cancer Inst. 2021;113:1670–82. https://doi.org/10.1093/jnci/djab192 .
Buja A, Francesconi P, Bellini I, Barletta V, Girardi G, Braga M, et al. Health and health service usage outcomes of case management for patients with long-term conditions: a review of reviews. Prim Heal Care Res Dev. 2020;21:1–21. https://doi.org/10.1017/S1463423620000080 .
Case Management Society of America. CMSA’s standards of practice for case management, Revised 2016. 2016. http://www.naylornetwork.com/cmsatoday/articles/index-v3.asp?aid=400028&issueID=53653 .
Google Scholar
Joo JY, Liu MF. Effectiveness of nurse-led case Management in Cancer Care: systematic review. Clin Nurs Res. 2019;28:968–91. https://doi.org/10.1177/1054773818773285 .
Aubin M, Giguère A, Martin M, Verreault R, Fitch MI, Kazanjian A, et al. Interventions to improve continuity of care in the follow-up of patients with cancer. Cochrane Database Syst Rev. 2012:1–193. https://doi.org/10.1002/14651858.CD007672.pub2 .
Chan RJ, Teleni L, McDonald S, Kelly J, Mahony J, Ernst K, et al. Breast cancer nursing interventions and clinical effectiveness: a systematic review. BMJ Support Palliat Care. 2020;10:276–86. https://doi.org/10.1136/bmjspcare-2019-002120 .
Aromataris E, Fernandez R, Godfrey C, Holly C, Khalil H, Tungpunkom P. JBI manual for evidence synthesis. In: Aromataris E, Munn Z, editors. JBI Manual for Evidence Synthesis. JBI; 2020. https://doi.org/10.46658/JBIMES-20-11 .
Chapter Google Scholar
Bougioukas KI, Liakos A, Tsapas A, Ntzani E, Haidich AB. Preferred reporting items for overviews of systematic reviews including harms checklist: a pilot tool to be used for balanced reporting of benefits and harms. J Clin Epidemiol. 2018;93:9–24. https://doi.org/10.1016/j.jclinepi.2017.10.002 .
Veritas Health Innovation. Covidence systematic review sofware. Covidence. 2016. https://get.covidence.org/systematic-review-software?campaignid=15030045989&adgroupid=130408703002&gclid=EAIaIQobChMIvc7KuJDO-QIVh97ICh1BeQKnEAAYASAAEgI4APD_BwE .
Aromataris E, Fernandez R, Godfrey C, Holly C, Kahlil H, Tungpunkom P. Summarizing systematic reviews: methodological development, conduct and reporting of an umbrella review approach. Int J Evid Based Heal. 2015;13:132–40 http://joannabriggs.org/research/critical-appraisal-tools.html .
Gifford W, Rowan M, Dick P, Modanloo S, Benoit M, Al AZ, et al. Interventions to improve cancer survivorship among indigenous peoples and communities : a systematic review with a narrative synthesis. Support Care Cancer. 2021;29:7029–48. https://doi.org/10.1007/s00520-021-06216-7 .
Article PubMed PubMed Central Google Scholar
Gifford W, Squires J, Angus D, Ashley L, Brosseau L, Craik J, et al. Managerial leadership for research use in nursing and allied health care professions : a systematic review. Implement Sci. 2018;13:1–23. https://doi.org/10.1186/s13012-018-0817-7 .
Popay J, Roberts H, Sowden A, Petticrew M, Arai L, Rodgers M, et al. Guidance on the conduct of narrative synthesis in systematic reviews: a product from the ESRC methods Programme. Lancaster: Lancaster University; 2006. https://www.lancaster.ac.uk/media/lancaster-university/content-assets/documents/fhm/dhr/chir/NSsynthesisguidanceVersion1-April2006.pdf .
Li Q, Lin Y, Liu X, Xu Y. A systematic review on patient-reported outcomes in cancer survivors of randomised clinical trials: direction for future research. Psychooncology. 2014;23:721–30. https://doi.org/10.1002/pon.3504 .
Article CAS PubMed Google Scholar
Pieper D, Antoine SL, Mathes T, Neugebauer EAM, Eikermann M. Systematic review finds overlapping reviews were not mentioned in every other overview. J Clin Epidemiol. 2014;67:368–75. https://doi.org/10.1016/j.jclinepi.2013.11.007 .
Choi J, Lee M, Lee JK, Kang D, Choi JY. Correlates associated with participation in physical activity among adults: a systematic review of reviews and update. BMC Public Health. 2017;17:1–13. https://doi.org/10.1186/s12889-017-4255-2 .
Joo JY, Huber DL. Case management effectiveness on health care utilization outcomes: a systematic review of reviews. West J Nurs Res. 2019;41:111–33. https://doi.org/10.1177/0193945918762135 .
Wulff CN, Thygesen M, Søndergaard J, Vedsted P. Case management used to optimize cancer care pathways: a systematic review. BMC Health Serv Res. 2008;8:1–7 http://www.biomedcentral.com/1472-6963/8/227 .
Yin YN, Wang Y, Jiang NJ, Long DR. Can case management improve cancer patients quality of life?: a systematic review following PRISMA. Medicine (Baltimore). 2020;99:1–7. https://doi.org/10.1097/MD.0000000000022448 .
Wu YL, Padmalatha KMS, Yu T, Lin YH, Ku HC, Tsai YT, et al. Is nurse-led case management effective in improving treatment outcomes for cancer patients? A systematic review and meta-analysis. J Adv Nurs. 2021;00:1–11. https://doi.org/10.1111/jan.14874 .
McQueen J, McFeely G. Case management for return to work for individuals living with cancer: a systematic review. Int J Ther Rehabil. 2017;24:203–10. https://doi.org/10.12968/ijtr.2017.24.5.203 .
Stokes J, Panagioti M, Alam R, Checkland K, Cheraghi-Sohi S, Bower P. Effectiveness of case management for “at risk” patients in primary care: a systematic review and meta-analysis. PLoS One. 2015;10:e0132340. https://doi.org/10.1371/journal.pone.0132340 .
Article CAS PubMed PubMed Central Google Scholar
Lemieux J, Goodwin PJ, Bordeleau LJ, Lauzier S, Théberge V. Quality-of-life measurement in randomized clinical trials in breast cancer: an updated systematic review (2001-2009). J Natl Cancer Inst. 2011;103:178–231. https://doi.org/10.1093/jnci/djq508 .
Montazeri A. Health-related quality of life in breast cancer patients: a bibliographic review of the literature from 1974 to 2007. J Exp Clin Cancer Res. 2008;27:1–31 https://jeccr.biomedcentral.com/articles/10.1186/1756-9966-27-32 .
Cella DF, Tulsky DS, Gray G, Sarafian B, Linn E, Bonomi A, et al. The functional assessment of cancer therapy scale: development and validation of the general measure. J Clin Oncol. 1993;11:570–9. https://doi.org/10.1200/JCO.1993.11.3.570 .
Webster K, Cella D, Yost K. The F unctional a ssessment of C hronic I llness T herapy (FACIT) measurement system: properties, applications, and interpretation. Health Qual Life Outcomes. 2003;1:79. https://doi.org/10.1186/1477-7525-1-79 .
Brady MJ, Cella DF, Mo F, Bonomi AE, Tulsky DS, Lloyd SR, et al. Reliability and validity of the functional assessment of cancer therapy- breast quality-of-life instrument. J Clin Oncol. 1997;15:974–86. https://doi.org/10.1200/jco.1997.15.3.974 .
Cella DF, Bonomi AE, Lloyd SR, Tulsky DS, Kaplan E, Bonomi P. Reliability and validity of the functional assessment of cancer therapy-lung (FACT-L) quality of life instrument. Lung Cancer. 1995;12:199–220. https://doi.org/10.1016/0169-5002(95)00450-F .
Darling G, Eton DT, Sulman J, Casson AG, Cella D. Validation of the functional assessment of cancer therapy esophageal cancer subscale. Cancer. 2006;107:854–63. https://doi.org/10.1002/cncr.22055 .
Aaronson NK, Ahmedzai S, Bergman B, Bullinger M, Cull A, Duez NJ, et al. The European organization for research and treatment of cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst. 1993;85:365–76. https://doi.org/10.1093/jnci/85.5.365 .
Ware J, Kosinski M, Dewey J. How to score version two of the SF-36® health survey. Lincoln: QualityMetric Incorporated; 2000.
Treanor C, Donnelly M. A methodological review of the short form health survey 36 (SF-36) and its derivatives among breast cancer survivors. Qual Life Res. 2015;24:339–62. https://doi.org/10.1007/s11136-014-0785-6 .
Lins L, Carvalho FM. SF-36 total score as a single measure of health-related quality of life: scoping review. SAGE Open Med. 2016;4:1–12. https://doi.org/10.1177/2050312116671725 .
Chan RJ, Crawford-Williams F, Crichton M, Joseph R, Hart NH, Milley K, et al. Effectiveness and implementation of models of cancer survivorship care: an overview of systematic reviews. J Cancer Surviv. 2021:1–25. https://doi.org/10.1007/s11764-021-01128-1 .
Joo J, Huber D. Community-based case management effectiveness in populations that abuse substances. Int Nurs Rev. 2015;62:536–46. https://doi.org/10.1111/inr.12201 .
Welch G, Garb J, Zagarins S, Lendel I, Gabbay RA. Nurse diabetes case management interventions and blood glucose control: results of a meta-analysis. Diabetes Res Clin Pract. 2010;88:1–6. https://doi.org/10.1016/j.diabres.2009.12.026 .
Hoffmann TC, Glasziou PP, Boutron I, Milne R, Perera R, Moher D, et al. Better reporting of interventions: template for intervention description and replication (TIDieR) checklist and guide. BMJ. 2014;348:g1687. https://doi.org/10.1136/bmj.g1687 .
Download references
Acknowledgements
Not applicable.
Dual (co-)authorship
We declared that no author has authored one or more of the included systematic reviews.
This study was supported by 1) Hunan Provincial Key Laboratory of Nursing (2017TP1004, PI: Jia Chen), Hunan Provincial Science and Technology Department, 2) Changsha Natural Science Foundation (kq2202365, PI: Nina Wang) Changsha Science and Technology Department, and 3) Management research foundation of Xiangya Hospital (2021GL12, PI: Nina Wang).
Author information
Authors and affiliations.
Department of Respiratory Medicine, Xiangya Hospital, Central South University, Changsha, China
Nina Wang, Zhengkun Shi & Huaping Yang
National Clinical Research Center for Geriatric Disorders, Xiangya Hospital, Changsha, China
Xiangya School of Nursing, Central South University, Changsha, China
Jia Chen, Chen Wang & Xianhong Li
School of Nursing, University of Ottawa, Ottawa, Canada
Wenjun Chen
Center for Research on Health and Nursing, University of Ottawa, Ottawa, Canada
Intensive Care Unit of Cardiovascular Surgery Department, Xiangya Hospital, Central South University, Changsha, China
Peng Liu & Xiangling Dong
The 956th Army Hospital, Linzhi, China
School of Nursing, Changsha Medical University, Changsha, China
You can also search for this author in PubMed Google Scholar
Contributions
All authors have contributed to the production of this review. NW and WC conceptualized and designed the study and are the guarantor of the paper. JC and ZS conducted the literature search. PL, XW and XD were involved in the study screening. NW, LM and XL participated in the quality appraisal and data extraction. NW, ZS and HY conducted the data analysis. NW drafted the manuscript. WC and XL revised the manuscript. All authors participated in the review of the manuscript and approved the final manuscript.
Corresponding author
Correspondence to Wenjun Chen .
Ethics declarations
Ethics approval and consent to participate, consent for publication, competing interests.
The authors declare no competing interests.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1., additional file 2..
Searching strategies.
Additional file 3.
Rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Wang, N., Chen, J., Chen, W. et al. The effectiveness of case management for cancer patients: an umbrella review. BMC Health Serv Res 22 , 1247 (2022). https://doi.org/10.1186/s12913-022-08610-1
Download citation
Received : 04 April 2022
Accepted : 21 September 2022
Published : 14 October 2022
DOI : https://doi.org/10.1186/s12913-022-08610-1
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Cancer patients
- Umbrella review
- Health care
- Outcome assessment
BMC Health Services Research
ISSN: 1472-6963
- General enquiries: [email protected]
Log in using your username and password
- Search More Search for this keyword Advanced search
- Latest content
- Current issue
- BMJ Journals
You are here
- Volume 64, Issue 7
- A case of small cell lung cancer treated with chemoradiotherapy followed by photodynamic therapy
- Article Text
- Article info
- Citation Tools
- Rapid Responses
- Article metrics
- Department of Internal Medicine; College of Medicine, Chungnam National University Hospital and Cancer Research Institute, Jungku, Daejeon, South Korea
- Professor J O Kim, Department of Internal Medicine, Chungnam National University Hospital and Cancer Research Institute, 640 Daesadong, Jungku, Daejeon 301-721, South Korea; jokim{at}cnu.ac.kr
Here, we present the case of a 51-year-old man with limited-stage small cell lung cancer (LS-SCLC) who received concurrent chemoradiotherapy and photodynamic therapy (PDT). The patient was diagnosed as having LS-SCLC with an endobronchial mass in the left main bronchus. Following concurrent chemoradiotherapy, a mass remaining in the left lingular division was treated with PDT. Clinical and histological data indicate that the patient has remained in complete response for 2 years without further treatment. This patient represents a rare case of complete response in LS-SCLC treated with PDT.
https://doi.org/10.1136/thx.2008.112912
Statistics from Altmetric.com
Request permissions.
If you wish to reuse any or all of this article please use the link below which will take you to the Copyright Clearance Center’s RightsLink service. You will be able to get a quick price and instant permission to reuse the content in many different ways.
Competing interests: None.
Patient consent: Obtained.
Read the full text or download the PDF:
- Get new issue alerts Get alerts
Secondary Logo
Journal logo.
Colleague's E-mail is Invalid
Your message has been successfully sent to your colleague.
Save my selection
A case report of metastatic lung adenocarcinoma with long-term survival for over 11 years
Editor(s): NA.,
Department of Respiratory Medicine, Tokyo Dental College, Ichikawa General Hospital, 5-11-13, Sugano, Ichikawa, Chiba, Japan.
∗Correspondence: Tatsu Matsuzaki, Department of Respiratory Medicine, Tokyo Dental College, Ichikawa General Hospital, 5-11-13, Sugano, Ichikawa, Chiba 272-8513, Japan (e-mail: [email protected] ).
Abbreviations: CBDCA = carboplatin, CDDP = cisplatin, CEA = carcinoembryonic antigen, DTX = docetaxel, ECOG-PS = Eastern Cooperative Oncology Group performance status, EGFR = epidermal growth factor receptor, EGFR-TKI = epidermal growth factor receptor tyrosine kinase inhibitor, NSCLC = non-small cell lung cancer, OS = overall survival, PD = progressive disease, PEM = pemetrexed, PFS = progression-free survival, RC = re-challenge chemotherapy, RR = response rate, UICC = Union of International Cancer Control.
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
The Ethics Committee of our hospital approved the study and provided permission to publish the results.
The patient provided written informed consent and has provided consent for publication of the case.
The authors declare that they have no conflict of interest.
This is an open access article distributed under the Creative Commons Attribution License 4.0 (CCBY), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. http://creativecommons.org/licenses/by/4.0
Rationale:
This is the first known report in the English literature to describe a case of metastatic non-small cell lung cancer that has been controlled for >11 years.
Patient concerns:
A 71-year-old man visited our hospital because of dry cough.
Diagnosis:
Chest computed tomography revealed a tumor on the left lower lobe with pleural effusion, and thoracic puncture cytology indicated lung adenocarcinoma.
Interventions:
Four cycles of carboplatin and docetaxel chemotherapy reduced the size of the tumor; however, it increased in size after 8 months, and re-challenge chemotherapy (RC) with the same drugs was performed. Repeated RC controlled disease activity for 6 years. After the patient failed to respond to RC, erlotinib was administered for 3 years while repeating a treatment holiday to reduce side effects. The disease progressed, and epidermal growth factor receptor ( EGFR ) gene mutation analysis of cells from the pleural effusion detected the T790 M mutation. Therefore, osimertinib was administered, which has been effective for >1 year.
Outcomes:
The patient has survived for >11 years since the diagnosis of lung cancer.
Lessons:
Long-term survival may be implemented by actively repeating cytotoxic chemotherapy and EGFR-tyrosine kinase inhibitor administration.
1 Introduction
The prognosis of patients with advanced non-small cell lung cancer (NSCLC) is poor, and their 1-year survival rate after cytotoxic chemotherapy is only 29%. [1] However, the development of epidermal growth factor receptor tyrosine kinase inhibitors (EGFR-TKIs) dramatically improves the prognosis of certain patients. Patients with EGFR-mutant advanced NSCLC receiving EGFR-TKIs have a median overall survival (OS) more than twice as long as those not receiving EGFR-TKIs (24.3 vs 10.8 months). [2] The 5-year survival rate of patients with EGFR-mutant metastatic lung adenocarcinoma treated with EGFR-TKIs is 14.6%. [3] However, metastatic NSCLC patients with long-term survival (>10 years) are still rare.
We treated an advanced NSCLC patient with malignant pleural effusion who survived for >11 years and for whom disease progression was controlled using drugs alone without surgery or radiation therapy.
2 Case presentation
A 71-year-old Japanese man experienced dry cough for 2 weeks and visited the Department of Respiratory Medicine at our hospital in August 2007. Enhanced chest-abdomen computed tomography revealed a tumor with a 3-cm diameter in the left lower lobe and left pleural effusion ( Fig. 1 ). A 5-mm nodule, considered to be lung metastasis, was detected in the left upper lobe. Cytological analysis of the left pleural effusion by thoracic puncture led to the diagnosis of lung adenocarcinoma. Gadolinium-enhanced brain magnetic resonance imaging and bone scintigraphy did not reveal any other metastases. The tumor was classified as clinical T4N0M1, stage IV according to the TNM classification of the Union of International Cancer Control (UICC), 6th edition. According to the UICC 8th edition, it was classified as clinical T4N0M1a, stage IV A. The patient had a history of hypertension and was a past smoker (60 pack-years) and a company employee. The Eastern Cooperative Oncology Group performance status (ECOG-PS) at the time of admission was 1. The carcinoembryonic antigen (CEA) level was 97.4 ng/mL (normal, 0–5 ng/ml).

Beginning in August 2007, the patient received carboplatin (CBDCA) and docetaxel (DTX). After 4 cycles, the tumor was reduced to 1 cm in diameter. The 5-mm nodule and pleural effusion had also decreased. According to the Response Evaluation Criteria in Solid Tumors version 1.1, partial response was achieved, but he experienced progressive disease (PD) after 8 months. Six cycles of re-challenge chemotherapy (RC) using the same regimen were started in August 2008 and were effective. Thereafter, at each recurrence of PD, 4 to 6 cycles of RC were administered, and by 2013, 38 cycles had been completed over 6 years of treatment ( Fig. 2 A). However, we could no longer control disease activity using the same chemotherapy regimen. Moreover, primary tumor size evaluation became difficult owing to massive pleural effusion; although not standard, we estimated the effect of treatment using the increase and decrease of CEA as an index. CEA increased from a minimum of 4.6 ng/ml to 33.3 ng/ml in October 2013 during repeated cytotoxic chemotherapy. Although his EGFR mutation status was unknown, we initiated erlotinib administration and the CEA level decreased. After 8 weeks, the patient developed grade 3 acneiform rash, assessed using the Common Terminology Criteria for Adverse Events version 5.0, and erlotinib administration was discontinued for 6 weeks. Cycles of medication and treatment holiday were repeated, and the patient was carefully observed for skin rash. Dose reduction was attempted once, but it was not effective, because we noted an elevated CEA level and intolerable skin rash. For 3 years, 4-week erlotinib administration was repeated with 4–6-week treatment holiday intervals ( Fig. 2 B). CEA increased from a minimum of 3.1 ng/ml to 30.4 ng/ml in January 2017 during treatment with erlotinib. We performed EGFR mutation analysis using adenocarcinoma cells from the pleural effusion and detected exon 19 deletion and exon 20 T790 M mutation; therefore, osimertinib was substituted for erlotinib.

We continued monthly CEA measurements after beginning osimertinib administration and noted that the level continued to decrease. In August 2018, the CEA level was 12.1 ng/ml and the ECOG-PS was 1. As of the last follow-up, the patient has survived for >11 years since the diagnosis of lung cancer.
3 Discussion
The clinical data of 10 patients with advanced NSCLC who survived for >5 years were retrospectively reviewed, and a good PS, adenocarcinoma, and a history of EGFR-TKI administration were the factors contributing to long-term survival. [4] According to another retrospective study, [3] 20 of 137 patients with EGFR-mutant lung adenocarcinoma survived for ≥5 years, and exon 19 deletion, absence of extrathoracic metastases, absence of brain metastasis, and current non-smoking status were reportedly good prognostic factors. Our case corroborated the good prognostic factors reported in these studies.
A case of metastatic NSCLC in which the patient survived for 10 years has already been reported; however, the patient underwent not only chemotherapy but also surgery and radiation therapy. [5] To the best of our knowledge, ours is the first report in the English literature to describe a metastatic NSCLC case controlled for >11 years. Moreover, our patient was only treated with chemotherapy and EGFR-TKIs.
We considered that 4 treatment policies may be the key to success:
- 1. RC with CBDCA plus DTX;
- 2. repeated re-challenge erlotinib administration;
- 3. osimertinib administration after T790 M mutation in exon 20, which confers resistance to erlotinib; and
- 4. use of both cytotoxic drugs and EGFR-TKIs. We will particularly focus on the first and second policies because of the non-standard methods.
The response rate (RR) to RC of platinum doublets containing pemetrexed (PEM) or taxanes is reportedly 27.5%, with a progression-free survival (PFS) of 3.9 months and an OS of 8.7 months. This RR is high, but the PFS and OS are similar to those seen with administration of a single-agent as second-line treatment. [6] Advanced NSCLC patients for whom RC with 2-drug combination therapy is performed have a longer median survival than those administered only DTX as second-line treatment. [7] The current evidence that RC is superior to a single agent second-line treatment is not sufficient, but if the side effects are acceptable, RC may be a suitable option.
To safely perform RC using platinum-based 2-drug therapy, it may be necessary to include a treatment holiday period for a certain duration to facilitate physical fitness recovery. Time to progression of >3 months after ending first-line chemotherapy is a predictor of long-term survival (>2 years) in advanced NSCLC patients who receive cytotoxic chemotherapy. [8] Advanced NSCLC patients who survive for >2 years have a good response to first-line cytotoxic chemotherapy, and a prolonged treatment-free interval increases long-term survival. [9] In the present case, the treatment holiday after cytotoxic chemotherapy was approximately 6 months. A prolonged treatment-free interval appears to be important for restoring physical fitness; therefore, the patient could tolerate the next treatment.
A meta-analysis of randomized control studies compared cisplatin (CDDP) and CBDCA in advanced NSCLC patients. Although regimens containing CDDP did not prolong OS, subgroup analysis demonstrated that, when combined with third-generation anticancer drugs, CDDP prolonged OS more than CBDCA in patients with non-squamous cell carcinoma. [10] In the TAX 326 trial, CBDCA and DTX combination therapy helped achieve an OS equivalent to that associated with CDDP and vinorelbine combination therapy; in addition, the CBDCA and DTX combination was well tolerated and facilitated a high quality of life. [11] Here, 38 cycles of CBDCA and DTX combination therapy were administered. To perform platinum-based 2-drug RC, it may be advantageous to repeatedly administer CBDCA rather than CDDP to facilitate tolerability.
When erlotinib toxicity is intolerable, as in our patient who developed a severe skin rash, the dosage is generally reduced. In patients with EGFR-mutant NSCLC, dose reduction (25 mg/day) of erlotinib can reduce the toxicity while maintaining efficacy. [12] In contrast, a prospective phase II trial involving low-dose erlotinib in patients with EGFR-mutant NSCLC revealed that dose reduction (50 mg/day) is not recommended because of reduced efficacy. [13] Intermittent erlotinib administration on alternate days successfully maintained efficacy while reducing toxicity. [14] Here, disease control was possible by RC of platinum doublets for approximately 6 cycles after the 6-month treatment holiday. Therefore, we applied the RC strategy to EGFR-TKI administration. Continued erlotinib administration was discontinued if side effects became intolerable or health-threatening and resumed when side effects disappeared. Toxicity and efficacy were balanced by allowing an approximately 4–6-week treatment holiday after the 4-week erlotinib administration. To the best of our knowledge, we were the first to employ erlotinib in RC; this method was named repeated re-challenge administration of erlotinib and was considered to alleviate suffering due to toxicity. Although there is little evidence to determine whether dose reduction, intermittent administration, or repeated re-challenge administration is better, it is important to avoid complete cessation of therapy.
The exon 20 mutation in EGFR , leading to the T790 M mutation, is a resistance mechanism to traditional EGFR-TKIs. Osimertinib treatment is associated with a longer median PFS than chemotherapy using PEM plus either CBDCA or CDDP as second-line treatment in T790M-mutant patients who received EGFR-TKIs. [15] Our patient with the exon 19 deletion and T790 M mutation received osimertinib after erlotinib. Even if the tumor develops resistance to conventional EGFR-TKIs, it is important to recognize that drugs such as osimertinib are available and may improve long-term survival.
In EGFR-mutant advanced NSCLC with exon 19 deletion and exon 21 (L858R) mutation, patients who receive sequential therapy with erlotinib and cytotoxic drugs experience a longer median OS than those who receive cytotoxic drugs or erlotinib alone. [16] It may be important to administer both EGFR-TKIs and cytotoxic drugs throughout the course of treatment.
Although more study is required, RC of CBDCA plus DTX and repeated re-challenge administration of erlotinib may be an empirical option for long-term survival. To understand the clinical and biological background of long-term survival cases, accumulation of future cases similar to ours is warranted.
Acknowledgments
We would like to thank Editage ( www.editage.jp ) for English language editing.
Author contributions
Supervision: Takeshi Terashima.
Writing – original draft: Tatsu Matsuzaki.
Writing – review & editing: Eri Iwami, Kotaro Sasahara, Aoi Kuroda, Takahiro Nakajima.
- Cited Here |
- Google Scholar
- View Full Text | PubMed | CrossRef |
- PubMed | CrossRef |
adenocarcinoma; EFGR-TKIs; EGFR gene mutation; long-term survival; non-small cell lung cancer; re-challenge chemotherapy; T790 M
- + Favorites
- View in Gallery
Readers Of this Article Also Read
Global and regional prevalence and burden for premenstrual syndrome and..., severe re-expansion pulmonary edema after chest tube insertion for the..., is intraoperative corticosteroid a good choice for postoperative pain relief in ..., the incidence and prognosis of thymic squamous cell carcinoma: a surveillance,..., klebsiella pneumoniae</em> k2-st86: case report', 'yamamoto hiroyuki md phd; iijima, anna ms; kawamura, kumiko phd; matsuzawa, yasuo md, phd; suzuki, masahiro phd; arakawa, yoshichika md, phd', 'medicine', 'may 22, 2020', '99', '21' , 'p e20360');" onmouseout="javascript:tooltip_mouseout()" class="ejp-uc__article-title-link">fatal fulminant community-acquired pneumonia caused by hypervirulent klebsiella ....
- Biomarker-Driven Lung Cancer
- HER2-Positive Breast Cancer
- Chronic Lymphocytic Leukemia
- Small Cell Lung Cancer
- Renal Cell Carcinoma

- CONFERENCES
- PUBLICATIONS
- CASE-BASED ROUNDTABLE
Case Presentation: A 57-Year-Old Woman With Ovarian Cancer
Lyndsay Willmott, MD, presents the case of a 57-year-old woman with ovarian cancer.

EP: 1 . Case Presentation: A 57-Year-Old Woman With Ovarian Cancer
Ep: 2 . molecular testing: ovarian cancer, ep: 3 . first-line therapies in ovarian cancer, ep: 4 . rucaparib maintenance therapy in ovarian cancer: the ariel3 trial, ep: 5 . toxicities with parp inhibitors for ovarian cancer, ep: 6 . future treatments in ovarian cancer.
Lyndsay Willmott, MD: We’d like to discuss a patient who’s presenting with classic symptoms of ovarian cancer. This is a 57-year-old woman who presented with progressive abdominal discomfort and bloating, as well as early satiety, new onset constipation, and unintentional weight loss. Her past medical history is significant for hypertension, but this has been controlled with medications. She also has a history of osteoarthritis. She comes in for her visit and physical exam reveals some right lower-quadrant tenderness. She is healthy with a performance status of 1.
To work up her symptoms, she undergoes imaging, including an ultrasound, which reveals a 4.5-cm ovarian mass. A CT scan reveals this mass, along with what appears to be lymphadenopathy and ascites. She undergoes a paracentesis for further evaluation, with cytology from that study revealing high-grade serous ovarian cancer. She then also has additional testing following this diagnosis, including germline testing, which reveals no evidence of a BRCA1 or BRCA2 mutation, and somatic testing, which is also negative for BRCA1 and BRCA2 . She has subsequent testing to look for homologous recombination deficiency, and this confirms that she is showing evidence of homologous recombination deficiency.
The patient underwent up-front surgery, which included a debulking with total abdominal hysterectomy, bilateral salpingo-oophorectomy, and resection of those involved lymph nodes. She was able to be resected to no gross residual disease. She was subsequently placed on adjuvant therapy with carboplatin and paclitaxel, which she had every 3 weeks for 6 cycles. She had normalization of her CA [cancer antigen]–125 and no evidence of disease at the completion of her treatment. Following this course of therapy, the patient and her physician had a conversation, and she was subsequently placed on bevacizumab maintenance. Following this, she was monitored. She experienced recurrence of symptoms 2 years later, which included, similar to her initial presentation, abdominal bloating and pain. She underwent imaging as well as continued CA-125 monitoring. CA-125 started to increase, and her imaging revealed recurrent retroperitoneal adenopathy. Because of her disease distribution, she was counseled about returning to chemotherapy. She was placed back onto carboplatin and paclitaxel and experienced a partial response. Following this, she was subsequently placed onto PARP inhibitor maintenance with rucaparib 600 mg twice daily. She experienced toxicity with some thrombocytopenia requiring interruption of her dose and subsequent dose reduction to 500 mg twice daily. The patient is doing well and is being monitored with a plan to continue this therapy until she experiences either additional toxicity or evidence of disease progression.
This young woman is a very classic presentation of ovarian cancer. Unfortunately, ovarian cancer has no screening modality; therefore, the majority of women who present with ovarian cancer present with these relatively innocuous symptoms and unfortunately are diagnosed in stage III or IV disease. The good news is that the majority of women are also platinum sensitive, so most women can get into remission despite presenting with advanced stage. We obviously understand that there are some prognostic factors that can help predict patients who may perform better, including certainly performance status. Other important things include BRCA status. For women who have a BRCA mutation or homologous recombination deficiency, this often means that they’re more likely to be platinum sensitive. The reason for that is related to the mechanism of action of a platinum drug. This causes DNA adducts and subsequently DNA double-stranded breaks. In women who have a BRCA mutation, they lack the ability to appropriately repair those breaks and are more likely to be more profoundly platinum sensitive. This patient did not have a BRCA mutation, but she was homologous recombination deficient, and that may also allow us to expect that she’d be more likely to have a more profound platinum-sensitive interval. And this person certainly did. This patient had 2 years from completion of her platinum-based chemotherapy before she had evidence of recurrence. These bigger platinum-free intervals are also associated with better likelihood to respond to a platinum rechallenge.
Case Overview: A 57-Year-Old Woman With Ovarian Cancer
Initial Presentation
A 57-year-old woman presented with progressive abdominal discomfort and bloating, early satiety, new-onset constipation, and unintentional weight loss
PMH: postmenopausal; hypertension—medially controlled; osteoarthritis
PE: right lower quadrant tenderness on palpation
Clinical work-up
Pelvic exam with ultrasound showed a ~4.5-cm right ovarian mass
Chest/abdomen/pelvis CT with contrast revealed a right adnexal mass with lymph node involvement and ascites
Paracentesis (1200cc) cytology confirmed high-grade serous epithelial ovarian cancer
Germline molecular testing: BRCA1/2 wt
Somatic testing: BRCA1/2 negative; HRD positive
CA-125, 360 U/mL
Diagnosis: Stage III, high-grade serous epithelial ovarian cancer
Patient underwent TAH/BSO, lymph node dissection, with optimal debulking; R0
Carboplatin/docetaxel q3 weeks for 6 cycles; CA-125 normalized; CR
Bevacizumab maintenance
At 2 years post chemotherapy, patient experienced abdominal bloating and pain, nausea and vomiting and progressive fatigue; CA-125 increased; imaging revealed progressive retroperitoneal adenopathy suggestive of recurrent disease; not deemed a candidate for secondary surgery
Rechallenged with carboplatin/docetaxel q3 weeks for 6 cycles; PR; predominantly enlarged lymph nodes
Rucaparib single-agent maintenance 600 mg bid
Patient developed clinically significant thrombocytopenia; dose reduced to 500 mg and patient remained on treatment until disease progression
Transcript edited for clarity.

Pembrolizumab Improves Surgical Outcomes and Response in Advanced Ovarian Cancer
Findings from the phase 2 NeoPembrOV study supported the addition of pembrolizumab to neoadjuvant chemotherapy before surgery in high-grade serous ovarian cancer.

FDA Approves Second Dose of CAR T-Cell Therapy in Ovarian Cancer Trial
The FDA has approved an individual patient investigational new drug application, allowing for a second dose of a novel CAR T-cell therapy for a patient with recurrent ovarian cancer.

Nivolumab Shows Promising Efficacy in dMMR Uterine and Ovarian Cancers
A phase 2 trial demonstrated that nivolumab is effective and has a manageable safety profile in patients with mismatch repair deficiency uterine or ovarian cancers.

FDA Approves Tepylute in Breast and Ovarian Cancer
The FDA approved Tepylute, a ready-to-dilute formulation of an existing treatment for breast and ovarian adenocarcinoma.

FDA Fast Track for TUB-040: ADC Shows Promise in Treating Ovarian Cancer
A phase 1/2a study is investigating the novel antibody-drug conjugate for the treatment of ovarian and non–small cell lung cancers.
2 Commerce Drive Cranbury, NJ 08512
609-716-7777

Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Open access
- Published: 11 August 2024
A qualitative study on the disease coping experiences of pancreatic cancer patients and their spouses
- Bo Zhang 1 ,
- Qigui Xiao 1 ,
- Jingtao Gu 1 ,
- Qingyong Ma 1 &
- Liang Han 1
Scientific Reports volume 14 , Article number: 18626 ( 2024 ) Cite this article
238 Accesses
Metrics details
- Health care
Cancer affects patients as well as their spouses. Patients and their spouses use different strategies to cope with cancer and the associated burden. This study aimed to gain a deeper and more differentiated understanding of support systems for patients and their spouses. This was an exploratory qualitative study conducted in China. The study was based on 20 semistructured face-to-face interviews. Ten pancreatic cancer patients and their spouses were interviewed. The interviews took place at a tertiary hospital from June 2023 to December 2023. The data were analysed using thematic analysis according to Braun and Clarke's methodology. This study was guided by the Consolidated Criteria for Reporting Qualitative Research (COREQ) checklist. Twenty participants of different ages (patients: range = 49–75 years; spouses: range = 47–73 years) participated. Patients with different cancer stages (e.g., potentially resectable, borderline resectable, locally advanced) and cancer types (initial diagnosis or relapse) participated in the study. Five themes emerged from the data, namely, denial and silence, fear and worry, struggle, coping strategies and cherishing the present. Active dyadic coping is conducive to promoting disease adaptation, and spouses seem to need more psychological support to improve their own well-being. Health care providers should pay attention to pancreatic cancer patients and their spouses in terms of five themes: denial and silence, fear and worry, struggle, coping strategies and cherishing the present. Future studies should use a combination of qualitative and quantitative methods to explore dyadic coping in greater depth.
Similar content being viewed by others

Exploring the spiritual needs of patients with advanced cancer in China: a qualitative study
A chain mediation model reveals the association between family sense of coherence and quality of life in caregivers of advanced cancer patients
Relationship among post-traumatic growth, spiritual well-being, and perceived social support in chinese women with gynecological cancer, introduction.
Pancreatic cancer is a malignant disease with high morbidity and a high mortality rate 1 . Pancreatic ductal adenocarcinoma (PDAC) accounts for approximately 90% of all cases of pancreatic cancer. Patients often have no obvious symptoms or signs in the early stage of disease, and more than half of patients are in the late stage at the time of diagnosis. According to the latest data of the American Cancer Society published in 2022, the incidence rate of pancreatic cancer ranks 10th among men and 7th among women among all cancer types; however, pancreatic cancer ranks 4th in cancer mortality, and the 5-year survival rate of pancreatic cancer is the lowest among all tumours, at only 11% 2 . Due to the great uncertainty about treatment effectiveness and the risk of cancer, pancreatic cancer causes a great psychological burden for patients’ spouses 3 . These couples face new challenges (e.g., being unable to effectively obtain information about the disease and its treatment, having high medical bills), role function transformation (e.g., having to stop working, managing family affairs) and self-concept changes (e.g., negative emotions, nervous about the future) 4 .
A cancer diagnosis has a profound impact on patients and their spouses, affecting multiple dimensions, such as the emotional, psychological, social, and economic dimensions. A cancer diagnosis often elicits a strong emotional response, encompassing shock, denial, anger, sadness, and fear 5 . Patients, as well as their spouses, may experience symptoms of anxiety and depression, which are normal reactions to the news of a cancer diagnosis. In some cases, professional mental health services may be necessary to help individuals manage these emotions 6 . A cancer diagnosis affects not only patients but also their family members and social networks. Patients’ spouses may need to take on more caregiving responsibilities while also dealing with their own emotional reactions. This additional stress can affect their work performance and social activities and may even lead to increased tension within the family 7 . Cancer itself, as well as its treatment, can have long-term, even permanent, effects on a patient's body. These effects include pain, fatigue, weight changes, skin changes, decreased libido, and fertility issues. Spouses may also neglect their own health while caring for patients. Patients and their spouses must communicate and cooperate when facing complex medical choices due to the diagnosis of cancer 8 . Effective communication can help both parties better understand their condition, treatment options, and potential risks and benefits, thereby helping them make informed decisions.
Some cancer patients also need support to cope with cooccurring mental health issues 9 . The multiple impacts of cancer on patients and their spouses have prompted researchers to conceptualize cancer as a dyadic stressor 10 . Taking spouses as the unit of analysis and considering the interactions that occur in the interdependent system between spouses can provide us with a new perspective on understanding the psychological distress and coping strategies of couples after cancer diagnosis 11 , 12 . How patients and their spouses deal with cancer affects the mental health of both parties and their assessments of relationship satisfaction 13 .
Research on coping styles for individuals with cancer has shifted from the individual level to the couple level 14 . Bodenmann noted that for couples, stress events do not affect the individual reactions of only one party but have common effects on both parties 15 . Coping with cancer and its related burdens requires collaboration, which is called dyadic coping (DC), which refers to the common reactions and strategies of spouses with intimate relationships when facing dual stress events 15 . The couple relationship is one of the most important resources for patients to cope with stressful events. Traditional models such as the systemic transactional model emphasize that both spouses should jointly perceive and evaluate stress, help each other, promote each other’s physical and mental health, and strengthen their intimate relationship 16 , 17 , 18 when coping with stress. Spouses play a key role in treatment decisions and in facilitating disease management 19 . According to dyadic disease management theory 20 , coping behaviour is a factor related to quality of life. The dyadic perspective of couples, focusing on the dyadic response of cancer patients and their spouses, is a new perspective for improving the physical and mental health of both parties and improving quality of life 21 , 22 . Couples facing cancer usually adopt a dyadic response 23 to reduce disease pressure.
Quantitative research on coping with cancer is very extensive, but there have been few qualitative studies in this field. A recent qualitative study of pancreatic cancer patients and their spouses reported coping strategies such as changing roles and identities, managing weight loss and addressing gastrointestinal problems 24 . A systematic review identified coping strategies such as symptom management, better clinical communication, support seeking, and maintaining a good attitude 25 . However, less is known about the use of different types of coping strategies by patients and their spouses and social support styles from outside couples.
In summary, we aimed to explore couples in which one spouse had been diagnosed with pancreatic cancer, including how the couple coped with the disease together and what coping and support strategies they used. In addition, we aimed to identify possible differences in coping behaviour between patients and their spouses. Insight into specific types of coping strategies can improve the development of more tailored and detailed intervention programs for cancer patients and their spouses.
Study design
A qualitative design was used in this study. Reporting was performed in accordance with the Consolidated Criteria for Reporting Qualitative Research (COREQ) reporting guidelines 26 .
Participants
Participants (patient-spouse dyads) from a tertiary hospital in China were recruited through random sampling from June 2023 to December 2023. The research team identified potential eligible participants through the hospital's patient registration system. Patients who met the preliminary conditions were invited to participate by the research team. Potential spouse participants were identified and recruited through direct communication with patients. Eligibility was based on the following criteria: having a diagnosis of pancreatic ductal adenocarcinoma (PDAC), being aged 18–75 years, being hospitalized for 2 weeks or more, and living with a spouse. The exclusion criteria for patients were severe cognitive impairment; serious heart, lung, kidney or other diseases; and consciousness disorders. The inclusion criterion for spouses was the ability to communicate normally. The exclusion criterion for spouses was cognitive impairment. Both patients and their spouses were required to provide written informed consent prior to enrolment.
Individual, audio-recorded, semistructured in-depth interviews 23 were conducted by creating a space for participants to openly share their perspectives. An interview guide, collaboratively developed after performing a literature review, aimed to explore the research interests of this study. Two participants were selected for a pilot interview, and the interview guide was revised and finalized accordingly (Table 1 ). Those meeting the inclusion criteria were provided with an interview explanatory statement outlining the study's aim and procedures. The statement emphasized voluntary participation, with the option to withdraw at any time without providing a reason. Participants were assured of anonymity, with their personal information remaining confidential and unidentified. Before the interviews, each participant signed an informed consent form.
The interviews were conducted by the first author in the VIP conference room of the hospital department. Patients and their spouses were interviewed separately. During the interviews, participants were encouraged to share their experiences and provide additional comments on the topic. There was no prior relationship between the interviewer and the participants, which helped to reduce bias and ensure the objectivity and accuracy of the research results. The interview ended when no new or surprising information was uncovered during further data collection, that is, when data saturation occurred 27 . The interviews lasted between 20 and 74 min, with an average duration of 30 min.
Data analysis
Sociodemographic information was reported with basic descriptive statistics using IBM SPSS Statistics 24. At the end of each interview, the authors independently transcribed the recordings into text within 24 h, and then imported the text into NVivo 11 software. We used Braun and Clarke's thematic analysis method 28 , 29 . The initial coding was independently carried out by two authors (B. Zhang and Q. Xiao), with a third author regularly monitoring and intervening in case of differing opinions. Encoding attempts to identify and describe aspects and their outcomes were considered to constitute contextual or mechanistic features. Then, the fourth author reviewed all coding results. Group discussions aim to compare, critique, and agree on external or internal factors related to patients and their spouses. In the process of inductive coding and deductive classification, if there was any inconsistency, the researcher discussed the differences with QGX and JTG and reached a consensus. Finally, the research group discussed and reviewed all the identified themes and categories.
After the interview, we transcribed the conversation verbatim and created detailed written records about every interview (i.e., interview memorandum). We also provided interview recordings and transcripts to the interviewees and their spouses for review. The transcripts were then reviewed by the research team to ensure their accuracy. Once research team were confident that the transcripts accurately reflected the interviews, we contacted the interviewees and their spouses to request their feedback. We did this to confirm that they agreed with the transcript's content and that we had not misrepresented any of their words or ideas. If the interviewee and his or her spouse agreed with the content of the transcript, we moved forward with our analysis. However, if there were any discrepancies or errors, research team made the necessary corrections based on their feedback. This ensured that the data we analysed were as accurate and reliable as possible.
The authors are all postgraduate students, who have received systematic training and mastered qualitative research methods and interview skills. During the interviews, voice recorders and field notes were used to ensure the quality of the recordings, questions were asked as neutrally as possible, and the participants' responses were neutral. At the end of each interview, two authors repeatedly listened to the recordings to check for accuracy, and then they encoded and analysed the transcripts. If there were new items or disagreements in the transcribed interview, this will be further elaborated in the next interview.
This study complied with the Declaration of Helsinki, was approved by the Ethics Committee of the First Affiliated Hospital of Xi’an Jiaotong University (XJTU1AF2019LSK086). Written informed consent was obtained from participants included in the study. All participants were informed that any personal information obtained in this study would remain confidential.
Among the 13 potential couples who expressed willingness for interviews, ten couples were included in the final analysis. Three couples refused to participate in the study for various reasons, including scheduling conflicts (n = 1), a lack of interest (n = 1) and unwillingness to discuss this topic (n = 1). Patients with pancreatic cancer had a median age of 62.5 (49–75) years. More than half of the patients were men (n = 7); the majority of patients had a secondary school education or below (n = 9) and all patients were married (n = 10). Approximately 50% (n = 5) of the patients were classified as having borderline resectable disease at the initial diagnosis. The duration of pancreatic cancer ranged from 0.5 to 5 years. The median age of the spouses was 63 years (47–73). The majority of spouses had a secondary school education or below (n = 9). Three individuals had previously worked in the field of nursing. Further characteristics are displayed in Table 2 and Supplementary Material.
Five themes were extracted from the interviews in this study, which described the experiences and perceptions of patients with pancreatic cancer and their spouses in coping with the disease. The five themes were denial and silence, fear and worry, struggle, coping strategies and cherishing the present. Figure 1 and Table 3 present the themes, subthemes, and representative quotes.
The process of participants' coping to illness. Patients and their spouses fell into denial and silence and filled with fear and anxiety at the beginning. As the disease progressed, they gradually struggled to cope with this situation. During this painful journey, they adopted two different coping strategies: one was a negative coexistence approach within couples, while the other involved actively adjusting their mindset and ultimately learning to cherish the present and harmoniously integrate into life.
Denial and silence
Denial and silence meant that the patients with cancer and/or their spouses did not want to communicate regarding any illness-related issues and that they had not communicated. The essential characteristic was the lack of intention to communicate about the illness, which includes denying the disease diagnosis and refusing to talk about the illness.
Denying the disease diagnosis
Patients and their spouses tended to be sceptical of the diagnosis of the illness and denied it through psychological defence mechanisms. Specifically, some patients admitted that they never expected to have cancer and that the unexpected diagnosis triggered strong feelings of disbelief and resistance. Spouses also mentioned that the patient's diagnosis of cancer was unexpected and refused to accept it.
“When I first got my diagnosis, I felt like the sky was falling, my legs were shaking, my heart was panicking…” (P5) “It never occurred to me that this would be my husband’s test result. He was only 54, so young, and in good health before.” (S6)
Refusing to talk about the illness
Some patients and their spouses were afraid to communicate about the malignant disease because they feared that it would cause shock or a psychological burden for their spouse and that they could not control their emotions. As spouses, when faced with sudden negative emotions from patients, some respondents were unsure how to comfort them. Some spouses chose to accommodate and remain silent, internalizing the negative emotions. This led to a certain degree of emotional exhaustion.
“I am afraid to tell my wife that her disease is advanced and incurable. I dare not tell her because I am worried that she cannot bear it.” (S4) “He was in a really bad mood, and I didn’t know how to comfort and console him.” (S1)
Fear and worry
This theme demonstrated participant’s emotion change as they become aware of burden and death as the disease progressed.
Increased family burdens
The diagnosis and treatment of pancreatic cancer had prompted a series of adjustments in family roles to adapt to new responsibilities. Following the diagnosis, the couples bore their respective responsibilities in the dyad and embarked on discussions regarding changes in their roles, considered revising established models for managing household duties, and navigated the interruptions to their life plans. For spouses, the responsibility was to serve the patients wholeheartedly. For patients, the responsibility was to minimize the burden on their spouses as much as possible. Some patients or their spouses also chose to bear pressure alone to alleviate the burden on others. Some families lost their primary sources of income. Additionally, they needed continuous treatment. All these factors increased the financial burden.
“I certainly hope she can help me, but it is already very difficult for her, and I don't want to burden her even more…” (P1) “My husband is the primary source of income in our family, but now he is unable to work…so the financial pressure is quite significant.” (S3) “Before she got sick, she used to do more housework at home. Now that she is ill, I must take care of her and do a lot of things every day. ” (S9)
Worrying about treatment effectiveness and the future
The participants were conflicted between having hope for the effectiveness of treatment and being fearful about the progression of the patient’s disease. In addition, they were conflicted about cancer as a disease and their life as a caregiver. Some spouses themselves had diseases. Therefore, they felt anxious about their health problems worsening and taking care of the patient with their health condition. Spouses reported that taking care of their own physical and emotional needs ensured they could continue to participate in caring for the patient. When they realized their limited emotional resources and time, they consciously chose to reduce their efforts towards others. Spouses reported a range of self-care activities, including exercise, dietary modification, and making time for hobbies.
“I always felt down, and… I also felt that I wanted to have hope.” (P3) “I have to take care of myself before I can start taking care of him.” (S1)
Fear of death
From the moment of diagnosis, the spouse perceived the threat of the patient's death as a real possibility. As they witnessed the changes in their partner's body caused by the disease, this possibility became increasingly concrete. Which prompted them to accept or prepare themselves for their new reality, to anticipate and imagine life without their partner. They accomplished this by seeking out information about the cancer, about the dying process and death. Spouses strived to comprehend the patient's experiences. They hoped that treatment could continue until they no longer felt overwhelmed when considering the remainder of their lives. In other words, their perspectives on life and death deepened when faced with the opportunity to contemplate living until death.
“I was prepared for his death when I discovered his cancer had metastasized. When the doctor told me he had terminal cancer, I knew he would die soon.” (S8) “We were all gradually coming to terms with the arrival of death, though it was a harsh reality (sobbing).” (P8)
At this stage, some patients and their spouses still avoided disease-related topics, and even felt hopelessness.
Avoidance/contradiction
This study showed that many couples tended to cope with stressors by avoiding problems. Some couples had poor adaptation to the diagnosis of the disease, experienced avoidance or contradiction, and perceived minimal dyadic support and greater adaptation difficulties. They rarely or never communicated their feelings about the disease, avoided or retreated from their spouse, and reacted negatively to the pain associated with the disease in others. This included reluctantly providing support, maintaining distance, and even ridiculing each other. Some couples reported that after the onset of illness, both the patient and their spouse prioritized each other's thoughts and feelings over their own in order to maintain normal marital life and avoided increasing the psychological burden on their partner.
“I sometimes look up some information and want to share it with him, but whenever I discuss his condition with him, he pretends to sleep and doesn't want to talk to me (sobbing).” (S6) “She was exhausted from taking care of me, so I silently endured the bad emotions. I don't want her to worry about me. ” (P5)

Hopelessness
Although patients and spouses were well aware of what to do, sometimes they failed to do the right thing and communicate well, resulting in poor adjustment to the disease. The physical condition of some patients was difficult to improve, and in this case, efforts may be futile. In their view, pancreatic cancer is impossible to cure and the disease is equivalent to a death sentence. Therefore, they believed that any effort was useless, which led to hopelessness. However, hope had proven to be a resource for spouses, helping them cultivate meaning and face adversity. Hope flowed with the progression of the disease and feedback from professionals on treatment outcomes, sometimes being strengthened, sometimes being tempered, but always present until the end of the patient's life.
“Treatment is useless. It's not going to get better; I’m just going to die. ” (P8) “I often search for information on others overcoming cancer, which brings us a little more hope in our hearts.” (S5)
Coping strategies
Patients and their spouses adopted two different coping strategies after they experienced heavy burdens and painful struggles: dyadic negative coping or positive coping.
Dyadic negative coping
Upon receiving a cancer diagnosis, patients faced a myriad of challenges including physical, cognitive, and psychological barriers, which were frequently accompanied by fluctuating emotions. The illness advanced swiftly, while the responses and engagement of both institution and the assisting professionals lagged behind, being neither timely nor adequate. This discrepancy resulted in profound hopelessness and a sense of incapacity. Concurrently, their spouses endured immense psychological pressure, rooted in anxiety over the patient's condition and the weight of their commitment to caretaking. Some patients and their spouses opted to manage this stress through evasion of difficult topics or by refraining from communication.
At times, partners transmit their emotions to each other, potentially leading to family crises. Patients or their spouses often hid the true condition of the illness and suppressed their own emotions and thoughts to protect their partner, disregarding their own feelings and health. Over time, some couples were more likely to form an evasive way of communicating with others regarding illness-related issues, which often persisted, thus hindering their adaptation to the disease. This deprived couples of opportunities for emotional and stress relief and had a negative impact on relationship quality and marital satisfaction.
“I dare not tell him the truth, worrying it might make him feel even worse.” (S1) “After my husband got sick, he would often get angry for no reason, and it really weighed on my heart.” (S6) “I felt like I was drowning, fighting for my life, but I didn't want to leave my husband stranded because of my illness.” (P2)
Dyadic positive coping
Some participants were able to face life setbacks and pain with composure and acceptance. The roles of the spouses were adjusted and adapted. Couples who openly communicated their feelings could better adapt to the disease. Nine patients and spouses expressed that they accepted the existence of the disease: some accepted "fate", and some accepted "disease", choosing to face it calmly. Some spouses were hopeful, believing that with the advancement of medical methods, the situation would improve. Information and instrumental support also play important roles. This included taking care of children, doing household chores, and cooking. In addition, participants obtained from medical staff or the internet and shared disease-related information. The patients experienced care and love and expressed great concern for their spouses. The couples achieved positive results in overall relationship satisfaction and intimacy.
Couples support each other in difficult times and live together for a lifetime, which is the concept of marriage. Spouses made their "being there" commitment in words or actions to support their partners with cancer. The couples stated that the disease was a part of their marriage journey and they were committed to supporting each other. Patients received immense emotional support from the verbal commitments of their spouses. Spouses spent more time with patients, which helped to enhance the intimacy between the couples and aided them in coping with the disease more effectively.
Actively seeking external support was also important. The patients reported that understanding and moral support from friendly, patient, and well-trained doctors and nurses was important. The spouses believed that the treatment of a psychological counsellor was equally important. Overall, many people suggested providing psychological support. The spouses expressed a hope for more proactive services in clinical settings.
“Later, I just resigned to fate. Whenever I had a solution, I would try my best to develop in a positive direction. I chose to face and accept reality. I'm already 68 years old now, and it's worth it.” (P3) “However, I am glad to have a psychological counsellor. A person who understands me. Someone said, "Yes, you're facing difficulties right now, but you can do it, you'll get rid of it.” (P2) “My wife gave me tremendous confidence and consistently supported me, affirming that regardless of the illness I had and whatever the outcome, she would always be there to support me in the end. ” (P10)
Cherishing the present
Everything has two sides, and terrible experiences can stimulate positive thinking. After experiencing this significant upheaval, the majority of the participants expressed varying degrees of cherishing the present moment and maintaining a positive outlook towards the future. Some couples came to accept and adapt to the consequences of the disease after receiving chemotherapy. They engaged in discussions about what the experience of cancer signified for themselves individually and for their relationship as a whole. This involved jointly envisioning the future, creating plans, and striving to bring their lives back onto a stable path.
This theme was observed among all participants, who expressed a desire to cherish the present moment and whose marital relationships were strengthened by the caregiving experience. Moreover, to experience prosperity for the rest of their lives, they also needed other significant others to help them live. Therefore, they needed connections with society, including family members. The patients and their spouses appreciated each other more and perceived warmth from family and friends. In the interviews, they all mentioned that the love and support of people around them were important sources of strength for them to deal with the cancer diagnosis. One patient reported that he was particularly grateful for his boss’s support. During his treatment, they also helped him pay for his pension insurance and provided a minimum living allowance of 2000 RMB per month.
“The genuine concern from family and friends that can be felt at all times, everyone is concerned about me…” (P7) “I believe we can move forward. My wife and I are doing everything we can to save money for the next stage of treatment.” (S9) “With the advancement of science and technology, I believe that tomorrow will be better.” (S4)
Characteristics of the DC styles of pancreatic cancer patients and their spouses
The findings of this study indicate that within the DC style of PDAC patients and their spouses, both positive coping and negative coping strategies coexist.
Dyadic coping is beneficial for the adaptation and recovery of patients' physiological functions 30 . Patients and spouses have diverse coping styles, most of which involve positive coping styles. They seek emotional comfort and meaning through their relationships with the present, themselves, and others, achieving personal growth.
Positive binary coping can shape patients’ and spouses’ optimistic attitudes towards the disease, which helps them cope with treatment. Medical staff can carry out interventions, such as life meaning therapy interventions 31 , by promoting an understanding of the present, reviewing life, and facing the future, and patients and spouses can reunderstand their responsibilities, tap into their potential, and find the meaning of life. Training programs such as communication skills training for couples 32 , cognitive-behavioural therapy 33 , and coping enhancement training 34 can also be carried out to improve communication skills and alleviate negative emotions between partners. Therefore, future research should emphasize training plans for pancreatic cancer patients and their spouses to improve their DC ability.
The dyadic relationships between partners influence each other 34 . This study revealed that patients and their spouses have consistent coping strategies for accepting reality and perceiving warmth, while there are differences in coping strategies for harbouring hope. In terms of consistent coping styles, patients’ and spouses’ coping styles resonate with each other and have a positive impact, while tension and pain are mostly related to inconsistent coping styles with coping with or releasing emotions between partners 35 . For example, when facing cancer, patients both avoid and accept it, while their spouses have high levels of anxiety. The anxiety of one partner influences the other partner, indicating that inconsistency in coping styles has an impact on disease management.
A lack of communication or fear of communication has a negative effect on dyadic coping. Open communication is crucial for couples dealing with cancer 36 . A previous study developed a communication skills training program for cancer patients and their spouses 37 , which could improve the avoidance of cancer-related topics and intimate relationships with spouses. This program is an effective intervention to promote communication and expression in couples. Our study reported the relaxing effect of communication about stress, while some spouses could not tolerate patients’ communication about stress. To improve this difference, couple-based supportive interventions should shift general communication to more individual approaches 38 . Because couples with good relationships may be more willing to participate in interviews, there are generally fewer reports of negative dyadic responses. In fact, the interviews were conducted jointly with both partners, which may have reduced the opportunity to observe negative dyadic responses.
Challenges to DC for PDAC patients and their spouses
This study showed that the five couples tended to cope with stressors by avoiding problems. This coping strategy will lower patients’ self-esteem and increase negative emotions. Therefore, healthcare professionals should adopt a comprehensive approach when treating couples, evaluate their attitudes towards the disease in a timely manner, encourage open communication, and cultivate confidence in overcoming the disease.
Most participants said that in addition to guidance from medical staff, the internet was a main source of information. Therefore, comprehensive explanations on disease progression and treatment processes should be provided to patients and spouses who have limited understanding of the disease. In addition, mobile device-based 39 communication and consulting platforms can be established to meet their information needs.
The research results also indicate that spouses have greater anxiety and unease than patients, but this is often overlooked 40 . During the interview process, the spouses mentioned that they always tolerated the patient's uncontrollable emotions. This shows that in the process of taking care of patients, spouses tend to hide their emotions, focus on the patient, and expect improvement of the disease. This suggests that medical personnel should pay attention to the needs of spouses and take effective measures to intervene in a timely manner, such as by providing music therapy 41 , mindfulness stress relief therapy 42 , and cognitive behavioural interventions 43 . This not only has positive effects on the treatment and rehabilitation of cancer patients but also reduces the burden of care, alleviates psychological distress, and improves the quality of life of spouses.
In this study, more than half of the households had a per capita monthly income below 5000 RMB, and the patients were at risk of not being able to resume their work. Economic conditions can affect family relationships, and lower income is associated with reduced intimacy between spouses 44 . This means that when there are multiple treatment options for cancer, health care professionals should fully consider the financial situation of the patient's family and encourage couples to actively participate in medical discussions. The purpose is to minimize treatment costs as much as possible. In addition, promoting public welfare measures can help alleviate the financial burden on patients whose families are facing economic difficulties.
Providing additional support for DC between patients and their spouses
This study indicated that the family is the most reliable resource for patients and spouses. Strong family relationships bring multiple benefits to individuals, including faith, hope, and peace 45 . At the same time, the binary coping styles of patients and spouses towards stress can affect their adjustment to life after illness. Negative binary coping is negatively correlated with the prognosis of patients and their relationship with their spouse 46 . The greater the quality of the relationship between spouses is, the less depressive symptoms they experience. Therefore, family interventions in clinical practice, such as psychological and spiritual integration therapy, meditation, and meaningful therapy, are recommended to alleviate patients’ psychological pain and enhance family relationships and functions 47 . Patients with decent abilities can also be encouraged to actively care for their family members, do what they can, and express their love to their family members more often; they can also give positive encouragement regarding their spouse's efforts, making them feel responsible and willing to maintain their strength and resilience.
During the coping process, the couple's cognition of cancer was self-assessed and restructured. Their trust in doctors could illustrate this. They readily adopted almost all of the doctor's advice, which implied that when they entrusted themselves to the doctor, their belief in the therapeutic effect and control over the disease was strengthened. They also cultivated interests (e.g., mindfulness meditation and watching television) to take their minds off the diagnosis of cancer. Couples dealing with cancer focused on moving forward, striving to improve the consequences and expectations of the disease. With their efforts and positive coping strategies, the threatening disease cognitions that hindered psychological adaptation were gradually replaced by constructive disease perceptions.
Spouses' distress could be indirectly alleviated by instrumental social support for patients 48 . Peer support from other patients (through sharing experiences and communication) and professional psychological counselling can effectively reduce psychological symptoms and improve cognitive abilities 49 . Therefore, health care professionals should recognize the importance of peer support and psychotherapy and encourage patients and their spouses to attend peer support groups for pancreatic cancer survivors to pay timely attention to their psychological status 50 . Psychological and social interventions for cancer patients and their spouses should be adjusted according to their needs and desires, as well as the nature and stage of the disease 51 . For pancreatic cancer, a malignant disease, intervention measures should focus on alleviating depression, demoralization, and distress about dying and death, as well as addressing multiple challenges of disease and treatment. These include making treatment decisions and communicating with healthcare providers; adapting to diseases on self-concept, personal relationships, and sense of meaning in life; and preparing for the end of life. Overall, interventions should be developed to strengthen couples’ social support systems.
Study strengths and limitations
The primary strength of this research is that it addressed an overlooked area in relation to coping experiences in pancreatic cancer patients and their spouses. Braun and Clarke’s thematic analysis method was used for analysis. By adhering to the six steps and closely involving all members of the research team, the quality of the analysis process of this research topic was ensured. This study also has several limitations. Firstly, we recruited a relatively small sample of participants. However, considering the relatively narrow focus of the study, the sample size was considered sufficient to achieve our goals. In addition, the team unanimously believed that the data was already saturated. Secondly, this study only collected data from one tertiary hospital. Therefore, the generalizability of our results might be limited. However, according to the discussion of the research team, the data consulted in this study could represent the views of a considerable number of patients and their spouses.
Clinical implications
The couples are supportive for and to each other. The results of this study revealed that spousal caregivers cared for patients while also experiencing health issues themselves. Healthcare professionals should provide support for couples coping with cancer, especially by focusing on spouses' psychological conditions because their distress is easily overlooked. These new insights can serve as a new direction for couples' interventions in the future. Spousal caregivers need education about the treatment and support in coping with pancreatic cancer. Thus, more attention is needed for awareness of the patient’s medical condition, managing the side effects of treatment, and the support system of the patients and their spouses. Furthermore, healthcare professionals can help in identifying and resolving the unmet needs of patients and their spouses.
This study showed a dynamic and complex picture of the coping experience between people with pancreatic cancer and their spouses. Patients and their spouses experienced diverse emotional changes and coping styles, that was, denial and silence, fear and worry, and struggle. They presented different coping strategies, that was, negative or positive coping, and finally chose to accept reality and cherish the present. A better understanding of the different conditions and experiences of coping with cancer could inform the design of a feasible and effective patient and spouse support program for coping with pancreatic cancer. Future studies should use a combination of qualitative and quantitative methods to explore dyadic coping in greater depth.
Data availability
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Li, M., Tang, D., Yang, T., Qian, D. & Xu, R. Apoptosis triggering, an important way for natural products from herbal medicines to treat pancreatic cancers. Front. Pharmacol. 12 , 796300. https://doi.org/10.3389/fphar.2021.796300 (2022).
Article PubMed PubMed Central CAS Google Scholar
Siegel, R. L., Miller, K. D., Fuchs, H. E. & Jemal, A. Cancer statistics, 2022. CA Cancer J. Clin. 72 , 7–33 (2022).
Article PubMed Google Scholar
Yanai, Y. et al. A feasibility study of a peer discussion group intervention for patients with pancreatobiliary cancer and their caregivers. Palliative Supp. Care 20 , 527–534 (2022).
Article Google Scholar
Dengso, K. E. et al. The psychological symptom burden in partners of pancreatic cancer patients: A population-based cohort study. Supp. Care Cancer 29 , 6689–6699 (2021).
Stenberg, U., Ruland, C. & Miaskowski, C. Review of the literature on the effects of caring for a patient with cancer. Psycho-Oncology 19 , 1013–1025. https://doi.org/10.1002/pon.1670 (2010).
Carlson, L. E., Waller, A., Groff, S. L., Giese-Davis, J. & Bultz, B. D. What Goes up does not always come down: Patterns of distress, physical and psychosocial morbidity in people with cancer over a one year period. Psycho-Oncology 22 , 168–176. https://doi.org/10.1002/pon.2068 (2011).
Caruso, R., Nanni, M. G., Riba, M., Sabato, S. & Grassi, L. The burden of psychosocial morbidity related to cancer: Patient and family issues. Int. Rev. Psychiatry 29 , 389–402. https://doi.org/10.1080/09540261.2017.1288090 (2017).
Bubis, L. D. et al. Symptom burden in the first year after Cancer diagnosis: An analysis of patient reported outcomes. J. Clin. Oncol. 36 , 1103–1111. https://doi.org/10.1200/JCO.2017.76.0876 (2018).
Article PubMed CAS Google Scholar
Singer, S. Psychosocial impact of cancer. In Psycho-Oncology: Recent Results in Cancer Research (eds. Goerling, U. & Mehnert, A.) (Springer, 2018).
Bodenmann, G.. Dyadic coping and its significance for marital functioning. In Couples Coping with Stress: Emerging Perspectives on Dyadic Coping (eds. Kayser, K., Bodenmann, G., Revenson, T. A.). 73–95 (American Psychological Association, 2005).
Regan, T. W. et al. Couples coping with cancer: Exploration of theoretical frameworks from dyadic studies. Psycho-Oncology 24 , 1605–1617. https://doi.org/10.1002/pon.3854 (2015).
Jacobs, J. M. et al. Distress is interdependent in patients and caregivers with newly diagnosed incurable cancers. Ann. Behav. Med. 51 , 519–531. https://doi.org/10.1007/s12160-017-9875-3 (2017).
Rottmann, N. et al. Dyadic coping within couples dealing with breast cancer: A longitudinal, population-based study. Health Psychol. 34 , 486–495. https://doi.org/10.1037/hea0000218 (2015).
Baucom, D. H., Porter, L. S., Kirby, J. S. & Hudepohl, J. Couple-based interventions for medical problems. Behav. Ther. 43 , 61–76 (2012).
Bodenmann, G. A systemic-transactional conceptualization of stress and coping in couples. Swiss J. Psychol. 54 , 34–49 (1995).
Google Scholar
Falconier, M. K., Jackson, J. B., Hilpert, P. & Bodenmann, G. Dyadic coping and relationship satisfaction: A meta-analysis. Clin. Psychol. Rev. 42 , 28–46. https://doi.org/10.1016/j.cpr.2015.07.002 (2015).
Traa, M. J., De Vries, J., Bodenmann, G. & Den Oudsten, B. L. Dyadic coping and relationship functioning in couples coping with cancer: A systematic review. Br. J. Health Psychol. 20 (1), 85–114. https://doi.org/10.1111/bjhp.12094 (2015).
Bodenmann, G. et al. Effects of stress on the social support provided by men and women in intimate relationships. Psychol. Sci. 26 , 1584–1594 (2015).
Meier, F., Cairo Notari, S., Bodenmann, G., Revenson, T. A. & Favez, N. We are in this together—Aren’t we? Congruence of common dyadic coping and psychological distress of couples facing breast cancer. Psychooncology 28 (12), 2374–2381. https://doi.org/10.1002/pon.5238 (2019).
Lyons, K. S. & Lee, C. S. The theory of dyadic illness management. J. Fam. Nurs. 24 , 8–28 (2018).
Rottmann, N. et al. Dyadic coping within couples dealing with breast cancer: A longitudinal, population-based study. Health Psychol. 34 , 486–495 (2015).
Stefanut, A. M., Vintila, M. & Sarbescu, P. Perception of disease, dyadic coping and the quality of life of oncology patients in the active treatment phase and their life partners: Study protocol of an approach based on the actor-partner interdependence model. Eur. J. Cancer Care (Engl.) 30 , e13374 (2021).
Falconier, M. K. & Kuhn, R. Dyadic coping in couples: A conceptual integration and a review of the empirical literature. Front. Psychol. 10 , 571 (2019).
Article PubMed PubMed Central Google Scholar
Wong, S. S., George, T. J., Godfrey, M., Le, J. & Pereira, D. B. Using photography to explore psychological distress in patients with pancreatic cancer and their caregivers: A qualitative study. Supp. Care Cancer 27 , 321–328 (2019).
Chong, E., Crowe, L., Mentor, K., Pandanaboyana, S. & Sharp, L. Systematic review of caregiver burden, unmet needs and quality-of-life among informal caregivers of patients with pancreatic cancer. Supp. Care Cancer. 31 (1), 74. https://doi.org/10.1007/s00520-022-07468-7 (2022).
Tong, A., Sainsbury, P. & Craig, J. Consolidated criteria for reporting qualitative research (COREQ): A 32-item checklist for interviews and focus groups. Int. J. Qual. Health Care 19 , 349–357 (2007).
Francis, J. J. et al. What is an adequate sample size? Operationalising data saturation for theory-based interview studies. Psychol. Health 25 , 1229–1245 (2010).
Braun, V. & Clarke, V. Using thematic analysis in psychology. Qual. Res. Psychol. 3 (2), 77–101. https://doi.org/10.1191/1478088706qp063oa (2006).
Kiger, M.E. & Varpio, L. Thematic analysis of qualitative data: AMEE Guide No. 131. Med Teach . 42 (8), 846–854 https://doi.org/10.1080/0142159X.2020.1755030 (2020).
Liu, W., Lewis, F. M., Oxford, M. & Kantrowitz-Gordon, I. Common dyadic coping and its congruence in couples facing breast cancer: The impact on couples’ psychological distress. Psychooncology. 33 (3), e6314. https://doi.org/10.1002/pon.6314 (2024).
Guerrero-Torrelles, M., Monforte-Royo, C., Rodríguez-Prat, A., Porta-Sales, J. & Balaguer, A. Understanding meaning in life interventions in patients with advanced disease: A systematic review and realist synthesis. Palliat. Med. 31 (9), 798–813. https://doi.org/10.1177/0269216316685235 (2017).
Porter, L. S. et al. A randomized pilot trial of a videoconference couples communication intervention for advanced GI cancer. Psychooncology 26 (7), 1027–1035 (2017).
Yasmin, N. & Riley, G. A. Psychological intervention for partners poststroke: A case report. NeuroRehabilitation. 47 (2), 237–245 (2020).
Zemp, M. et al. Couple relationship education: A randomized controlled trial of professional contact and self-directed tools. J. Fam. Psychol. 31 (3), 347–357 (2017).
Bannon, S. M., Grunberg, V. A. & Reichman, M. Thematic analysis of dyadic coping in couples with young-onset dementia. JAMA Netw. Open 4 (4), e216111 (2021).
Landolt, S. A., Weitkamp, K., Roth, M., Sisson, N. M. & Bodenmann, G. Dyadic coping and mental health in couples: A systematic review. Clin. Psychol. Rev. 106 , 102344. https://doi.org/10.1016/j.cpr.2023.102344 (2023).
Yang, F. et al. The experiences of family resilience in patients with permanent colostomy and their spouses: A dyadic qualitative study. Eur. J. Oncol. Nurs. 70 , 102590. https://doi.org/10.1016/j.ejon.2024.102590 (2024).
Gremore, T. M. et al. Couple-based communication intervention for head and neck cancer: A randomized pilot trial. Supp. Care Cancer 29 , 3267–3275 (2021).
Kor, P., Leung, A. & Parial, L. L. Are people with chronic diseases satisfied with the online health information related to COVID-19 during the pandemic?. J. Nurs. Scholar. 53 (1), 75–86 (2021).
Brosseau, D. C., Peláez, S., Ananng, B. & Körner, A. Obstacles and facilitators of cancer-related dyadic efficacy experienced by couples coping with non-metastatic cancers. Front. Psychol. 14 , 949443. https://doi.org/10.3389/fpsyg.2023.949443 (2023).
Umbrello, M. et al. Music therapy reduces stress and anxiety in critically ill patients: A systematic review of randomized clinical trials. Minerva Anestesiol. 85 (8), 886–898 (2019).
Goldberg, S. B., Tucker, R. P. & Greene, P. A. Mindfulness-based interventions for psychiatric disorders: A systematic review and meta-analysis. Clin. Psychol. Rev. 59 , 52–60 (2018).
Carpenter, J. K. et al. Cognitive behavioral therapy for anxiety and related disorders: A meta-analysis of randomized placebo-controlled trials. Depress. Anxiety 35 (6), 502–514 (2018).
Thomson, M. D., Wilson-Genderson, M. & Siminoff, L. A. Cancer patient and caregiver communication about economic concerns and the effect on patient and caregiver partners’ perceptions of family functioning. J. Cancer Surviv. 18 (3), 941–949. https://doi.org/10.1007/s11764-023-01341-0 (2024).
Bodschwinna, D. et al. Couples coping with hematological cancer: Support within and outside the couple—Findings from a qualitative analysis of dyadic interviews. Front. Psychol. 13 , 855638. https://doi.org/10.3389/fpsyg.2022.855638 (2022).
Palmer Kelly, E., Meara, A., Hyer, M., Payne, N. & Pawlik, T. M. Understanding the type of support offered within the caregiver, family, and spiritual/religious contexts of cancer patients. J. Pain Symptom Manag. 58 , 56–64. https://doi.org/10.1016/j.jpainsymman (2019).
Greer, J. A., Applebaum, A. J., Jacobsen, J. C., Temel, J. S. & Jackson, V. A. Understanding and addressing the role of coping in palliative care for patients with advanced cancer. J. Clin. Oncol. 38 (9), 915–925. https://doi.org/10.1200/JCO.19.00013 (2020).
Vodermaier, A. & Linden, W. Social support buffers against anxiety and depressive symptoms in patients with cancer only if support is wanted: A large sample replication. Supp. Care Cancer 27 , 2345–2347 (2019).
Hu, J. et al. Peer support interventions for breast cancer patients: A systematic review. Breast Cancer Res. Treat. 174 (2), 325–341. https://doi.org/10.1007/s10549-018-5033-2 (2019).
Adlard, K. N. et al. Peer support for the maintenance of physical activity and health in cancer survivors: The PEER trial—A study protocol of a randomised controlled trial. BMC Cancer. 19 (1), 656. https://doi.org/10.1186/s12885-019-5853-4 (2019).
Rodin, G., An, E., Shnall, J. & Malfitano, C. Psychological interventions for patients with advanced disease: Implications for oncology and palliative care. J. Clin. Oncol. 38 (9), 885–904. https://doi.org/10.1200/JCO.19.00058 (2020).
Download references
Acknowledgements
The authors sincerely thank the hospital that provided the research site. We also would like to sincerely thank all patients and spouses who participated in this study.
Author information
Authors and affiliations.
Department of Hepatobiliary Surgery, The First Affiliated Hospital, Xi’an Jiaotong University, Xi’an, 710061, China
Bo Zhang, Qigui Xiao, Jingtao Gu, Qingyong Ma & Liang Han
You can also search for this author in PubMed Google Scholar
Contributions
All authors had a substantial contribution to the manuscript. Bo Zhang: Conceptualisation, Study design, Data collection, Data analysis, Data interpretation, Writing—original draf, Review and editing, Final approval; Qigui Xiao and Jingtao Gu: Conceptualisation, Study design; Qingyong Ma: Study design, Review and editing; Liang Han: Study design, Review and editing.
Corresponding authors
Correspondence to Qingyong Ma or Liang Han .
Ethics declarations
Competing interests.
The authors declare no competing interests.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Supplementary information., rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/ .
Reprints and permissions
About this article
Cite this article.
Zhang, B., Xiao, Q., Gu, J. et al. A qualitative study on the disease coping experiences of pancreatic cancer patients and their spouses. Sci Rep 14 , 18626 (2024). https://doi.org/10.1038/s41598-024-69599-7
Download citation
Received : 17 February 2024
Accepted : 07 August 2024
Published : 11 August 2024
DOI : https://doi.org/10.1038/s41598-024-69599-7
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Pancreatic cancer
- Coping experience
- Qualitative study
By submitting a comment you agree to abide by our Terms and Community Guidelines . If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing: Cancer newsletter — what matters in cancer research, free to your inbox weekly.
What Every Cancer Patient Faces: Finding The Most Effective Treatments
Being diagnosed with cancer is an unnerving event that most people never want to experience. Unfortunately, 10 million worldwide died from cancer in 2020, and this number will rise as the world’s population ages.
In America, doctors are doing their best to provide each patient with the care they need. However, policies and procedures that are standard aren’t always suitable for every patient.
Since doctors see dozens of cancer patients a day, they don’t have the time to confirm that each one is receiving the best type of care possible. Additionally, most patients think they’re being given tailored treatments for their situation. In reality, their doctors are following the same route they do for all patients, and that’s not their fault.
Doctors are adhering to guidelines and rules that have been proven effective by research, but a cancer patient who isn’t responding to chemotherapy and other traditional types of care begins to wonder if they should be pushing for more personalized treatments.
As stated by many resources , clinical trials can offer benefits that cancer patients can’t access with normal treatment. These trials allow people to be treated with the most advanced medicinal approaches, receive hands-on care, and generally at a lower cost. About 70% of Americans with cancer reported being very open or willing to participate in clinical trials. Yet, less than one in 20 adult cancer patients enroll in these studies.
Many of these patients never participate in clinical trials because they’re not being offered or there are too many barriers. Additionally, some physicians may be biased against these trials, causing them to not recommend them even if they know about the science that supports them.
Despite these difficulties, patients still have hope. Advocacy groups and companies have begun to match patients to clinical trials that may help them. However, these services don’t address the multiple barriers patients face when trying to access these trials. Sagely Health, a company providing personalized guidance and resources to patients aims to close this gap through its dual approach of offering guidance and treatment evaluation.
Sagely Health was founded in 2014 by Dr. Jason Sager, an oncologist and drug developer who is passionate about helping patients get the best possible care. The company provides comprehensive services for guiding patients to the best doctors who can offer them top-tier care, including clinical trials.
Once a patient is onboarded, Sagely Health completes a rigorous intake and evaluation process to determine if a patient’s treatments are the most effective option. The company’s expert team formulates simple documentation for patients to use when advocating for better care. Sagely Health goes the extra mile by researching heavily into databases of over 13,000 standard therapies, clinical trials, and accessible drugs to see which may benefit the patient most.
In addition to providing a treatment roadmap and details about which doctors to see and treatments to receive, Sagely Health provides step by step guidance to maximize success. The company introduces a client’s case to a specialized doctor who can explain to them the positives and negatives of the treatments they’re being recommended. This guidance, which is not available using clinical trial matching services, is crucial for patients to fully understand the options they have. In addition, every patient gets a one-hour call with Dr. Sager to review treatment recommendations and ask any questions they may have.
"I started Sagely Health 10 years ago because of a gap that was becoming more and more obvious to me,” says Dr. Jason Sager, “The standard of care approach hospitals use for cancer patients is dissatisfying for the majority because they will not receive the care they need to beat their cancer. I always believed that more could be done to help these patients and their families succeed in getting access to advanced therapies that could translate into a longer, better quality of life. Sagely Health exists to not only guide patients toward those treatments but to also give them the knowledge they need to make an informed decision. Our team loves what we do and we plan to continue on our journey until we can help every single cancer patient know what is the best possible treatment for them and know how to go about getting it.”
With the ever growing number of Americans in need of effective cancer treatments, patients urgently require knowledge that can help them transform their outcomes. While it’s difficult to advocate for better care for yourself or your loved ones, it’s worth the time. Sagely Health is encouraging and educating more patients and their families to take action today. The sooner patients begin researching and consulting doctors, the better treatments they will be able to receive, and the more comfortable their lives will be.
Official websites use .gov
A .gov website belongs to an official government organization in the United States.
Secure .gov websites use HTTPS
A lock ( ) or https:// means you've safely connected to the .gov website. Share sensitive information only on official, secure websites.
Health Care Use Among Cancer Patients With Diabetes, National Health and Nutrition Examination Survey, 2017–2020
ORIGINAL RESEARCH — Volume 21 — August 8, 2024
Ara Jo, PhD 1 ; Sarina Parikh, BHS 2 ,3 ; Nathalie Sawczuk, MHA 1 ; Kea Turner, PhD, MPH, MA 4 ,5 ; Young-Rock Hong, PhD, MPH 1 ( View author affiliations )
Suggested citation for this article: Jo A, Parikh S, Sawczuk N, Turner K, Hong Y. Health Care Use Among Cancer Patients With Diabetes, National Health and Nutrition Examination Survey, 2017–2020. Prev Chronic Dis 2024;21:240066. DOI: http://dx.doi.org/10.5888/pcd21.240066 .
PEER REVIEWED
Introduction
Acknowledgments, author information.
What is already known on this topic?
Cancer patients with multiple chronic diseases have unplanned hospitalizations because of a lack of appropriate care management. Multiple chronic diseases among people with cancer are associated with worse clinical outcomes and survivorship than among people with cancer only.
What is added by this report?
Patients with cancer and prediabetes had higher levels of health care use than patients with cancer only. A diagnosis of type 2 diabetes did not significantly affect health care use among patients with cancer.
What are the implications for public health practice?
Optimal care coordination and early management of prediabetes among patients with cancer via primary care may contribute to improving cancer survivorship.
Diabetes is a common comorbidity among people with cancer. The objective of our study was to examine patterns of health care use among patients with cancer and either type 2 diabetes or prediabetes.
We used data from the National Health and Nutrition Examination Survey (NHANES) for 2017–2020. The study population included US adults aged 18 years or older who were diagnosed with any cancer and type 2 diabetes or prediabetes (established by self-report and/or hemoglobin A 1c measurement). We used Poisson and multivariate logistic regression models to determine the effect of comorbidity on health care use, defined as health care visits and overnight stays in a hospital.
Of 905 cancer patients representing 27,180,715 people in the US, 24.4% had a type 2 diabetes diagnosis, and 25.8% had a prediabetes diagnosis. Patients with cancer and prediabetes had a significantly higher rate of health care visits (incidence rate ratio = 1.11; 95% CI, 1.01–1.22; P = .03) than patients with cancer only. We found no significant association between having cancer and type 2 diabetes and the number of health care visits or overnight hospital stays compared with patients with cancer only.
More emphasis should be placed on optimal care coordination among people with cancer and other conditions, such as diabetes and prediabetes, to reduce the impact of comorbidity on health care use. Interventions integrated with technology to provide timely access to education on preventing or managing diabetes and prediabetes among cancer patients are warranted.
Diabetes is a common comorbidity among people with cancer. As patients with cancer live longer due to advances in cancer treatment, rates of chronic conditions, such as diabetes, are expected to rise among people with cancer. People with type 2 diabetes (hereinafter, diabetes) have a substantially higher risk of cancer incidence and death, leading to poorer survivorship compared with people without diabetes (1,2). For example, people with diabetes, compared with people who do not have diabetes, have double the risk for liver and pancreatic cancers and have a higher risk of developing bladder, colon, and breast cancers (3). In addition, as cancer incidence and death rates have risen consistently over time, the comorbidity of cancer with other chronic diseases has gained attention (4,5). Despite these clinical outcomes, the research is limited on care delivery for people with cancer and other comorbidities.
People with cancer and comorbidities, compared with those who have cancer and no comorbidities, have greater unplanned use of health care services, including higher rates of unplanned hospital readmissions (6,7) and revisits to the emergency department (8). One study showed that among people with cancer and comorbidities, diabetes was the top reason for emergency department revisits (24% of all revisit encounters) (8). Another study found that the average length of hospital stay among people with cancer and diabetes was significantly longer than among patients with no comorbidity (9). In that study, the average length of a hospital stay among patients with colorectal cancer and diabetes who underwent surgery was almost 17 days, which is 3 days longer than among patients with cancer only (9). Furthermore, health care costs are of critical concern. A national study, which used 5 years of data from the Medical Expenditure Panel Survey (2010–2014), found that cancer patients spent on average 4 times more in annual health expenditures than noncancer patients (10). Early initiation of chronic disease prevention and management with a primary care physician can mitigate this financial burden.
Many patients with cancer face the challenges of comanaging cancer and chronic diseases. In a qualitative study conducted in 2021 and 2022 at 3 New York City hospitals among 15 women with breast cancer and either diabetes or prediabetes, participants reported a lack of information and education on managing chronic diseases and the burden of co-management with different providers (11). In addition, patients tended to prioritize cancer treatment over diabetes management with their primary care physician (11). These struggles may be more detrimental for patients who are at a higher-than-average risk of developing diabetes. For example, a national cohort study in Korea found that a diagnosis of cancer increased the risk of subsequent diabetes (12). A case-cohort study in Israel that investigated the association between hormone therapy and diabetes risk among 2,246 female breast cancer survivors found that 48% of diabetes incidence could have been prevented had patients not received hormone therapy (13). Early implementation of a diabetes prevention strategy, particularly for patients with cancer and prediabetes, elevated blood glucose, or active engagement with a primary care physician during cancer treatment, could prevent comorbidity and improve survivorship. Furthermore, cancer treatments such as chemotherapy, radiation, or immunotherapy are associated with a higher prevalence of prediabetes (14).
Comorbidities or complications associated with cancer are linked to increased health care costs and various kinds of health care use, including ambulatory care visits and emergency department visits (15,16). However, evidence that focuses on the effects of specific kinds of comorbidity, such as diabetes, on health care use is limited. One study that used data from a statewide electronic health record database from 2007 to 2017 in the US found a significant association of having both diabetes and colorectal cancer with emergency department visits but did not examine other outcomes, such as hospitalization, which is a major driver of health care costs (17). Furthermore, little is known about how patterns of health care use differ across stages of diabetes. Addressing these gaps may help to improve the delivery of effective clinical care and preventive services for people with cancer and diabetes.
The objective of this study was to examine the association of health care use patterns among patients with cancer, stratified by diagnosis of diabetes or prediabetes. Findings from the current study may guide research to develop an optimal coordinated care model for early detection of prediabetes or diabetes and to enhance cancer survivorship for people with cancer and comorbidities.
Our study used a cross-sectional design and data from the National Health and Nutrition Examination Survey (NHANES) for the 3-year cycle of 2017–2020, before the pandemic. NHANES has been conducted since 1960 and is designed to assess the health and nutritional status of adults and children in the US. It collects nationally representative data through clinical examinations, selected medical and laboratory tests, and self-reported data. NHANES uses a stratified, multistage probability sample design and recommends using weights, stratification, and cluster variables to account for the complex sample design (18). Thus, we applied these variables to the statistical analyses to generate population estimates.
Study population
Our study population comprised adults aged 18 years or older who were diagnosed with any cancer and had physician-diagnosed diabetes or prediabetes. Those with a cancer history were identified by using the question, “Have you ever been told by a doctor or other health professional that you had cancer or a malignancy of any kind?” Physician-diagnosed diabetes and prediabetes were identified through self-report on the NHANES questionnaire. In addition, to reduce the risk of recall bias, we used NHANES laboratory results of the hemoglobin A 1c (HbA 1c ) test. We excluded data on undiagnosed diabetes because the sample size was too small for generating population estimates. We classified people into 3 categories: 1) those with a cancer history only, 2) those with any cancer history and prediabetes, and 3) those with any cancer history and diabetes. We excluded records that had missing data for these variables.
A primary outcome was the number of visits to a physician’s office, a clinic, or “some other place” in the previous 12 months. This visit did not include hospitalizations, emergency department visits, home visits, or telephone calls. A secondary outcome was an overnight stay in a hospital in the previous 12 months. It excluded overnight stays in the emergency department.
Independent variable
A primary independent variable was comorbidity status. We categorized the study population into 3 groups: 1) cancer only, 2) cancer and prediabetes, and 3) cancer and diabetes. Control variables were demographic characteristics (age, sex, and race and ethnicity), education, body mass index (BMI), and having a usual source of care (yes or no). We treated age as a continuous variable. Sex was a dichotomous variable (male or female). We categorized race and ethnicity into 4 categories: 1) Hispanic or Latino, 2) non-Hispanic Black, 3) non-Hispanic White, and 4) Other (American Indian or Alaska Native, Asian, and Native Hawaiian or Pacific Islander) or multiracial. We converted education into a dichotomous variable (less than high school and high school graduate or above). Financial status was measured by the ratio of income to poverty (total family income divided by the poverty threshold) and dichotomized into 2 levels: 1) poor (ratio <1) and 2) rich (ratio ≥1). Health status was measured by self-reported general health condition and grouped into 2 levels: 1) fair or above (excellent, very good, good, or fair) and 2) poor. BMI was categorized into 3 levels: 1) normal (BMI, 18.5–24.9), 2) overweight (25.0–29.9), and 3) obese (≥30.0). Health insurance status was categorized into 2 levels: 1) yes, insured, and 2) no, uninsured. We excluded underweight people due to a high risk of mortality and little relevance to our study. Lastly, we treated usual source of care as a dichotomous variable (has a usual source or does not have a usual source of care). We counted the number of other chronic diseases reported by the survey respondent, such as arthritis, cancer (if the respondent has ≥1 cancers), cardiovascular diseases (eg, congestive heart failure, coronary heart disease, angina, or stroke), chronic kidney disease, depression, hypertension, and pulmonary diseases (eg, emphysema, chronic bronchitis, or asthma). We categorized these data into 4 groups: 1) no other comorbidity, 2) 1 additional comorbidity, 3) 2 additional comorbidities, and 4) ≥3 additional comorbidities.
Statistical analysis
We conducted a descriptive analysis of the baseline characteristics of the 3 groups of NHANES respondents (cancer only, cancer and prediabetes, and cancer and diabetes). We used χ 2 tests and t tests to determine significant differences between groups, with P < .05 considered significant. We used a Poisson regression model to determine the effect of comorbidity status (cancer only, cancer and prediabetes, and cancer and diabetes) on the number of health care visits in the previous 12 months. We used a multivariate logistic regression model to examine the risk of an overnight hospital stay associated with comorbidity status. We conducted both unadjusted and adjusted models. The Poisson regression model produced incident rate ratios (IRRs) and 95% CIs, and the multivariate logistic regression model produced odds ratios (ORs) and 95% CIs. The Pearson χ 2 test was used to evaluate the goodness-of-fit for the Poisson regression model, and the Akaike Information Criterion (AIC) was used to evaluate the goodness-of-fit for the multivariate logistic regression model. We used SAS version 9.4 (SAS Institute, Inc) for all analyses. This study was exempted from the University of Florida Institutional Review Board review because of the use of publicly available data. We followed the STROBE statement in conducting methods and reporting results (19).
The unweighted sample size was 905, representing 27,180,715 people in the US. Of these cancer patients, 24.4% (weighted percentage) had a type 2 diabetes diagnosis, and 25.8% (weighted percentage) had a prediabetes diagnosis ( Table 1 ). The mean age of the total study population was 63.9 years. People with cancer and diabetes (mean age, 68.8 y) and people with cancer and prediabetes (mean age, 66.7 y) were older, on average, than people with cancer only (mean, 59.9 y). The percentage of people with less than a high school diploma was significantly larger among people with cancer and diabetes (10.2%) and cancer and prediabetes (9.4%) than people with cancer only (5.2%). The percentage of people who had a BMI in the obese range was significantly larger among people with cancer and diabetes (63.3%) and cancer and prediabetes (43.9%) than people with cancer only (30.7%). The percentage of people with 3 or more additional comorbidities was significantly larger among people with cancer and diabetes (51.0%) and cancer and prediabetes (30.3%) than among people with cancer only (17.1%). Regardless of comorbidity status, more than 95% of people had health insurance. The percentage of people with a usual source of care was significantly larger among people with cancer and diabetes (98.3%) and cancer and prediabetes (97.2%) than among people with cancer only (91.6%).
In the unadjusted Poisson regression model, the IRR for the number of health care visits in the previous 12 months was significantly higher among people with cancer and diabetes (IRR = 1.19; 95% CI, 1.12–1.27; P < .001) than among people with cancer only ( Table 2 ). However, after controlling for covariates, the comorbidity of cancer and diabetes was not significantly associated with increases in the number of health care visits (IRR = 1.04; 95% CI, 0.94–1.15; P = .44). After controlling for covariates, the comorbidity of cancer and prediabetes was associated with increases in the number of health care visits in the previous 12 months (IRR = 1.11; 95% CI, 1.01–1.22; P = .03). The results of the goodness-of-fit test for both unadjusted and adjusted models were not significant, indicating that neither model fit the data well.
In the multivariate logistic regression, the unadjusted model showed that people with diabetes and cancer were 2.5 times more likely than people with cancer only to stay overnight in a hospital (OR = 2.55; 95% CI, 1.54–4.21). However, after controlling for covariates, this association was not significant (OR = 1.57; 95% CI, 0.82–3.02). Moreover, we found no significant association in comorbidity with prediabetes for the risk of an overnight stay in a hospital in either the unadjusted or adjusted model ( Table 3 ). The goodness-of-fit test for the adjusted model had a lower AIC value than the unadjusted model, indicating a better fitting model.
The objective of our study was to examine patterns of health care use among people with cancer and either prediabetes or diabetes. In our nationally representative sample, patients with cancer and diabetes had 19% more health care visits than people with cancer only according to the unadjusted regression model, and patients with cancer and prediabetes had 11% more health care visits than people with cancer only according to the adjusted regression model. Future studies may be needed to test strategies to improve care coordination and early initiation of preventive care strategies for people with cancer at risk of developing prediabetes and diabetes.
Having diabetes and cancer increased the risk for an overnight stay in a hospital in the unadjusted regression models, whereas having prediabetes and cancer increased the number of health care visits in the adjusted regression model only. These findings indicate that different stages of diabetes may drive different health care needs. In the qualitative study conducted in 2021 and 2022 at 3 New York City hospitals among 15 women with breast cancer and either diabetes or prediabetes, 7 participants reported glucose levels of more than 200 mg/dL (normal is 70–90 mg/dL) and 9 participants indicated a lack of glucose control during cancer treatment (11). In addition, as cancer treatment tends to be prioritized over other treatment, diabetes prevention and management led by a primary care physician may be paused (20). Medication adherence for chronic diseases may also decline due to the priority of cancer treatment (21,22). In addition, many cancer patients with comorbidities may not receive self-management education or guidelines for preventive care, negatively affecting cancer survivorship (23). Moreover, our study found that patients with cancer and diabetes were 2 times more likely to be hospitalized, whereas patients with cancer and prediabetes did not have significantly higher rates of hospitalization. This finding was supported by literature showing that patients with cancer and at least 1 comorbidity were more likely than patients with no comorbidities to be hospitalized (6,24). Clinical guidelines for managing patients with cancer and prediabetes are lacking, and communication guidelines for coordinated care between oncologists and primary care physicians are limited. Because many patients with cancer tend to prioritize cancer treatment over primary care for prediabetes or diabetes, detrimental clinical outcomes and increased health care use may not be preventable without early prevention or ongoing management. In response to increases in the prevalence of prediabetes and cancer, it is important to develop a systematic preventive care model for early-stage chronic diseases (eg, prediabetes, prehypertension) that includes collaboration between oncologists and primary care physicians. Such a model could be a cost-effective strategy for improving cancer survivorship.
Our study also found that more than 80% of comorbid people were overweight or obese (compared with 67.5% among those with cancer only). It is well established that obesity is significantly associated with cancer incidence and mortality (25) and is a risk factor for cancer and chronic diseases (eg, diabetes, prediabetes) (26,27). Excessive body fat causes chronic inflammation that may be attributed to cancer treatment–associated adverse outcomes (25). Thus, it is important to control overweight and obesity during cancer treatment. A combination of diet and exercise was identified as a more effective intervention for weight loss than a standard of care for patients with cancer (28). Clinicians need to provide self-management guidelines for lifestyle changes when a cancer diagnosis is first made, especially among overweight or obese patients. In the qualitative study among 15 women with breast cancer and either diabetes or prediabetes, participants indicated not receiving guidance on self-management or having a designated clinician who continuously monitored them (11). One in-depth patient interview found that a patient searched for diet or exercise information on Google (11). This research suggests a need for self-management guidelines provided by clinicians for controlling overweight or obesity and monitoring chronic disease progression.
Educational attainment was significantly associated with comorbidity status. Among patients with less than a high school diploma, the percentage of patients with a comorbidity was twice the percentage of patients with no comorbidity (9.4% and 10.2% vs 5.2%). Education may be key to health behaviors and the prevention of adverse outcomes. It is well established that education inequality is associated with cancer survivorship (29,30). For example, a study in The Netherlands showed that among patients with cancer and comorbidity, those with a low level of education (equivalent to primary school) had a 3 times higher risk of death than those with a university degree (29). A study of education differentials in cancer deaths in Lithuania found an inverse educational gradient for selected cancer sites among men and women, noting that substantial shares of cancer deaths (8% to 35%) could have been avoided or postponed (30). Increasing access to resources for patients with low levels of education may help to minimize the number of comorbidities that can arise and ultimately improve their cancer survivorship. Particularly, providing more resources may benefit from developing effective and structured communication strategies with providers.
Optimal coordinated care is crucial to mitigate the burden of comorbidities on health care use and costs among patients with cancer. Despite the growing need for increased care coordination between primary care physicians and oncologists, no standardized care coordination model exists for managing the comorbidity of cancer and chronic diseases such as prediabetes or diabetes (31). Additionally, the involvement of primary care physicians in cancer care is limited, especially during active cancer treatment (32). Previous research identified some barriers to effective cancer care coordination, including inadequate communication between oncologists and primary care providers and between patients and primary care providers; geographic limitations; and limited interoperability of the electronic health record among health care providers (32,33). Fortunately, the recent rapid technological evolution has provided new opportunities to reduce these barriers. Studies conducted at the Johns Hopkins Primary Care for Cancer Survivors clinic in 2015 and the Duke Cancer Institute during 2020–2021 found that comorbid patients were more likely to use telehealth for cancer and primary care, and telehealth improved outcomes such as patient satisfaction and survivorship (34,35). Using artificial intelligence in the care coordination process and communication will become pivotal to improving an efficient and effective care coordination model. An optimal care coordination model integrated with technology can be achieved by using standardized communication channels among health care providers and between health care providers and patients and the interoperability of electronic health records. Moreover, appropriate data privacy and security regulation will be essential to ensure patient trust in the care coordination model. To leverage these benefits, standardized clinical guidelines for managing comorbidities in patients with cancer should be developed. These guidelines would provide clear recommendations on integrating care coordination.
Limitations
Our study has several limitations. First, the diagnosis information obtained from a self-reported survey may be subject to recall bias, and we could not determine the exact timing of the diagnosis of diabetes or prediabetes and cancer. Second, our study used cross-sectional data, which prevented us from following disease progression over time and examining the effects of various treatments. A study that uses longitudinal data is needed to understand the effect of comorbidity on health care use among cancer patients. Third, we could not identify the reasons for health care use because of a lack of data. A study that incorporates electronic health records may identify patient-centered health care needs for those with comorbidities. Lastly, while the study identified patients with cancer who had undiagnosed diabetes, the sample size was too small to generate population estimates. Studies that use larger data sets could examine the role of undiagnosed diabetes on cancer prognosis and outcomes.
Among people with cancer, diabetes was significantly associated with an increased risk of an overnight hospital stay, whereas prediabetes was significantly associated with an increase in the number of health care visits. Our findings suggest that it may be beneficial to prioritize preventive measures (eg, screening) to prevent prediabetes from progressing to diabetes in patients with cancer and develop optimal coordinated care, which could help alleviate the strain on the health care system and improve oncology care.
The authors received no external financial support for the research, authorship, or publication of this article. The authors declared no potential conflicts of interest with respect to the research, authorship, or publication of this article. No copyrighted material, surveys, instruments, or tools were used in the research described in this article.
Corresponding Author: Ara Jo, PhD, Department of Health Services Research, Management and Policy, University of Florida, Health Sciences Center, PO Box 100195, Gainesville, FL 32610-0195 ( [email protected] ).
Author Affiliations: 1 Department of Health Services Research, Management and Policy, University of Florida, Gainesville. 2 College of Public Health and Health Professions, University of Florida, Gainesville. 3 Now with School of Dental Medicine, University of Pennsylvania, Philadelphia. 4 Department of Health Outcomes and Behavior, Moffitt Cancer Center, Tampa, Florida. 5 Department of Gastrointestinal Oncology, Moffitt Cancer Center, Tampa, Florida.
- Pearson-Stuttard J, Papadimitriou N, Markozannes G, Cividini S, Kakourou A, Gill D, et al. . Type 2 diabetes and cancer: an umbrella review of observational and Mendelian randomization studies. Cancer Epidemiol Biomarkers Prev . 2021;30(6):1218–1228. PubMed doi:10.1158/1055-9965.EPI-20-1245
- Ranc K, Jørgensen ME, Friis S, Carstensen B. Mortality after cancer among patients with diabetes mellitus: effect of diabetes duration and treatment. Diabetologia . 2014;57(5):927–934. PubMed doi:10.1007/s00125-014-3186-z
- Rahman I, Athar MT, Islam M. Type 2 diabetes, obesity, and cancer share some common and critical pathways. Front Oncol . 2021;10:600824. PubMed doi:10.3389/fonc.2020.600824
- Centers for Disease Control and Prevention. An update on cancer deaths in the United States. Published February 28, 2022. Accessed July 11, 2023. https://stacks.cdc.gov/view/cdc/119728
- National Cancer Institute. Cancer statistics. Published April 2, 2015. Accessed July 10, 2023. https://www.cancer.gov/about-cancer/understanding/statistics
- Bur AM, Brant JA, Mulvey CL, Nicolli EA, Brody RM, Fischer JP, et al. . Association of clinical risk factors and postoperative complications with unplanned hospital readmission after head and neck cancer surgery. JAMA Otolaryngol Head Neck Surg . 2016;142(12):1184–1190. PubMed doi:10.1001/jamaoto.2016.2807
- Goel AN, Raghavan G, St John MA, Long JL. Risk factors, causes, and costs of hospital readmission after head and neck cancer surgery reconstruction. JAMA Facial Plast Surg . 2019;21(2):137–145. PubMed doi:10.1001/jamafacial.2018.1197
- Nene RV, Brennan JJ, Castillo EM, Tran P, Hsia RY, Coyne CJ. Cancer-related emergency department visits: comparing characteristics and outcomes. West J Emerg Med . 2021;22(5):1117–1123. PubMed doi:10.5811/westjem.2021.5.51118
- Zhang X, Wang X, Wang M, Gu J, Guo H, Yang Y, et al. . Effect of comorbidity assessed by the Charlson Comorbidity Index on the length of stay, costs, and mortality among colorectal cancer patients undergoing colorectal surgery. Curr Med Res Opin . 2023;39(2):187–195. PubMed doi:10.1080/03007995.2022.2139053
- Park J, Look KA. Health care expenditure burden of cancer care in the United States. Inquiry . 2019;56:46958019880696. PubMed doi:10.1177/0046958019880696
- Pinheiro LC, Cho J, Rothman J, Zeng C, Wilson M, Kern LM, et al. . Diabetes and cancer co-management: patient-reported challenges, needs, and priorities. Support Care Cancer . 2023;31(2):145. PubMed doi:10.1007/s00520-023-07604-x
- Hwangbo Y, Kang D, Kang M, Kim S, Lee EK, Kim YA, et al. . Incidence of diabetes after cancer development: a Korean national cohort study. JAMA Oncol . 2018;4(8):1099–1105. PubMed doi:10.1001/jamaoncol.2018.1684
- Hamood R, Hamood H, Merhasin I, Keinan-Boker L. Diabetes after hormone therapy in breast cancer survivors: a case‐cohort study. J Clin Oncol . 2018;36(20):2061–2069. PubMed doi:10.1200/JCO.2017.76.3524
- Ose DJ, Viskochil R, Holowatyj AN, Larson M, Wilson D, Dunson WA, et al. . Understanding the prevalence of prediabetes and diabetes in patients with cancer in clinical practice: a real-world cohort study. J Natl Compr Canc Netw . 2021;19(6):709–718. PubMed doi:10.6004/jnccn.2020.7653
- May P, Garrido MM, Aldridge MD, Cassel JB, Kelley AS, Meier DE, et al. . Prospective cohort study of hospitalized adults with advanced cancer: associations between complications, comorbidity, and utilization. J Hosp Med . 2017;12(6):407–413. PubMed doi:10.12788/jhm.2745
- Rotbain EC, Rostgaard K, Andersen MA, Da Cunha-Bang C, Niemann CU, Frederiksen H, et al. . Healthcare utilization and comorbidity in chronic lymphocytic leukemia. Clin Epidemiol . 2021;13:1155–1165. PubMed doi:10.2147/CLEP.S337495
- Storey S, Zhang Z, Luo X, Von Ah D, Metzger M, Zhang J, et al. . Association of comorbid diabetes with clinical outcomes and healthcare utilization in colorectal cancer survivors. Oncol Nurs Forum . 2021;48(2):195–206. PubMed doi:10.1188/21.ONF.195-206
- Centers for Disease Control and Prevention. NHANES survey methods and analytic guidelines. Accessed August 11, 2023. https://wwwn.cdc.gov/nchs/nhanes/analyticguidelines.aspx
- von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP; STROBE Initiative. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. BMJ . 2007;335(7624):806–808. PubMed doi:10.1136/bmj.39335.541782.AD
- Cho J, Nilo D, Sterling MR, Kern LM, Safford MM, Pinheiro LC. Eliciting primary care and oncology provider perspectives on diabetes management during active cancer treatment. Support Care Cancer . 2021;29(11):6881–6890. PubMed doi:10.1007/s00520-021-06264-z
- Dashputre AA, Gatwood KS, Schmidt J, Gatwood J. Impact of oral oncolytic initiation on medication adherence for pre-existing comorbid chronic conditions. J Oncol Pharm Pract . 2020;26(4):835–845. PubMed doi:10.1177/1078155219875206
- Gatwood J, Dashputre A, Rajpurohit A, Gatwood K, Mackler E, Wallace L, et al. . Medication adherence among adults with comorbid chronic conditions initiating oral anticancer agent therapy for multiple myeloma. JCO Oncol Pract . 2022;18(9):e1475–e1483. PubMed doi:10.1200/OP.22.00008
- Earle CC, Neville BA. Under use of necessary care among cancer survivors. Cancer . 2004;101(8):1712–1719. PubMed doi:10.1002/cncr.20560
- Christodoulou I, Ukert B, Vavuranakis MA, Kum HC, Giannouchos TV. Adult cancer-related emergency department utilization: an analysis of trends and outcomes from emergency departments in Maryland and New York. JCO Oncol Pract . 2023;19(5):e683–e695. PubMed doi:10.1200/OP.22.00525
- Pati S, Irfan W, Jameel A, Ahmed S, Shahid RK. Obesity and cancer: a current overview of epidemiology, pathogenesis, outcomes, and management. Cancers (Basel) . 2023;15(2):485. PubMed doi:10.3390/cancers15020485
- Leach CR, Weaver KE, Aziz NM, Alfano CM, Bellizzi KM, Kent EE, et al. . The complex health profile of long-term cancer survivors: prevalence and predictors of comorbid conditions. J Cancer Surviv . 2015;9(2):239–251. PubMed doi:10.1007/s11764-014-0403-1
- Gallagher EJ, LeRoith D. Obesity and diabetes: the increased risk of cancer and cancer-related mortality. Physiol Rev . 2015;95(3):727–748. PubMed doi:10.1152/physrev.00030.2014
- LeVasseur N, Cheng W, Mazzarello S, Clemons M, Vandermeer L, Jones L, et al. . Optimising weight-loss interventions in cancer patients — a systematic review and network meta-analysis. PLoS One . 2021;16(2):e0245794. PubMed doi:10.1371/journal.pone.0245794
- Aarts MJ, Kamphuis CBM, Louwman MJ, Coebergh JWW, Mackenbach JP, van Lenthe FJ. Educational inequalities in cancer survival: a role for comorbidities and health behaviours? J Epidemiol Community Health . 2013;67(4):365–373. PubMed doi:10.1136/jech-2012-201404
- Jasilionis D, Smailyte G, Vincerzevskiene I, Shkolnikov VM. Educational differentials in cancer mortality and avoidable deaths in Lithuania, 2001–2009: a census-linked study. Int J Public Health . 2015;60(8):919–926. PubMed doi:10.1007/s00038-015-0745-0
- Hohmann NS, McDaniel CC, Mason SW, Cheung WY, Williams MS, Salvador C, et al. . Patient perspectives on primary care and oncology care coordination in the context of multiple chronic conditions: a systematic review. Res Social Adm Pharm . 2020;16(8):1003–1016. PubMed doi:10.1016/j.sapharm.2019.11.014
- Petrovic B, Morgan S, Afkham A, O’Brien MA, Sussman J, Fitch M, et al. . Cancer specialist perspectives on implementing an online communication system with primary care providers. Ann Fam Med . 2022;20(20 Suppl 1):3246. PubMed
- Walsh J, Harrison JD, Young JM, Butow PN, Solomon MJ, Masya L. What are the current barriers to effective cancer care coordination? A qualitative study. BMC Health Serv Res . 2010;10(1):132. PubMed doi:10.1186/1472-6963-10-132
- Choi Y, Parrillo E, Wenzel J, Grabinski VF, Kabani A, Peairs KS. Optimizing cancer survivorship in primary care: patient experiences from the Johns Hopkins Primary Care for Cancer Survivors clinic. J Cancer Surviv . 2023;17(5):1286–1294. PubMed doi:10.1007/s11764-022-01166-3
- Golla V, Frascino N, Wilson LE, Oakes M, Oeffinger KC, Check DK, et al. . Telehealth patterns in primary and onco-primary care. Cancer Survivorship Research & Care. 2023;1(1):2288879.
| Characteristic | Cancer only | Cancer and prediabetes | Cancer and diabetes | value |
|---|---|---|---|---|
| 403 | 248 | 254 | — | |
| 13,532,512 (49.8) | 7,024,691 (25.8) | 6,623,512 (24.4) | — | |
| 59.9 | 66.7 | 68.8 | <.001 | |
| Male | 40.8 | 38.1 | 46.4 | .51 |
| Female | 59.2 | 61.9 | 53.6 | |
| Hispanic | 6.0 | 19.4 | 8.6 | .33 |
| Non-Hispanic Black | 5.8 | 32.1 | 6.3 | |
| Non-Hispanic White | 82.2 | 26.3 | 79.3 | |
| Other | 6.1 | 19.3 | 5.8 | |
| Less than high school | 5.2 | 9.4 | 10.2 | .02 |
| High school graduate or above | 94.8 | 90.6 | 89.8 | |
| Poor | 6.3 | 5.7 | 8.9 | .33 |
| Rich | 93.7 | 94.3 | 91.1 | |
| Normal (18.5–24.9) | 32.5 | 16.0 | 6.5 | <.001 |
| Overweight (25.0–29.9) | 36.8 | 40.1 | 30.2 | |
| Obese (≥30.0) | 30.7 | 43.9 | 63.3 | |
| Fair or above | 95.7 | 95.2 | 89.1 | .02 |
| Poor | 4.3 | 4.8 | 10.9 | |
| 0 | 30.9 | 19.2 | 5.6 | <.001 |
| 1 | 30.8 | 21.1 | 17.6 | |
| 2 | 21.3 | 29.4 | 25.8 | |
| ≥3 | 17.1 | 30.3 | 51.0 | |
| No | 8.4 | 2.81 | 1.8 | .02 |
| Yes | 91.6 | 97.2 | 98.2 | |
| No | 3.5 | 1.4 | 4.8 | .25 |
| Yes | 96.5 | 98.6 | 95.2 | |
| 3.4 | 3.8 | 3.8 | .13 | |
| No | 15.3 | 15.5 | 31.6 | <.001 |
| Yes | 84.7 | 84.5 | 68.4 | |
a All values are weighted percentages, unless otherwise indicated. b Determined by t test for continuous variable and χ 2 tests for categorical variables. c Includes American Indian or Alaska Native, Asian, and Native Hawaiian or Pacific Islander, and multiracial. d Measured by the ratio of income to poverty (total family income divided by the poverty threshold) and dichotomized into 2 levels: 1) poor (ratio < 1) and 2) rich (ratio ≥ 1). e Visits to a physician’s office, a clinic, or some other place in the previous 12 months, not including hospitalizations, emergency department visits, home visits, or telephone calls. f Excludes overnight stays in the emergency department.
| Characteristic | Unadjusted IRR (95% CI) [ value] | Adjusted IRR (95% CI) [ value] |
|---|---|---|
| Cancer only | Reference | Reference |
| Cancer and prediabetes | 1.05 (0.98–1.12) [.14] | 1.11 (1.01–1.22) [.03] |
| Cancer and diabetes | 1.19 (1.12–1.27) [<.001] | 1.04 (0.94–1.15) [.44] |
Abbreviation: IRR, incidence rate ratio. a Visits to a physician’s office, a clinic, or some other place in the previous 12 months, not including hospitalizations, emergency department visits, home visits, or telephone calls. b Controlled for age, sex, race and ethnicity, education, poverty-to-income ratio, body mass index, number of additional comorbidities, and health insurance.
| Characteristic | Unadjusted odds ratio (95% CI) | Adjusted odds ratio (95% CI) |
|---|---|---|
| Cancer only | 1 [Reference] | 1 [Reference] |
| Cancer and prediabetes | 1.01 (0.63–1.64) | 0.84 (0.42–1.65) |
| Cancer and diabetes | 2.55 (1.54–4.21) | 1.57 (0.82–3.02) |
a Excludes overnight stays in the emergency department. b An odds ratio with a 95% CI that includes 1 indicates no significant effect on risk. c Controlled for age, sex, race and ethnicity, education, poverty-to-income ratio, body mass index, number of comorbidities, and health insurance.
The opinions expressed by authors contributing to this journal do not necessarily reflect the opinions of the U.S. Department of Health and Human Services, the Public Health Service, the Centers for Disease Control and Prevention, or the authors’ affiliated institutions.
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
- My Bibliography
- Collections
- Citation manager
Save citation to file
Email citation, add to collections.
- Create a new collection
- Add to an existing collection
Add to My Bibliography
Your saved search, create a file for external citation management software, your rss feed.
- Search in PubMed
- Search in NLM Catalog
- Add to Search
Coconut Milk Consumption and Breast Cancer Risk in Thai Women: A Case-Control Study
Affiliations.
- 1 Master of Science Program in Toxicology and Nutrition for Food Safety, Institute of Nutrition, Mahidol University, Nakhon Pathom, Thailand.
- 2 Institute of Nutrition, Mahidol University, Nakhon Pathom, Thailand.
- PMID: 39132930
- DOI: 10.1080/01635581.2024.2390202
Coconut milk contains plant-based saturated fat and phytochemicals with antioxidant activities. However, its role in breast cancer risk remains unclear. A case-control study was conducted on 244 participants to study the association. The Case group includes 61 newly diagnosed breast cancer patients receiving < 6 months of therapies. The Control group includes 183 healthy people with matched characteristics. A new questionnaire was developed, validated, and used in this study to estimate the frequency of coconut milk-containing food intake. Results show that the questionnaire has satisfactory content validity, test-retest reliability, and criterion-related validity. From the case-control study, either consuming 1-3 or 4-6 times/week of coconut-milk-containing curry or consuming 4-6 times/week of coconut milk-topped desserts are associated with increased risk of breast cancer (OR = 5.23, 5.6, and 2.6 respectively, p < 0.01). Consuming less than half of coconut milk liquid in desserts correlated with a reduced risk (OR = 0.43, p < 0.05). The findings suggest that moderate (less than half of a serving) and infrequent (less than once a week) consumption of coconut milk may be beneficial for breast cancer prevention. A larger scale study is warranted to confirm the findings and provide evidence for dietary recommendations.
PubMed Disclaimer
Similar articles
- Dietary intake and the risk of coronary heart disease among the coconut-consuming Minangkabau in West Sumatra, Indonesia. Lipoeto NI, Agus Z, Oenzil F, Wahlqvist M, Wattanapenpaiboon N. Lipoeto NI, et al. Asia Pac J Clin Nutr. 2004;13(4):377-84. Asia Pac J Clin Nutr. 2004. PMID: 15563444
- Nutritional Content of Non-Dairy Frozen Desserts. Craig WJ, Brothers CJ. Craig WJ, et al. Nutrients. 2022 Oct 6;14(19):4150. doi: 10.3390/nu14194150. Nutrients. 2022. PMID: 36235801 Free PMC article.
- Milk Consumption Decreases Risk for Breast Cancer in Korean Women under 50 Years of Age: Results from the Health Examinees Study. Shin WK, Lee HW, Shin A, Lee JK, Kang D. Shin WK, et al. Nutrients. 2019 Dec 21;12(1):32. doi: 10.3390/nu12010032. Nutrients. 2019. PMID: 31877693 Free PMC article.
- The Duration, Frequency, and Volume of Exclusive Human Milk and/or Infant Formula Consumption and Overweight and Obesity: A Systematic Review [Internet]. Dewey K, Bazzano L, Davis T, Donovan S, Taveras E, Kleinman R, Güngör D, Madan E, Venkatramanan S, Terry N, Butera G, Obbagy J. Dewey K, et al. Alexandria (VA): USDA Nutrition Evidence Systematic Review; 2020 Jul. Alexandria (VA): USDA Nutrition Evidence Systematic Review; 2020 Jul. PMID: 35315996 Free Books & Documents. Review.
- Beverage Consumption and Growth, Size, Body Composition, and Risk of Overweight and Obesity: A Systematic Review [Internet]. Mayer-Davis E, Leidy H, Mattes R, Naimi T, Novotny R, Schneeman B, Kingshipp BJ, Spill M, Cole NC, Bahnfleth CL, Butera G, Terry N, Obbagy J. Mayer-Davis E, et al. Alexandria (VA): USDA Nutrition Evidence Systematic Review; 2020 Jul. Alexandria (VA): USDA Nutrition Evidence Systematic Review; 2020 Jul. PMID: 35349233 Free Books & Documents. Review.
Related information
Linkout - more resources, full text sources.
- Taylor & Francis
- Citation Manager
NCBI Literature Resources
MeSH PMC Bookshelf Disclaimer
The PubMed wordmark and PubMed logo are registered trademarks of the U.S. Department of Health and Human Services (HHS). Unauthorized use of these marks is strictly prohibited.
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Int J Surg Case Rep
A case of an elderly patient with high-grade colorectal cancer in poor general condition who showed near complete response to chemotherapy and achieved long-term survival
Yoshiaki kanemoto.
a Department of Surgery, IMSUT Hospital, The Institute of Medical Science, The University of Tokyo, Japan
b Division of Molecular Pathology, The Institute of Medical Science, The University of Tokyo, Japan
Giichiro Tsurita
Tomohiro kurokawa, kentaro yazawa, yoshinori murakami.
- • Systemic therapy can achieve good treatment outcomes in advanced CRC.
- • Suitable chemotherapeutics can markedly improve the prognosis of unresectable CRC.
- • Unresectable CRC can now be treated with systemic chemotherapy instead of BSC.
Introduction
Chemotherapy is difficult to administer in patients with poor performance status (PS), advanced metastatic lesion, and unresectable colon cancer. We report herein our experience of a patient who showed complete response to chemotherapy and marked PS improvement. The patient presented with the following adverse factors poor PS, advanced progression of metastatic lesions, advanced unresectable colorectal cancer with severe stricture, and old age.
Presentation of case
The patient was an 80-year-old male diagnosed with occlusive cancer of the descending colon with multiple metastases in the liver, Stage Ⅳb (National Comprehensive Cancer Network guidelines version 2. 2018). A 5-fluorouracil, leucovorin, and oxaliplatin (mFOLFOX6) + panitumumab (Pmab) regimen was successfully administered and led to decreased tumor marker levels; oral intake also became possible. Additional examinations showed that the primary lesion and distant metastatic lesions had almost disappeared; the patient had achieved a near complete response (CR). Currently, 35 cycles of mFOLFOX6+Pmab have been administered, and his near CR has been maintained for 32 months.
Best supportive care (BSC) is the recommended option for elderly patients with advanced unresectable colon cancer. This is the first case in which an elderly patient with poor PS and advanced unresectable colorectal cancer was treated with combination chemotherapy of mFOLFOX6 + Pmab.
Although the use of chemotherapy for elderly with advanced unresectable colorectal cancer or those with poor PS is limited, this case shows that systemic chemotherapy is now an option for such cases previously managed with BSC.
1. Introduction
Initiation of chemotherapy is difficult in patients with poor performance status (PS), advanced metastatic lesion, and unresectable colon cancer, and best supportive care (BSC) is the standard treatment of choice in these patients. However, advancements in anti-cancer drugs have been remarkable, and several case reports have demonstrated that if anti-cancer drugs are properly selected and complications are prevented, chemotherapeutic drugs may have substantial effects [ 1 ].
This is a report of a patient with many adverse factors: old age, advanced unresectable colon cancer with poor PS, and advanced liver metastases.
The work in this case has been reported in line with the SCARE criteria [ 2 ].
2. Presentation of case
An 80-year-old man presented with complaints of general malaise, loss of appetite, and weight loss. A full physical examination revealed occlusive cancer of the descending colon with liver metastasis. Two hospitals recommended BSC. However, the patient had a strong desire to undergo anti-cancer treatment.
Upon admission, he weighed 47.9 kg, had a height of 170 cm, and had an Eastern Cooperative Oncology Group (ECOG) PS 3. The alkaline phosphatase (937 U/L), lactate dehydrogenase (1190 U/L), and γ-glutamyltransferase (494 U/L) levels were so elevated. Carcinoembryonic antigen (921 ng/mL) and CA19-9 (32,963 U/mL) tumor markers were also elevated.
Abdominal contrast-enhanced computed tomography scan revealed advanced descending colon cancer and swelling of the paracolic lymph nodes. Numerous masses in the liver were found. No peritoneal seeding was observed ( Fig. 1 ). Colonoscopy revealed circumferential type 2 lesions in the descending colon. Histopathological examination of the biopsy sample revealed highly-to-moderately differentiated wild-type KRAS adenocarcinoma.

The occlusion of the descending colon improved, and no malignant findings were observed in the biopsy. CT scan also showed marked decrease in lesions, particularly, liver metastasis.
Upon admission, after instituting fasting and parenteral nutrition and confirming improved large intestine obstruction symptoms, mFOLFOX6 + Pmab were administered. mFOLFOX6 (80% dose) was started 7 days later and mFOLFOX6(80% dose) + Pmab (100% dose) at 21 days. The treatment led to decreased tumor marker levels and enabled the patient to resume oral intake after five cycles completed. A follow-up computed tomography scan and colonoscopy performed 192 days after examination confirmed near CR (per Response Evaluation Criteria in Solid Tumors criteria). At 2 years and 8 months after the examination, he completed a total of 35 cycles and maintained the near CR status. The patient also developed mild cholangitis during that period, which was treated with antibiotics. Furthermore, a central venous port infection was observed; hence, the port was changed. No other serious adverse events were observed. The use of mFOLFOX6 + Pmab in this patient led to near CR that has been maintained without surgical treatment at the time of writing this report at 32 weeks after treatment ( Fig. 2 ).

Clinical course.
3. Discussion
According to the National Comprehensive Cancer Network guidelines, anti-cancer drugs selected for advanced, unresectable, and recurrent colorectal cancer can differ based on the indication for intensive therapy [ 3 ]. In general, BSC is the recommended option for elderly patients with advanced unresectable colon cancer who have risk factors, poor PS, and multiple distant metastases. Sargent et al. [ 4 ]. analyzed the actual PS and the results of first-line treatment against metastatic colorectal cancer and described the need for new approaches due to a significantly lower progression-free survival, overall survival (OS), and success rates in patients with PS 2 cancer compared to those with PS 0 or PS 1. They also reported high rates of adverse events and 60-day mortality among patients with grade 3 or higher.
PubMed was searched using the following keywords: ‘metastatic colon cancer,’ ‘elderly’, ‘poor performance status’, and ‘liver dysfunction’, which yielded a total of four case reports on elderly patients (age >65 years) with advanced colorectal cancer with liver metastasis and underwent chemotherapy with a PS of 2 or higher ( Table 1 ) [ [5] , [6] , [7] , [8] ].
Literature review.
| Patient 1 | Patient 2 | Patient 3 | Patient 4 | Present case | |
|---|---|---|---|---|---|
| Age (years), sex | 72, M | 71, F | 74, F | 67, F | 80, M |
| ECOG performance status | 3 | 3 | 3 | 2 | 3 |
| Cancer site | Sigmoid colon | Sigmoid colon | Ascending colon | Ascending colon | Descending colon |
| Pathology | Adenocarcinoma | Adenocarcinoma | Neuroendocrine carcinoma | – | Adenocarcinoma |
| Metastatic site | Liver | Liver | Liver | Liver | Liver |
| Prior chemotherapy | Cmab Cmab + FOLFOX | HAI of 5-FU mFOLFOX + Bev | Cisplatin/Irinotecan | Irinotecan + Cmab | mFOLFOX6 mFOLFOX6 + Pmab |
| Reason for choice of chemotherapy | Absence of liver disorder | Improvement of PS with topical therapy | Poor PS | Poor PS | Poor PS Absence of liver disorder |
| Total bilirubin (mg/dL) | 6.2 | 0.4 | 1.1 | – | 0.9 |
| Aspartate transaminase (U/L) | 258 | 51 | 79 | – | 86 |
| Alanine transaminase (U/L) | 98 | 22 | 28 | – | 75 |
| Alkaline phosphatase (IU/L) | 2,085 | 1,028 | 1,679 | – | 937 |
| KRAS status | Wild | – | – | Wild | Wild |
| CEA (ng/mL) | 894.9 | 1,408.8 | – | 976.4 | 921 |
| Drop in bilirubin level | Yes | – | – | – | No |
| Drop in CEA level | Yes | – | – | Yes | Yes |
| Maximal toxicity, grade | Skin toxicity, G2 | Hand-foot syndrome, G3 | Neutropenia, G3 | Diarrhea, G2 | Rash, G1 |
| Survival (months) | 18.0 | 29.0 | 8.0 | 8.0 | 32.0 |
Abbreviations: M, male; F, female; ECOG, Eastern Cooperative Oncology Group; Cmab, cetuximab; HAI, hepatic arterial infusion; Bev, bevacizumab; Pmab, panitumumab; mFOLFOX6, modified 5-FU + leucovorin + oxaliplatin.
Decrease in bilirubin or CEA levels represents a 50% decrease in serum total bilirubin or CEA levels. The bar indicates that the measurement is not described in this paper.
Modified from: Elsoueidi R, Craig J, Mourad H, Richa E. Safety and efficacy of FOLFOX followed by cetuximab for metastatic colorectal cancer with severe liver dysfunction. J Natl Compr Canc Netw. 2014; 12:155–160. https://doi.org/10.6004/jnccn.2014.0016 .
In one of the four cases, systematic therapy performed after hepatic arterial infusion resulted in improved liver function. In that case, the primary lesion was resected first; however, his PS became poor in the postoperative period. In two of the four cases, cetuximab was used (in combination), while the amount of cisplatin/irinotecan was reduced by 50% in one of the four cases, which was a case of primary small cell carcinoma in the large intestine.
This is the first case in which an elderly patient with poor PS and advanced unresectable colorectal cancer was treated with combination chemotherapy of Pmab. Chan et al. [ 9 ] studied 1013 young (<70 years) and elderly (≥70 years) patients with metastatic colon cancer with PS 0–1 administered with systemic therapy. The results of their analysis showed no significant difference in OS durations between the young and elderly.
Additionally, Naeim et al. [ 1 ] analyzed the combined use of capecitabine and bevacizumab treatment in frail (ECOG PS 2) or elderly patients with ECOG PS 1, and several patients with PS 2 achieved an overall response rate equivalent to those treated with fluorouracil (5-FU) + bevacizumab. This result also suggests the potential efficacy of using chemotherapy in elderly patients with poor PS.
Moreover, Fleming et al. [ 10 ] showed that combined therapy using a continuous infusion of 5-FU and leucovorin could be safely performed even in patients with jaundice, and liver function is also thought to not affect the pharmacokinetics of oxaliplatin. Moreover, the NCCN guidelines [ 3 ] recommend single administration of Pmab as the first-line treatment in patients with poor general condition; however, no studies on the onset of serious liver dysfunction were conducted based on this recommendation [ 11 ], leading to a conclusion that single administration of Pmab could also be a treatment option in patients who cannot be administered with other standard treatments due to liver dysfunction.
When planning the treatment for the present patient, the following information was considered. Based on the reports by Chan et al. [ 9 ], the systematic chemotherapy was expected to extend the OS in elderly patients. Naeim et al.’s [ 1 ] report suggested that performing systematic chemotherapy, even in cases of poor PS, may improve the OS and overall response rate. Fleming et al. [ 10 ] and Van Cutsem et al. [ 11 ] indicated that oxaliplatin, Pmab, or combined treatment of continuous 5-FU infusion and leucovorin can be used, even in patients with impaired liver function. For the initial administration, the dosage of mFOLFOX6 (80% dose) was reduced and the absence of major adverse events was confirmed. Then, we began a combined treatment with mFOLFOX6 (80% dose) + Pmab (100% dose). Because the patient progressed with no major side effects, the 35-cycle regimen was continued until his condition worsened to PS 4.
Although reports of using chemotherapy to treat advanced unresectable colorectal cancer in elderly patients or those with poor PS are limited, the information obtained in this case led us to conclude that systemic therapy was an option. Future accumulation of cases that use this therapy will lead to a comparison of the associated results with those of traditional therapies, such as BSC.
4. Conclusion
We encountered a case of near CR that was maintained long-term after using mFOLFOX6 (80% dose) + Pmab (100% dose) in an elderly patient with poor PS. The advanced unresectable colorectal cancer was accompanied by stricture, and multiple liver metastases were accompanied by elevated hepatobiliary system enzymes. Although BSC would typically have been chosen for this patient, chemotherapy was attempted, and long-term survival without requiring surgery was achieved. This suggests that advances in chemotherapy have made it possible to consider aggressive treatment as an option for severe advanced colorectal cancer that was managed with BSC in the past.
Conflict of interest
We have nothing to declare in any categories.
Sources of funding
This research does not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Ethical approval
On Ethical Guidelines for Medical and Health Research Involving Human Subjects, case report is not classified in the research study in Japan.
Written informed consent was obtained from the patient’s family for publication of this case report and accompanying images. The patient is currently in poor general condition due to pneumonia and cannot show consent. A copy of the written consent is available for review by the Editor-in-Chief of this journal on request”.
Author contribution
Yoshiaki Kanemoto: conceptualization, validation, investigation, writing-original draft, writing-review & editing, visualization.
Giichiro Tsurita: conceptualization, methodology, writing-review & editing, supervision, project administration.
Tomohiro Kurokawa: conceptualization, methodology, validation, writing-review & editing, supervision.
Yuki Azuma: supervision.
Kentaro Yazawa: supervision.
Yoshinori Murakami: supervision.
Registration of research studies
researchregistry4664.
Giichiro Tsurita.
Provenance and peer review
Not commissioned, externally peer-reviewed.
Acknowledgments
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Watch CBS News
Cancer deaths among men predicted to increase 93% by 2050, study finds
By Sara Moniuszko
Edited By Allison Elyse Gualtieri
Updated on: August 12, 2024 / 7:41 PM EDT / CBS News
Cancer cases and deaths among men are expected to surge globally by 2050, according to a new study.
In the study , published Monday in Cancer, a peer-reviewed journal of the American Cancer Society, researchers projected an 84% increase in cancer cases and a 93% increase in cancer deaths among men worldwide between between 2022 and 2050.
The increases were greater among men 65 and older and in countries and territories with a low or medium human development index. The index measures each country's development in health, knowledge and standard of living, according to the study.
Using data from the Global Cancer Observatory, the study analyzed more than 30 different types of cancers across 185 countries and territories worldwide to make demographic projections.
"We know from previous research in 2020 that cancer death rates around the world are about 43% higher in men than in women," said CBS News chief medical correspondent Dr. Jon LaPook. "So this study today looked at, OK, what do we expect over the next 25 years? And it turns out that it translates to about 5 million more deaths per year in men in 2050, compared to today."
This isn't the first study to paint a less-than-optimistic outlook at the future of cancer case numbers.
Earlier this year, the World Health Organization predicted we will see more than 35 million new cancer cases by 2050, a 77% increase from the estimated 20 million cases in 2022. The survey looked at both men and women in 115 countries.
The organization pointed to several factors behind the projected global cancer increase, including:
- Population aging and growth
- Changes to people's exposure to risk factors, with air pollution a key driver of environmental risk factors
- Tobacco and alcohol use
In the latest study, authors also pointed to smoking and alcohol consumption as modifiable risk factors prevalent among men.
"By far, not smoking is the single most important thing" people can do do reduce their risk, LaPook said.
Other factors that may help explain why men face higher rates of cancer compared to women include lower participation in cancer prevention activities and underuse of screening and treatment options, the study authors said.
Improving access to cancer prevention, screening, diagnosis and treatment options, especially for older men, could help improve cancer outcomes, lead author Habtamu Mellie Bizuayehu said in a news release .
Sara Moniuszko is a health and lifestyle reporter at CBSNews.com. Previously, she wrote for USA Today, where she was selected to help launch the newspaper's wellness vertical. She now covers breaking and trending news for CBS News' HealthWatch.
More from CBS News

Biden touts Cancer Moonshot, announcing $150 million in new funding
Mpox outbreaks in Africa are a global health emergency, WHO declares

Joro spiders seem to know how to stay chill in big cities, study finds

Whooping cough is spreading nationwide. Here's what to know.
- Study protocol
- Open access
- Published: 05 August 2024
A pragmatic, stepped-wedge, hybrid type II trial of interoperable clinical decision support to improve venous thromboembolism prophylaxis for patients with traumatic brain injury
- Christopher J. Tignanelli ORCID: orcid.org/0000-0002-8079-5565 1 , 2 , 3 , 4 ,
- Surbhi Shah 5 ,
- David Vock 6 ,
- Lianne Siegel 6 ,
- Carlos Serrano 6 ,
- Elliott Haut 7 ,
- Sean Switzer 8 ,
- Christie L. Martin 9 ,
- Rubina Rizvi 2 , 3 ,
- Vincent Peta 1 ,
- Peter C. Jenkins 10 ,
- Nicholas Lemke 1 ,
- Thankam Thyvalikakath 11 , 12 ,
- Jerome A. Osheroff 13 ,
- Denise Torres 14 ,
- David Vawdrey 15 ,
- Rachael A. Callcut 16 ,
- Mary Butler 3 , 17 &
- Genevieve B. Melton 1 , 2 , 3
Implementation Science volume 19 , Article number: 57 ( 2024 ) Cite this article
274 Accesses
2 Altmetric
Metrics details
Venous thromboembolism (VTE) is a preventable medical condition which has substantial impact on patient morbidity, mortality, and disability. Unfortunately, adherence to the published best practices for VTE prevention, based on patient centered outcomes research (PCOR), is highly variable across U.S. hospitals, which represents a gap between current evidence and clinical practice leading to adverse patient outcomes.
This gap is especially large in the case of traumatic brain injury (TBI), where reluctance to initiate VTE prevention due to concerns for potentially increasing the rates of intracranial bleeding drives poor rates of VTE prophylaxis. This is despite research which has shown early initiation of VTE prophylaxis to be safe in TBI without increased risk of delayed neurosurgical intervention or death. Clinical decision support (CDS) is an indispensable solution to close this practice gap; however, design and implementation barriers hinder CDS adoption and successful scaling across health systems. Clinical practice guidelines (CPGs) informed by PCOR evidence can be deployed using CDS systems to improve the evidence to practice gap. In the Scaling AcceptabLE cDs (SCALED) study, we will implement a VTE prevention CPG within an interoperable CDS system and evaluate both CPG effectiveness (improved clinical outcomes) and CDS implementation.
The SCALED trial is a hybrid type 2 randomized stepped wedge effectiveness-implementation trial to scale the CDS across 4 heterogeneous healthcare systems. Trial outcomes will be assessed using the RE 2 -AIM planning and evaluation framework. Efforts will be made to ensure implementation consistency. Nonetheless, it is expected that CDS adoption will vary across each site. To assess these differences, we will evaluate implementation processes across trial sites using the Exploration, Preparation, Implementation, and Sustainment (EPIS) implementation framework (a determinant framework) using mixed-methods. Finally, it is critical that PCOR CPGs are maintained as evidence evolves. To date, an accepted process for evidence maintenance does not exist. We will pilot a “Living Guideline” process model for the VTE prevention CDS system.
The stepped wedge hybrid type 2 trial will provide evidence regarding the effectiveness of CDS based on the Berne-Norwood criteria for VTE prevention in patients with TBI. Additionally, it will provide evidence regarding a successful strategy to scale interoperable CDS systems across U.S. healthcare systems, advancing both the fields of implementation science and health informatics.
Trial registration
Clinicaltrials.gov – NCT05628207. Prospectively registered 11/28/2022, https://classic.clinicaltrials.gov/ct2/show/NCT05628207 .
Contributions to the Literature
This paper provides a study protocol for a new and novel stepped wedge study variation which includes external control sites to take into account external influences on the uptake of traumatic brain injury guidelines nationally
This paper provides a study design for one of the largest trauma pragmatic trials in the U.S. of 9 heterogenous hospitals
This study is also unique and first-in-kind feature as the guideline may change over time during the study due to the “living” nature of the guideline being implemented.
Introduction
Venous thromboembolism (VTE) is a preventable complication of traumatic brain injury (TBI), which has a substantial impact on patient morbidity, mortality, disability. It is also associated with significant economic burden > $1.5 billion per year [ 1 , 2 ]. VTE is considered a preventable medical condition in the majority of cases [ 2 , 3 ]. Unfortunately, adherence with patient centered outcomes research (PCOR)-informed VTE prevention best practices is highly variable and often poor across U.S. hospitals. Compliance with best practice is especially relevant in the case of TBI as 54% of TBI patients will develop a VTE if they do not receive appropriate anticoagulation [ 4 ]. The delivery of appropriate VTE prophylaxis to TBI patients is such an important quality measure that adherence is tracked nationally and benchmarked by the American College of Surgeons Trauma Quality Improvement Program (ACS-TQIP) [ 5 ]. We have previously shown that instituting a hospital-wide VTE prevention initiative modeled after the Berne-Norwood criteria for VTE prophylaxis in TBI was associated with significantly increased compliance with VTE-related process and improved outcome metrics [ 6 ]. Specifically, we observed improved adherence with the Berne-Norwood criteria [ 7 , 8 ], reduced time to initiation of VTE prophylaxis, and reduced VTE events [ 9 ]. Multiple studies have shown that VTE prophylaxis in trauma patients not only reduces VTE events, but also significantly reduces mortality [ 10 ]. We noted the same reduction in mortality for TBI patients following the initiation of a VTE prophylaxis guideline for patients with TBI [ 11 ]. Unfortunately, despite widely published PCOR-informed best practice, nationally there is reluctance to initiate VTE prevention due to concerns for progression of intracranial hemorrhage. This is despite research which has shown early initiation of VTE prophylaxis to be safe in TBI without increased risk of delayed neurosurgical intervention or death [ 12 , 13 , 14 , 15 , 16 ].
Since approximately 40% of TBI patients do not receive DVT prophylaxis in a timely manner, there is a critical and timely need to close the gap between current PCOR evidence and clinical practice. [ 17 , 18 , 19 , 20 , 21 , 22 , 23 ]. Clinical decision support (CDS) systems are an indispensable solution to close this practice gap; however, design and implementation barriers hinder CDS adoption [ 24 , 25 ]. Another significant challenge to the implementation of CDS is that health information technology (IT) needs a common language for PCOR evidence to translate it into practice across multiple organizations [ 26 ]. Because of these challenges, we will deploy CDS using fast healthcare interoperability resources (FHIR) standards to rapidly implement PCOR evidence into practice [ 27 , 28 ]. We hypothesize that, FHIR standards will reduce CDS development and maintenance costs, increase PCOR uptake in rural and other underserved sites, and speed the development timeline to build a comprehensive suite of CDS for PCOR evidence [ 29 ].
Few studies have investigated specific barriers to and facilitating factors for adoption of interoperable FHIR-based CDS [ 30 ]. For example, many current studies investigating barriers and facilitators for interoperable CDS are limited to expert opinion [ 30 , 31 ] or lack a formal implementation science framework-guided investigation [ 32 , 33 ]. Barriers to and facilitating factors for adoption of interoperable CDS following real-life implementation and multicenter scaling guided by validated implementation science frameworks should be rigorously investigated. This study will facilitate comprehensive exploration of clinician and environmental (internal and external) contextual elements that influence interoperable CDS implementation success. In this study, we will scale and assess the effectiveness of a CDS system for a VTE prophylaxis guideline in patients with TBI and evaluate implementation across 9 sites within 4 U.S. trauma systems.
Study aims and implementation framework
This trial consists of a stepped wedge hybrid effectiveness-implementation trial to scale the CDS system across 4 trauma systems and in parallel evaluate implementation strategy guided by the Exploration, Preparation, Implementation, and Sustainment (EPIS) implementation framework (Fig. 1 a) [ 34 ]. We anticipate variability in CDS adoption across sites during the implementation trial. This variation represents a unique opportunity to study implementation at each site and understand what strategies, system factors, and engagement of specific stakeholders are associated with improved CDS adoption. We will rigorously evaluate each implementation phase, guided by The EPIS Implementation Framework [ 34 ], our determinant framework (Fig. 1 b). We will apply the EPIS framework to guide assessment of implementation phases, barriers, and facilitators (Fig. 2 ) [ 34 ]. EPIS comprises 16 constructs over 4 domains (outer context, inner context, bridging factors, and innovation factors). We selected EPIS as our determinant framework as it includes clearly delineated implementation stages and allows for examination of change at multiple levels, across time, and through phases that build toward implementation. While EPIS was initially developed for implementation in public service, it has since been translated to healthcare, especially for complex multi-institutional healthcare interventions [ 34 , 35 , 36 ].
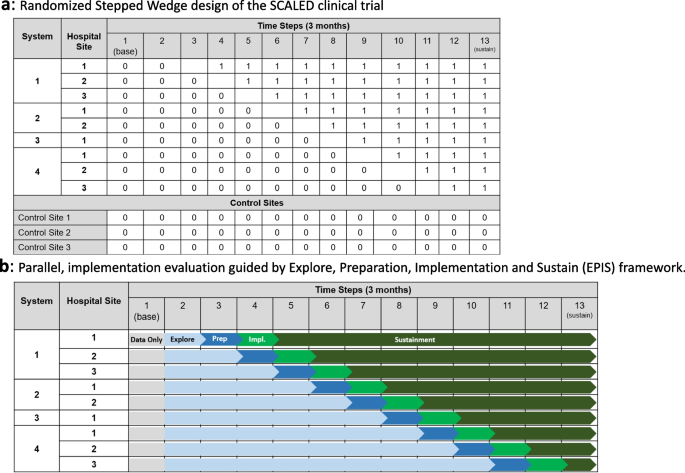
a Randomized Stepped Wedge design of the SCALED clinical trial. b Parallel, implementation evaluation guided by Explore, Preparation, Implementation and Sustain (EPIS) framework
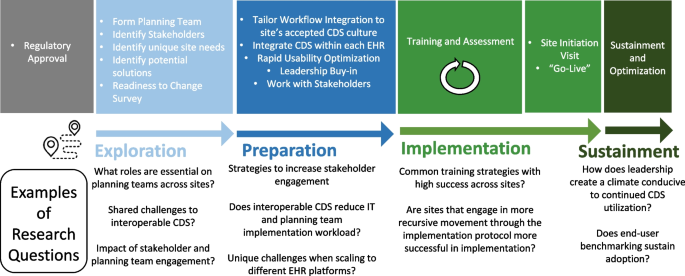
Implementation evaluation across study sites
Trial overview, setting, and inclusion/exclusion criteria
This trial will be conducted at 4 healthcare systems with 1–3 hospitals per system and is projected to occur over a 3 to 4-year period. The trial uses a randomized stepped-wedge design to scale an interoperable CDS system for the Berne-Norwood TBI CPG. Figure 1 a provides a schematic for the trial design. The order of health systems and sites will be randomly determined. This study will include a heterogeneous number of hospitals by trauma verification status, electronic health record (EHR) platform, bed size, and setting (Table 1 ). Our target population is adult patients admitted with an acute TBI defined as International Classification of Disease 10 Clinical Modification (ICD-10-CM): S06.1 – S06.9 or S06.A. Patients who die within 24 h of hospital admission and patients documented as “comfort cares” during the first 72 h of hospitalization will be excluded, as they would have a limited opportunity to receive adherence with the Berne-Norwood criteria. Additionally, patients with a pre-existing VTE or inferior vena cava (IVC) filter at the time of admission, and patients with a mechanical heart valve or ventricular assist device will be excluded from final analysis.
This study will also include up to 3 control sites (Fig. 1 a), a feature not typically included with historic stepped-wedge trial designs, which will strengthen our ability to understand external influences on the study findings. These control sites, which do not receive the CDS intervention and do not have any planned initiatives around guideline implementation, will allow the study to assess baseline adherence and variation in clinical practice over the study period.
CDS Intervention
TBI diagnosis upon admission will activate an interoperable CDS system leveraging the Stanson Health (Charlotte, NC) CDS platform [ 37 ], which is being expanded to include interoperable offerings for TBI VTE prophylaxis. This system provides a knowledge representation framework to faithfully express the intent of the Berne-Norwood prevention criteria computationally (Table 2 ). The interoperable FHIR data standard will be used for bi-directional data transfer between each site’s EHR and the CDS platform. Workflow integration includes a combination of both passive and interruptive provider and trauma system leader information and “nudges”. Table 2 represents the Standards-based, Machine-readable, Adaptive, Requirements-based, and Testable (SMART) L2 layer [ 38 ] of the Berne-Norwood criteria.
CDS user-centered design
We will complete a rapid cycle CDS evaluation to optimize CDS workflow integration by conducting a user-driven simulation and expert-driven heuristic usability optimization as we have previously done [ 39 ]. For rapid cycle CDS evaluation, multidisciplinary trauma end-user “teams” will complete up to 3 scenarios designed to represent various extremes in TBI VTE prevention decision making. Simulation usability testing will be overseen by usability experts, who will catalogue usability issues that arise during simulation. Via consensus ranking, the development and planning teams will rank usability issues from 0 (cosmetic) to 5 (usability catastrophe). Using 10 predefined heuristics for usability design [ 40 ], we will conduct a heuristic evaluation of the CDS, then catalogue and rank usability issues. These results will inform CDS application design, optimized for TBI workflow integration.
Implementation strategy
Following CDS development, our healthcare system relies on a time-tested approach for the implementation and scaling of user-centered CDS: this approach is called the Scaling AcceptabLE cDs (SCALED) Strategy [ 41 ]. This framework integrates multiple evidence-based implementation strategies (Table 3 ).
Study outcomes
The primary implementation outcome is patient-level adherence with the CPG: Specifically, did the patient received guideline-concordant care? Adherence will be measured as an all-or-none measure (binary endpoint at the encounter/patient-level). Thus, if a patient is low-risk for TBI progression, by 24 h they should have risk-specific VTE prevention ordered; if they receive this after 24 h, or if they receive the intermediate risk VTE prevention regimen, this would be deemed non-adherent. The primary effectiveness outcome is VTE (binary endpoint at the patient-encounter level). Safety outcomes evaluated include: TBI progression, in-hospital mortality, and bleeding events. A secondary hypothesis is that as the trial scales to additional sites, iterative implementations will be more efficient (reduced implementation time) and more effective (improved adoption). Secondary hypotheses will be evaluated using the RE 2 -AIM framework [ 42 , 43 ] and are displayed in Table 4 .
Clinical trial data collection methods
Data sources used in this trial include the Stanson Health CDS eCaseReport and site trauma registry. The eCaseReport is a living registry of all patients, and their associated clinical trial data elements, that were eligible for the CDS. All sites also maintain a trauma registry adhering to the National Trauma Data Standards [ 44 ], a requirement for ACS trauma center verification. This dataset is manually annotated by trained clinical abstractors. Data will be sent to the biostatistical team at 6-month intervals. Control and pre-implementation sites will provide their trauma registry in addition to supplemental standards-based EHR extraction of clinical trial data elements or manual abstraction. A data dictionary has been created for the study and will be made available on the trial webpage.
Multiple methods evaluation of implementation success at each EPIS phase
Survey instruments will be prepared using Likert-type scales. Outcomes will be calculated based on scoring guides for the following validated scales: Program Sustainability Assessment Tool (PSAT) [ 45 ], Clinical Sustainability Assessment Tool (CSAT) [ 46 ], Implementation Leadership Scale (ILS) [ 47 ], and Evidenced-based Practice Attitude Scale-36 (EBPAS-36) [ 48 ]. Two scales do not have scoring rubrics: the Organizational Readiness for Change Questionnaire [ 49 , 50 ] and the Normalization Measure Development (NoMAD) Questionnaire [ 51 , 52 , 53 ]. Since both of these scales group questions into constructs, they will be analyzed by generating mean Likert scores and standard deviations per construct, and a mean across constructs, at each of the four implementation phases [ 54 ].
To deeply investigate barriers and facilitators of successful implementation, semi-structured qualitative interviews of key personnel (clinical leadership and end-users, IT leadership and staff) will be conducted at each of the 4 implementation phases. Studies suggest saturation of new ideas occurs after approximately 12 interviews [ 55 ]. Additional samples will be added as needed if thematic saturation is not achieved. Following informed consent, interviews will be performed by a trained qualitative research assistant, audio recorded, and transcribed verbatim. An interview guide, informed by the EPIS framework, was developed to collect key informant experiences with CDS implementation with a focus on inner and outer context factors [ 56 ]. A hybrid approach, primarily deductive and secondarily inductive, approach will be applied. All interviews will be independently double-coded and coding discrepancies will be resolved through discussion. A descriptive thematic analysis approach [ 57 ] will be used to characterize the codes into themes and sub-themes representing the barriers and facilitators to implementation success.
Results for all instruments will be primarily stratified according to site implementation success at each study phase. Additional stratifications may include respondent role, discipline, and hospital system. Bar charts displaying mean survey domains with integrative quotations from the qualitative analysis will be used to facilitate data visualization and understanding of key themes representing barriers and facilitators to successful CDSS implementation.
Statistical analysis
Mixed-effects logistic regression models will be fit to test whether or not CDS implementation changes the likelihood of a VTE event during TBI admission (effectiveness outcome) and the likelihood that the clinical guideline was followed (implementation outcome). The models for these outcomes include fixed-effects for month (when available, to account for secular trends) and an indicator variable for whether the center had the CDS integrated in the EHR. The primary test statistic will be a Wald test of the coefficient for this treatment indicator. We will include random center-specific intercepts to account for correlation within center. Assuming there are 9 sites enrolled with an average of 400 TBI admissions per year and the typical site has between 20%-40% adherence to the clinical guidelines, we will have > 80.0% and > 99.9% power to detect a 5 and 10 percentage point increase in the adherence. Similarly, assuming the typical site has between a VTE event rate of 5–6%, we will have > 80.0% power to detect a 40%-50% reduction in VTE consistent with our published data [ 11 ].
Study oversight
This study is overseen by the University of Minnesota Surgical Clinical Trials Office and by an independent Data Safety Monitoring Board (DSMB). Even though this intervention is deploying a TBI clinical guideline that is currently considered best practice, we believe the addition of a DSMB will improve trial safety, data quality, and trial integrity [ 58 ]. DSMB membership will be independent from the study investigators and will consist of 3 members including: 1 trauma surgeon, 1 informaticist, and 1 statistician. Annual reports including data from all sites, including control sites, will be shared with the DSMB to assure timely monitoring of safety and data quality. The trial will not be stopped early in the event of CDS efficacy because a critical secondary outcome focuses on studying implementation and effectiveness over time.
VTE guideline monitoring and maintenance
Given the potential for a changing evidence-base, it is possible that best practice VTE prevention guidance may change during the study period or afterwards. A critical element in improving adherence with PCOR evidence is updating guidance based on this evidence – in this study, this requires ensuring that the CDS system remains current.
We will pilot a model for producing and maintaining TBI VTE prophylaxis 'Living Guidance and CDS' to ensure that the CDS remains current (Fig. 3 ). The University of Minnesota Evidence-based Practice Center (EPC) Evidence Generation team will conduct and maintain a “living” systematic review. Systematic review data will be uploaded to the AHRQ’s Systematic Review Data Repository (SRDR). “Living” implies that every 6 months the EPC team will evaluate and synthesize new evidence related to TBI VTE prophylaxis, update the existing systematic review and deliver it to a multi-stakeholder Guideline Committee. The Guideline Committee will then use the GRADE (Grading of Recommendations, Assessment, Development and Evaluations) evidence-to-decision (EtD) framework to develop VTE prophylaxis guidelines for patients with TBI [ 59 , 60 , 61 ]. A computational representation of these guidelines will be updated and maintained within the CDS platform by Stanson Health, the CDS Vendor.
Pilot process for “Living Guideline”
Spreading successful results beyond study sites
The ultimate goal of this study is to spread successful CDS tools and strategies to broadly improve TBI VTE-related care processes and outcomes. The research outlined above will surface sharable insights about what information needs to be presented to which people in what formats through what channels at what times to reliably deliver guideline-based care – i.e., specific instantiations of the “CDS 5 Rights Framework” applied to this target [ 62 ]. We will use Health Service Blueprint tools to describe our recommended implementation approaches; these tools are being applied in an increasing number of public and private care delivery organizations as a structured approach to ‘get the CDS 5 Right right’ for various improvement targets. We will further adapt and apply Health Service Blueprint foundations supported by VA and AHRQ [ 63 ] to capture VTE care transformation guidance in Health Service Blueprint tooling [ 64 ]. Presenting recommended CDS-enabled workflow, information flow – as well as and related implementation considerations and broader healthcare ecosystem implications – in this structured format will help organizations beyond the initial study participants put study results into action efficiently and effectively.
In this paper, we present the protocol for the SCALED trial, a stepped-wedge cluster randomized trial of a CDS intervention to improve adherence with VTE prevention best practices for patients with TBI. As a hybrid type 2 trial, this study will evaluate both implementation and effectiveness outcomes. In addition to investigating effectiveness, we will also be able to provide insight into the implementation challenges for deploying interoperable CDS across heterogenous health systems. In our pilot study [ 9 ], while patients who received guideline-concordant care had significantly improved outcomes, we noted that not all patients receive guideline concordant care following implementation. Additionally, best strategies for scaling interoperable CDS systems are poorly studied. Thus, this study represents one of the earliest implementation evaluations of scaling interoperable CDS systems across heterogeneous health systems.
This study has several strengths. First, it will rigorously test implementation of a CPG for VTE prevention across 9 U.S. trauma centers using a multi-faceted CDS platform supporting both passive and interruptive decision support. Second, it will rigorously investigate scalable and interoperable CDS strategies to deploy CPGs. Third, this study leverages a centralized eCaseReport generated by the CDS system, a solution which can drive data collection for future pragmatic trials. Importantly, this study takes place at trauma centers which are geographically distinct, utilize different EHR vendors, include both ACS-verified level 1 through level 3 trauma centers, and include rural, community, and university-based trauma centers. In addition to helping spread recommended care transformation strategies beyond additional study sites, documenting these approaches in Health Service Blueprint tools will also support creation of learning communities for sharing, implementing, and enhancing these strategies.
This study also has limitations. First, we are only investigating 4 trauma systems which already have fairly advanced informatics divisions and experience implementing interoperable CDS systems. Thus, these findings may not be broadly applicable to health systems with less informatics experience and expertise. Second, we are only investigating implementation across two EHR vendors: Epic and Cerner, thus these findings may not be applicable to health systems with different EHR vendors such as Meditech or Allscripts. However, the Health Service Blueprint implementation strategy representations should still enable users of other systems to glean valuable insights about components of the transformation approach less dependent on specific EHRs used.
In summary, this study will implement and scale a CDS-enabled care transformation approach across a diverse collaborative CDS community, serving as an important demonstration of this critical healthcare challenge. We will integrate lessons learned for a planned national scaling in collaboration with U.S. trauma societies. Finally, we will pilot an approach for the “Living Guideline” and use that to maintain evidenced-based decision logic within CDS platforms.
Availability of data and materials
Following trial completion data will be made available upon request through the University of Minnesota Data Repository.
Heit JA. Venous thromboembolism: disease burden, outcomes and risk factors. J Thromb Haemost. 2005;3(8):1611–7.
Article CAS PubMed Google Scholar
Yorkgitis BK, Berndtson AE, Cross A, Kennedy R, Kochuba MP, Tignanelli C, Tominaga GT, Jacobs DG, Marx WH, Ashley DW, Ley EJ, Napolitano L, Costantini TW. American Association for the Surgery of Trauma/American College of Surgeons-Committee on Trauma Clinical Protocol for inpatient venous thromboembolism prophylaxis after trauma. J Trauma Acute Care Surg. 2022;92(3):597–604.
Article PubMed Google Scholar
Nicholson M, Chan N, Bhagirath V, Ginsberg J. Prevention of Venous Thromboembolism in 2020 and Beyond. J Clin Med. 2020;9(8):2467.
Article CAS PubMed PubMed Central Google Scholar
Geerts WH, Code KI, Jay RM, Chen E, Szalai JP. A prospective study of venous thromboembolism after major trauma. N Engl J Med. 1994;331(24):1601–6.
Nathens AB, Cryer HG, Fildes J. The American College of Surgeons Trauma Quality Improvement Program. Surg Clin North Am. 2012;92(2):441–54, x−xi.
Ingraham NE, Lotfi-Emran S, Thielen BK, Techar K, Morris RS, Holtan SG, Dudley RA, Tignanelli CJ. Immunomodulation in COVID-19. Lancet Respir Med. 2020;8(6):544–6.
Phelan HA, Eastman AL, Madden CJ, Aldy K, Berne JD, Norwood SH, Scott WW, Bernstein IH, Pruitt J, Butler G, Rogers L, Minei JP. TBI risk stratification at presentation: a prospective study of the incidence and timing of radiographic worsening in the Parkland Protocol. J Trauma Acute Care Surg. 2012;73(2 Suppl 1):S122–7.
Pastorek RA, Cripps MW, Bernstein IH, Scott WW, Madden CJ, Rickert KL, Wolf SE, Phelan HA. The Parkland Protocol’s modified Berne-Norwood criteria predict two tiers of risk for traumatic brain injury progression. J Neurotrauma. 2014;31(20):1737–43.
Article PubMed PubMed Central Google Scholar
Tignanelli CJ, Gipson J, Nguyen A, Martinez R, Yang S, Reicks PL, Sybrant C, Roach R, Thorson M, West MA. Implementation of a Prophylactic Anticoagulation Guideline for Patients with Traumatic Brain Injury. Jt Comm J Qual Patient Saf. 2020;46(4):185–91.
PubMed Google Scholar
Jacobs BN, Cain-Nielsen AH, Jakubus JL, Mikhail JN, Fath JJ, Regenbogen SE, Hemmila MR. Unfractionated heparin versus low-molecular-weight heparin for venous thromboembolism prophylaxis in trauma. J Trauma Acute Care Surg. 2017;83(1):151–8.
Tignanelli CJ, Silverman GM, Lindemann EA, Trembley AL, Gipson JC, Beilman G, Lyng JW, Finzel R, McEwan R, Knoll BC, Pakhomov S, Melton GB. Natural language processing of prehospital emergency medical services trauma records allows for automated characterization of treatment appropriateness. J Trauma Acute Care Surg. 2020;88(5):607–14.
Kim J, Gearhart MM, Zurick A, Zuccarello M, James L, Luchette FA. Preliminary report on the safety of heparin for deep venous thrombosis prophylaxis after severe head injury. J Trauma. 2002;53(1):38–42; discussion 3.
Cothren CC, Smith WR, Moore EE, Morgan SJ. Utility of once-daily dose of low-molecular-weight heparin to prevent venous thromboembolism in multisystem trauma patients. World J Surg. 2007;31(1):98–104.
Norwood SH, Berne JD, Rowe SA, Villarreal DH, Ledlie JT. Early venous thromboembolism prophylaxis with enoxaparin in patients with blunt traumatic brain injury. J Trauma. 2008;65(5):1021–6; discussion 6-7.
CAS PubMed Google Scholar
Scudday T, Brasel K, Webb T, Codner P, Somberg L, Weigelt J, Herrmann D, Peppard W. Safety and efficacy of prophylactic anticoagulation in patients with traumatic brain injury. J Am Coll Surg. 2011;213(1):148–53; discussion 53-4.
Byrne JP, Mason SA, Gomez D, Hoeft C, Subacius H, Xiong W, Neal M, Pirouzmand F, Nathens AB. Timing of Pharmacologic Venous Thromboembolism Prophylaxis in Severe Traumatic Brain Injury: A Propensity-Matched Cohort Study. J Am Coll Surg. 2016;223(4):621-31e5.
Lau R, Stevenson F, Ong BN, Dziedzic K, Eldridge S, Everitt H, Kennedy A, Kontopantelis E, Little P, Qureshi N, Rogers A, Treweek S, Peacock R, Murray E. Addressing the evidence to practice gap for complex interventions in primary care: a systematic review of reviews protocol. BMJ Open. 2014;4(6): e005548.
Tignanelli CJ, Vander Kolk WE, Mikhail JN, Delano MJ, Hemmila MR. Noncompliance with American College of Surgeons Committee on Trauma recommended criteria for full trauma team activation is associated with undertriage deaths. J Trauma Acute Care Surg. 2018;84(2):287–94.
Robbins AJ, Ingraham NE, Sheka AC, Pendleton KM, Morris R, Rix A, Vakayil V, Chipman JG, Charles A, Tignanelli CJ. Discordant Cardiopulmonary Resuscitation and Code Status at Death. J Pain Symptom Manage. 2021;61(4):770–780.e1.
Tignanelli CJ, Watarai B, Fan Y, Petersen A, Hemmila M, Napolitano L, Jarosek S, Charles A. Racial Disparities at Mixed-Race and Minority Hospitals: Treatment of African American Males With High-Grade Splenic Injuries. Am Surg. 2020;86(5):441–9.
Tignanelli CJ, Rix A, Napolitano LM, Hemmila MR, Ma S, Kummerfeld E. Association Between Adherence to Evidence-Based Practices for Treatment of Patients With Traumatic Rib Fractures and Mortality Rates Among US Trauma Centers. JAMA Netw Open. 2020;3(3): e201316.
Oliphant BW, Tignanelli CJ, Napolitano LM, Goulet JA, Hemmila MR. American College of Surgeons Committee on Trauma verification level affects trauma center management of pelvic ring injuries and patient mortality. J Trauma Acute Care Surg. 2019;86(1):1–10.
Tignanelli CJ, Wiktor AJ, Vatsaas CJ, Sachdev G, Heung M, Park PK, Raghavendran K, Napolitano LM. Outcomes of Acute Kidney Injury in Patients With Severe ARDS Due to Influenza A(H1N1) pdm09 Virus. Am J Crit Care. 2018;27(1):67–73.
Khairat S, Marc D, Crosby W, Al SA. Reasons For Physicians Not Adopting Clinical Decision Support Systems: Critical Analysis. JMIR Med Inform. 2018;6(2): e24.
Jones EK, Ninkovic I, Bahr M, Dodge S, Doering M, Martin D, Ottosen J, Allen T, Melton GB, Tignanelli CJ. A novel, evidence-based, comprehensive clinical decision support system improves outcomes for patients with traumatic rib fractures. J Trauma Acute Care Surg. 2023;95(2):161–71.
Marcos M, Maldonado JA, Martinez-Salvador B, Bosca D, Robles M. Interoperability of clinical decision-support systems and electronic health records using archetypes: a case study in clinical trial eligibility. J Biomed Inform. 2013;46(4):676–89.
FHIR Clinical Guidelines. http://build.fhir.org/ig/HL7/cqf-recommendations/ . Accessed 14 Sep 2021.
Mandel JC, Kreda DA, Mandl KD, Kohane IS, Ramoni RB. SMART on FHIR: a standards-based, interoperable apps platform for electronic health records. J Am Med Inform Assoc. 2016;23(5):899–908.
Goldberg HS, Paterno MD, Rocha BH, Schaeffer M, Wright A, Erickson JL, Middleton B. A highly scalable, interoperable clinical decision support service. J Am Med Inform Assoc. 2014;21(e1):e55-62.
Marcial LH, Blumenfeld B, Harle C, Jing X, Keller MS, Lee V, Lin Z, Dover A, Midboe AM, Al-Showk S, Bradley V, Breen J, Fadden M, Lomotan E, Marco-Ruiz L, Mohamed R, O’Connor P, Rosendale D, Solomon H, Kawamoto K. Barriers, Facilitators, and Potential Solutions to Advancing Interoperable Clinical Decision Support: Multi-Stakeholder Consensus Recommendations for the Opioid Use Case. AMIA Annu Symp Proc. 2019;2019:637–46.
Lomotan EA, Meadows G, Michaels M, Michel JJ, Miller K. To Share is Human! Advancing Evidence into Practice through a National Repository of Interoperable Clinical Decision Support. Appl Clin Inform. 2020;11(1):112–21.
Dolin RH, Boxwala A, Shalaby J. A Pharmacogenomics Clinical Decision Support Service Based on FHIR and CDS Hooks. Methods Inf Med. 2018;57(S 02):e115–23.
Dorr DA, D’Autremont C, Pizzimenti C, Weiskopf N, Rope R, Kassakian S, Richardson JE, McClure R, Eisenberg F. Assessing Data Adequacy for High Blood Pressure Clinical Decision Support: A Quantitative Analysis. Appl Clin Inform. 2021;12(4):710–20.
Moullin JC, Dickson KS, Stadnick NA, Rabin B, Aarons GA. Systematic review of the Exploration, Preparation, Implementation, Sustainment (EPIS) framework. Implement Sci. 2019;14(1):1.
Becan JE, Bartkowski JP, Knight DK, Wiley TRA, DiClemente R, Ducharme L, Welsh WN, Bowser D, McCollister K, Hiller M, Spaulding AC, Flynn PM, Swartzendruber A, Dickson MF, Fisher JH, Aarons GA. A model for rigorously applying the Exploration, Preparation, Implementation, Sustainment (EPIS) framework in the design and measurement of a large scale collaborative multi-site study. Health Justice. 2018;6(1):9.
Idalski Carcone A, Coyle K, Gurung S, Cain D, Dilones RE, Jadwin-Cakmak L, Parsons JT, Naar S. Implementation Science Research Examining the Integration of Evidence-Based Practices Into HIV Prevention and Clinical Care: Protocol for a Mixed-Methods Study Using the Exploration, Preparation, Implementation, and Sustainment (EPIS) Model. JMIR Res Protoc. 2019;8(5): e11202.
Jackson JM, Witek MA, Hupert ML, Brady C, Pullagurla S, Kamande J, Aufforth RD, Tignanelli CJ, Torphy RJ, Yeh JJ, Soper SA. UV activation of polymeric high aspect ratio microstructures: ramifications in antibody surface loading for circulating tumor cell selection. Lab Chip. 2014;14(1):106–17.
Mazzag B, Tignanelli CJ, Smith GD. The effect of residual Ca2+ on the stochastic gating of Ca2+-regulated Ca2+ channel models. J Theor Biol. 2005;235(1):121–50.
Jones EK, Hultman G, Schmoke K, Ninkovic I, Dodge S, Bahr M, Melton GB, Marquard J, Tignanelli CJ. Combined Expert and User-Driven Usability Assessment of Trauma Decision Support Systems Improves User-Centered Design. Surgery. 2022;172(5):1537–48.
Jakob N. Enhancing the explanatory power of usability heuristics. In Proceedings of the SIGCHI Conference on Human Factors in Computing Systems (CHI '94). New York: Association for Computing Machinery; 1994. p. 152–8. https://doi.org/10.1145/191666.191729 .
Shah S, Switzer S, Shippee ND, Wogensen P, Kosednar K, Jones E, Pestka DL, Badlani S, Butler M, Wagner B, White K, Rhein J, Benson B, Reding M, Usher M, Melton GB, Tignanelli CJ. Implementation of an Anticoagulation Practice Guideline for COVID-19 via a Clinical Decision Support System in a Large Academic Health System and Its Evaluation: Observational Study. JMIR Med Inform. 2021;9(11): e30743.
Ingraham NE, Jones EK, King S, Dries J, Phillips M, Loftus T, Evans HL, Melton GB, Tignanelli CJ. Re-Aiming Equity Evaluation in Clinical Decision Support: A Scoping Review of Equity Assessments in Surgical Decision Support Systems. Ann Surg. 2023;277(3):359–64.
Holtrop JS, Estabrooks PA, Gaglio B, Harden SM, Kessler RS, King DK, Kwan BM, Ory MG, Rabin BA, Shelton RC, Glasgow RE. Understanding and applying the RE-AIM framework: Clarifications and resources. J Clin Transl Sci. 2021;5(1): e126.
https://www.facs.org/-/media/files/quality-programs/trauma/ntdb/ntds/data-dictionaries/ntds_data_dictionary_2022.ashx . Accessed 14 Sep 2021. ACoSNTDSDDA.
https://www.cdc.gov/pcd/issues/2014/13_0184.htm . Accessed 1/3/2021.
Malone S, Prewitt K, Hackett R, Lin JC, McKay V, Walsh-Bailey C, Luke DA. The Clinical Sustainability Assessment Tool: measuring organizational capacity to promote sustainability in healthcare. Implement Sci Commun. 2021;2(1):77.
Aarons GA, Ehrhart MG, Farahnak LR. The Implementation Leadership Scale (ILS): development of a brief measure of unit level implementation leadership. Implement Sci. 2014;9(1):45.
Rye M, Torres EM, Friborg O, Skre I, Aarons GA. The Evidence-based Practice Attitude Scale-36 (EBPAS-36): a brief and pragmatic measure of attitudes to evidence-based practice validated in US and Norwegian samples. Implement Sci. 2017;12(1):44.
Holt DT, Armenakis AA, Feild HS, Harris SG. Readiness for Organizational Change. J Appl Behav Sci. 2007;43(2):232–55.
Article Google Scholar
Weiner BJ. A theory of organizational readiness for change. Implement Sci. 2009;4:67.
Goodridge D, Rana M, Harrison EL, Rotter T, Dobson R, Groot G, Udod S, Lloyd J. Assessing the implementation processes of a large-scale, multi-year quality improvement initiative: survey of health care providers. BMC Health Serv Res. 2018;18(1):237.
Vis C, Ruwaard J, Finch T, Rapley T, de Beurs D, van Stel H, van Lettow B, Mol M, Kleiboer A, Riper H, Smit J. Toward an Objective Assessment of Implementation Processes for Innovations in Health Care: Psychometric Evaluation of the Normalization Measure Development (NoMAD) Questionnaire Among Mental Health Care Professionals. J Med Internet Res. 2019;21(2): e12376.
NoMAD. https://www.implementall.eu/17-nomad.html . Accessed 1/2/2021.
Ng F, McGrath BA, Seth R, et al. Measuring multidisciplinary staff engagement in a national tracheostomy quality improvement project using the NoMAD instrument. Br J Anesth. 2019;123(4):e506.
Guest G, Bunce A, Johnson L. How Many Interviews Are Enough?: An Experiment with Data Saturation and Variability. Field Methods. 2006;18:59–82.
Beidas RS, Stewart RE, Adams DR, Fernandez T, Lustbader S, Powell BJ, Aarons GA, Hoagwood KE, Evans AC, Hurford MO, Rubin R, Hadley T, Mandell DS, Barg FK. A Multi-Level Examination of Stakeholder Perspectives of Implementation of Evidence-Based Practices in a Large Urban Publicly-Funded Mental Health System. Adm Policy Ment Health. 2016;43(6):893–908.
Braun V, Clarke V. Thematic analysis. In Cooper H, Camic PM, Long DL, Panter AT, Rindskopf D, Sher KJ, editors. APA handbooks in psychology®. APA handbook of research methods in psychology, vol. 2. Research designs: Quantitative, qualitative, neuropsychological, and biological. American Psychological Association; 2012. p. 57–71.
Fiscella K, Sanders M, Holder T, Carroll JK, Luque A, Cassells A, Johnson BA, Williams SK, Tobin JN. The role of data and safety monitoring boards in implementation trials: When are they justified? J Clin Transl Sci. 2020;4(3):229–32.
Alonso-Coello P, Schunemann HJ, Moberg J, Brignardello-Petersen R, Akl EA, Davoli M, Treweek S, Mustafa RA, Rada G, Rosenbaum S, Morelli A, Guyatt GH, Oxman AF, Group GW. GRADE Evidence to Decision (EtD) frameworks: a systematic and transparent approach to making well informed healthcare choices. 1: Introduction. BMJ. 2016;353:i2016.
Rosenbaum SE, Moberg J, Glenton C, Schunemann HJ, Lewin S, Akl E, Mustafa RA, Morelli A, Vogel JP, Alonso-Coello P, Rada G, Vasquez J, Parmelli E, Gulmezoglu AM, Flottorp SA, Oxman AD. Developing Evidence to Decision Frameworks and an Interactive Evidence to Decision Tool for Making and Using Decisions and Recommendations in Health Care. Glob Chall. 2018;2(9):1700081.
Alonso-Coello P, Oxman AD, Moberg J, Brignardello-Petersen R, Akl EA, Davoli M, Treweek S, Mustafa RA, Vandvik PO, Meerpohl J, Guyatt GH, Schunemann HJ, Group GW. GRADE Evidence to Decision (EtD) frameworks: a systematic and transparent approach to making well informed healthcare choices. 2: Clinical practice guidelines. BMJ. 2016;353:i2089.
Osheroff JA. CDS and and the CDS & LHS 5 Rights. CDS/PI Collaborative: Getting Better Faster Together.
ACTS Project Team. Patient Journey and Service Blueprint How Tos. AHRQ evidence-based Care Transformation Support (ACTS) Home. [Online] October 2021. https://cmext.ahrq.gov/confluence/display/PUB/Patient+Journey+and+Service+Blueprint+How+Tos .
CDS Approach for Optimizing VTE Prophylaxis (VTEP) Society of Hospital Medicine (SHM) Recommendations1 Version 2; March, 2013. [online] https://www.healthit.gov/sites/default/files/cds/Detailed%20Inpatient%20CDS-QI%20Worksheet%20-%20VTE%20Example%20-%20Recommendations.xlsx .
Download references
This research was supported by the Agency for Healthcare Research and Quality (AHRQ), grant R18HS028583, the University of Minnesota Center for Learning Health System Sciences – a partnership between the University of Minnesota Medical School and the School of Public Health. The authors have no other conflicts of interest.
Author information
Authors and affiliations.
Department of Surgery, University of Minnesota, 420 Delaware St SE, MMC 195, Minneapolis, MN, 55455, USA
Christopher J. Tignanelli, Vincent Peta, Nicholas Lemke & Genevieve B. Melton
Institute for Health Informatics, University of Minnesota, Minneapolis, MN, USA
Christopher J. Tignanelli, Rubina Rizvi & Genevieve B. Melton
Center for Learning Health Systems Science, University of Minnesota, Minneapolis, MN, USA
Christopher J. Tignanelli, Rubina Rizvi, Mary Butler & Genevieve B. Melton
Center for Quality Outcomes, Discovery and Evaluation, University of Minnesota, Minneapolis, MN, USA
Christopher J. Tignanelli
Department of Medicine, Mayo Clinic, Scottsdale, AZ, USA
Surbhi Shah
Division of Biostatistics and Health Data Science, University of Minnesota, Minneapolis, MN, USA
David Vock, Lianne Siegel & Carlos Serrano
Department of Surgery, Johns Hopkins University, Baltimore, MD, USA
Elliott Haut
M Health Fairview, Minneapolis, MN, USA
Sean Switzer
School of Nursing, University of Minnesota, Minneapolis, MN, USA
Christie L. Martin
Department of Surgery, Indiana University School of Medicine, Indianapolis, IN, USA
Peter C. Jenkins
Center for Biomedical Informatics, Regenstrief Institute, Indianapolis, IN, USA
Thankam Thyvalikakath
Indiana University School of Dentistry, Indianapolis, IN, USA
TMIT Consulting, LLC, Naples, FL, USA
Jerome A. Osheroff
Department of Surgery, Geisinger Health, Danville, PA, USA
Denise Torres
Department of Biomedical Informatics, Geisinger Health, Danville, PA, USA
David Vawdrey
Department of Surgery, UC Davis School of Medicine, Sacramento, CA, USA
Rachael A. Callcut
School of Publish Health, University of Minnesota, Minneapolis, MN, USA
Mary Butler
You can also search for this author in PubMed Google Scholar
Contributions
CT conceived and jointly designed the study protocol and helped write and critically revise this protocol paper, SS conceived and jointly designed the study protocol and helped write and critically revise this protocol paper, DV jointly designed the study protocol and helped write and critically revise this protocol paper, LS jointly designed the study protocol and helped write and critically revise this protocol paper, CS jointly designed the study protocol and helped write and critically revise this protocol paper, EH jointly designed the study protocol and helped write and critically revise this protocol paper, SS jointly designed the study protocol and helped write and critically revise this protocol paper, CM jointly designed the study protocol and helped write and critically revise this protocol paper, RR jointly designed the study protocol and helped write and critically revise this protocol paper, VP jointly designed the study protocol and helped write and critically revise this protocol paper, PJ jointly designed the study protocol and helped write and critically revise this protocol paper, NL jointly designed the study protocol and helped write and critically revise this protocol paper, TT jointly designed the study protocol and helped write and critically revise this protocol paper, JO jointly designed the study protocol and helped write and critically revise this protocol paper, DT jointly designed the study protocol and helped write and critically revise this protocol paper, DV jointly designed the study protocol and helped write and critically revise this protocol paper, RC jointly designed the study protocol and helped write and critically revise this protocol paper, MB jointly designed the study protocol and helped write and critically revise this protocol paper, GM conceived and jointly designed the study protocol and helped write and critically revise this protocol paper.
Corresponding author
Correspondence to Christopher J. Tignanelli .
Ethics declarations
Ethics approval and consent to participate.
This study protocol was given the determination of “Exempt” as secondary research for which consent is not required. The Mixed Methods investigation was given the determination of “Not Human Research” as a quality improvement activity.
Consent for publication
Not applicable.
Competing interests
The authors have no conflicts of interest to report.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/ .
Reprints and permissions
About this article
Cite this article.
Tignanelli, C.J., Shah, S., Vock, D. et al. A pragmatic, stepped-wedge, hybrid type II trial of interoperable clinical decision support to improve venous thromboembolism prophylaxis for patients with traumatic brain injury. Implementation Sci 19 , 57 (2024). https://doi.org/10.1186/s13012-024-01386-4
Download citation
Received : 09 June 2024
Accepted : 14 July 2024
Published : 05 August 2024
DOI : https://doi.org/10.1186/s13012-024-01386-4
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Traumatic brain injury
- Prophylaxis
- Venous thromboembolism
- Stepped wedge
- Implementation science
- Mixed methods
- Clinical decision support
- Randomized controlled trial
- Learning health system
- Health informatics
Implementation Science
ISSN: 1748-5908
- Submission enquiries: Access here and click Contact Us
- General enquiries: [email protected]

COMMENTS
In one study, 69.8% of the patients had a partial or complete response after receiving an ... Mehta V, Goel S, Kabarriti R, et al. Case fatality rate of cancer patients with COVID-19 in a New York ...
Presentation of Case. Dr. Jonathan E. Eisen: A 47-year-old woman presented to this hospital early during the pandemic of coronavirus disease 2019 (Covid-19), the disease caused by severe acute ...
Cancer is a risk factor for venous thromboembolism, and this patient presents with chest pain, dyspnea, and unexplained tachycardia. In the context of cancer, an elevated d -dimer level can ...
Introduction. Cervical cancer is one of the major causes of cancer-related death in women worldwide [].It is the fourth most prevalent cause of malignancy in women after breast, colorectal, and lung cancer [].]. The incidence of cervical cancer and mortality rate has declined by 70% in the United States since the 1950s because of age-appropriate screening [].
Case Report: We report here a case of a very young woman with diagnosis of early-stage lung adenocarcinoma harboring EML4-ALK rearrangement; she underwent radical surgery and adjuvant chemotherapy according to the pathologic stage. Potential risk factors for lung cancer in our patient are discussed and clinico-pathologic features and outcomes of lung cancer in the young population compared to ...
This is a qualitative study in which 21 women with breast cancer or survivors were interviewed. Participants were recruited at 9 large hospitals in Spain and intentional sampling methods were applied. Data were collected using a semi-structured interview that was elaborated with the help of medical oncologists, nurses, and psycho-oncologists.
This Case Study describes a patient with breast cancer who was treated with capecitabine and experienced a severe adverse event when treated with brivudin for a herpes infection.
Background Hereditary diffuse gastric cancer is a rare condition that accounts for approximately 1-3% of all gastric cancer cases. Due to its rapid and invasive growth pattern, it is associated with a very poor prognosis. As a result, comprehensive genetic testing is imperative in patients who meet the current testing criteria in order to identify relatives at risk. This case report ...
Case management (CM) is widely utilized to improve health outcomes of cancer patients, enhance their experience of health care, and reduce the cost of care. While numbers of systematic reviews are available on the effectiveness of CM for cancer patients, they often arrive at discordant conclusions that may confuse or mislead the future case management development for cancer patients and ...
rJOHN MULLEN, MDMassachusetts General HospitalPRESENTATION OF CASEA 76-year-old woman was seen in our multidisciplinary gastrointestinal oncology clinic f. r further management of an adenocarcinoma of the body of the stomach.The patient presented to her primary care physician with a f. ur-week history of intermittent chest pressure radiating to ...
It's my pleasure to walk us through the first case, which is small cell lung cancer. This is a case with a 72-year-old woman who presents with shortness of breath, a productive cough, chest pain, some fatigue, anorexia, a recent 18-pound weight loss, and a history of hypertension. She is a schoolteacher and has a 45-pack-a-year smoking ...
example, in a series of 8422 patients enrolled on International Breast Cancer Study Group trials between 1978 and 1999, the rate of node-negativity for medial compared to lateral/central tumors was 44 versus 33 percent, respectively. The most likely explanation for this difference is preferential drainage of some medial tumors to the IM nodes.
Here, we present the case of a 51-year-old man with limited-stage small cell lung cancer (LS-SCLC) who received concurrent chemoradiotherapy and photodynamic therapy (PDT). The patient was diagnosed as having LS-SCLC with an endobronchial mass in the left main bronchus. Following concurrent chemoradiotherapy, a mass remaining in the left lingular division was treated with PDT.
A 44-year-old woman presented with cough, dyspnea, and chest pain. On examination, she had tachycardia and hypotension. Evaluation revealed SARS-CoV-2 RNA in a nasopharyngeal swab, as well as eleva...
The patient was diagnosed with stage IV colorectal cancer, and the ECOG PS [performance status] of the patient was 1. At that time, the patient was started on treatment with bevacizumab and FOLFOX [5-fluorouracil, leucovorin, oxaliplatin]. The patient received about 4 months of this treatment with scans at 2- and 4-months showing response.
Their practice uses a care model they call the Breast Cancer Multidisciplinary Clinic, which in one appointment gathers the patient and several specialists—in Simona's case, a surgical oncologist, medical oncologist, and radiation oncologist—all at the same time to evaluate newly diagnosed breast cancer patients and develop a personalized ...
Methods. A case study approach was used because it allowed us to examine the complexity of cancer management from the perspective of one person's case as interpreted by multiple people, retaining its holistic and meaningful characteristics while being studied 17 answering how and why questions. 18 Interviews from four participants presented multiple perspectives of the same interested topic ...
Our case corroborated the good prognostic factors reported in these studies. A case of metastatic NSCLC in which the patient survived for 10 years has ... et al. Cisplatin- versus carboplatin-based chemotherapy in first-line treatment of advanced non-small-cell lung cancer: an individual patient data meta-analysis. J Natl Cancer Inst 2007;99: ...
Lyndsay Willmott, MD: We'd like to discuss a patient who's presenting with classic symptoms of ovarian cancer. This is a 57-year-old woman who presented with progressive abdominal discomfort and bloating, as well as early satiety, new onset constipation, and unintentional weight loss. Her past medical history is significant for hypertension ...
Patients with different cancer stages (e.g., potentially resectable, borderline resectable, locally advanced) and cancer types (initial diagnosis or relapse) participated in the study.
Yet, less than one in 20 adult cancer patients enroll in these studies. ... The company introduces a client's case to a specialized doctor who can explain to them the positives and negatives of ...
Complete pathological response to NAC is relatively rare in this cancer, the case authors noted. The positive outcome reported in their patient's case reflects the limited experience in this ...
Presentation of Case. Dr. Christopher T. Chen: A 34-year-old woman was evaluated in the oncology clinic of this hospital for the management of relapsed, metastatic intrahepatic cholangiocarcinoma ...
A case-cohort study in Israel that investigated the association between hormone therapy and diabetes risk among 2,246 female breast cancer survivors found that 48% of diabetes incidence could have been prevented had patients not received hormone therapy (13). Early implementation of a diabetes prevention strategy, particularly for patients with ...
The case was settled in the low range (<$200,000). Analysis Documentation is critical. A patient's refusal of treatment, the risks related to the refusal, and any alternative treatments offered should always be documented in the patient's medical record. Once a claim is asserted, poor documentation increases provider risk.
A case-control study was conducted on 244 participants to study the association. The Case group includes 61 newly diagnosed breast cancer patients receiving < 6 months of therapies. The Control group includes 183 healthy people with matched characteristics. A new questionnaire was developed, validated, and used in this study to estimate the ...
This is the first case in which an elderly patient with poor PS and advanced unresectable colorectal cancer was treated with combination chemotherapy of Pmab. Chan et al. studied 1013 young (<70 years) and elderly (≥70 years) patients with metastatic colon cancer with PS 0-1 administered with systemic therapy. The results of their analysis ...
Dr. Mathew S. Lopes: A 65-year-old woman was transferred to this hospital because of chest pain. Six months before the current presentation, the patient presented to a hospital affiliated with ...
Cancer cases and deaths among men are expected to surge globally by 2050, according to a new study. In the study, published Monday in Cancer, a peer-reviewed journal of the American Cancer Society ...
Venous thromboembolism (VTE) is a preventable medical condition which has substantial impact on patient morbidity, mortality, and disability. Unfortunately, adherence to the published best practices for VTE prevention, based on patient centered outcomes research (PCOR), is highly variable across U.S. hospitals, which represents a gap between current evidence and clinical practice leading to ...