Information
- Author Services

Initiatives
You are accessing a machine-readable page. In order to be human-readable, please install an RSS reader.
All articles published by MDPI are made immediately available worldwide under an open access license. No special permission is required to reuse all or part of the article published by MDPI, including figures and tables. For articles published under an open access Creative Common CC BY license, any part of the article may be reused without permission provided that the original article is clearly cited. For more information, please refer to https://www.mdpi.com/openaccess .
Feature papers represent the most advanced research with significant potential for high impact in the field. A Feature Paper should be a substantial original Article that involves several techniques or approaches, provides an outlook for future research directions and describes possible research applications.
Feature papers are submitted upon individual invitation or recommendation by the scientific editors and must receive positive feedback from the reviewers.
Editor’s Choice articles are based on recommendations by the scientific editors of MDPI journals from around the world. Editors select a small number of articles recently published in the journal that they believe will be particularly interesting to readers, or important in the respective research area. The aim is to provide a snapshot of some of the most exciting work published in the various research areas of the journal.
Original Submission Date Received: .
- Active Journals
- Find a Journal
- Proceedings Series
- For Authors
- For Reviewers
- For Editors
- For Librarians
- For Publishers
- For Societies
- For Conference Organizers
- Open Access Policy
- Institutional Open Access Program
- Special Issues Guidelines
- Editorial Process
- Research and Publication Ethics
- Article Processing Charges
- Testimonials
- Preprints.org
- SciProfiles
- Encyclopedia

Article Menu
- Subscribe SciFeed
- Recommended Articles
- Google Scholar
- on Google Scholar
- Table of Contents
Find support for a specific problem in the support section of our website.
Please let us know what you think of our products and services.
Visit our dedicated information section to learn more about MDPI.
JSmol Viewer
Recent trends of sunscreen cosmetic: an update review.

Graphical Abstract
1. Introduction
2. classification of sunscreen agents, 2.1. organic uv filters, 2.2. inorganic uv filters, 2.3. hybrid uv filters (organic/inorganic agents), 2.4. botanical agents, 2.5. safety and health hazards of sunscreen agents, 3. sunscreen formulations, 3.1. emulsion sunscreen, 3.2. gel sunscreen, 3.3. aerosol sunscreen, 3.4. sun stick, 4. novel properties of commercial sun protection products, 4.1. sunscreen with antioxidants and anti-aging, 4.2. sunscreen combined with dna repair enzymes, 4.3. sunscreen against environmental pollutants, 4.4. sunscreen against blue light, 4.5. sunscreen against thermal ir, 5. conclusions and outlook, author contributions, conflicts of interest.
- Hidaka, H.; Horikoshi, S.; Serpone, N.; Knowland, J. In vitro photochemical damage to DNA, RNA and their bases by an inorganic sunscreen agent on exposure to UVA and UVB radiation. J. Photochem. Photobiol. A Chem. 1997 , 111 , 205–213. [ Google Scholar ] [ CrossRef ]
- D’Orazio, J.; Jarrett, S.; Amaro-Ortiz, A.; Scott, T. UV radiation and the skin. Int. J. Mol. Sci. 2013 , 14 , 12222–12248. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Juzeniene, A.; Moan, J. Beneficial effects of UV radiation other than via vitamin D production. Dermato-Endocrinol. 2012 , 4 , 109–117. [ Google Scholar ] [ CrossRef ] [ PubMed ] [ Green Version ]
- Bintsis, T.; Litopoulou-Tzanetaki, E.; Davies, R.; Robinson, R.K. The antimicrobial effects of long-wave ultra-violet light and furocoumarins on some micro-organisms that occur in cheese brines. Food Microbiol. 2000 , 17 , 687–695. [ Google Scholar ] [ CrossRef ]
- Fleury, N.; Geldenhuys, S.; Gorman, S. Sun exposure and its effects on human health: Mechanisms through which sun exposure could reduce the risk of developing obesity and cardiometabolic dysfunction. Int. J. Environ. Res. Public Health 2016 , 13 , 999. [ Google Scholar ] [ CrossRef ]
- Weller, R.B. Sunlight has cardiovascular benefits independently of vitamin D. Blood Purif. 2016 , 41 , 130–134. [ Google Scholar ] [ CrossRef ]
- Taylor, B.R. Ultraviolet radiation and the eye: An epidemiologic study. Tr. Am. Ophth. Soc. 1989 , 87 , 802. [ Google Scholar ]
- Hitchin, V.M.; Withrow, T.J.; Olvey, K.M.; Harleston, B.A.; Ellingson, O.L.; Bostrom, A.R.G. The cytotoxic and mutagenic effects of UVA radiation on L5178Y mouse lymphoma cells. J. Photochem. Photobiol. 1986 , 44 , 53–57. [ Google Scholar ] [ CrossRef ]
- Sambandan, D.R.; Ratner, D. Sunscreens: An overview and update. J. Am. Acad. Dermatol. 2011 , 64 , 748–758. [ Google Scholar ] [ CrossRef ]
- Manikrao Donglikar, M.; Laxman Deore, S. Sunscreens: A review. Pharmacogn. J. 2016 , 8 , 171–179. [ Google Scholar ] [ CrossRef ] [ Green Version ]
- Palm, M.D.; O’Donoghue, M.N. Update on photoprotection. Dermatol. Ther. 2007 , 20 , 360–376. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Singer, S.; Karrer, S.; Berneburg, M. Modern sun protection. Curr. Opin. Pharmacol. 2019 , 46 , 24–28. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Mistry, N. Guidelines for formulating anti-pollution products. Cosmetics 2017 , 4 , 57. [ Google Scholar ] [ CrossRef ]
- Lee, S.-H. New Technical Developments in Sun Care and Blue Light Defense ; SUNJIN Beauty Science: Gyeonggi-do, Korea, 2018; p. 134. [ Google Scholar ]
- Ham, W.T.; Mueller, H.A.; Sliney, D.H. Retinal sensitivity to damage from short wavelength light. Nature 1976 , 260 , 153–155. [ Google Scholar ] [ CrossRef ]
- Schieke, S.M.; Schroeder, P.; Krutmann, J. Cutaneous effects of infrared radiation: From clinical observations to molecular response mechanisms. Photodermatol. Photoimmunol. Photomed. 2003 , 19 , 228–234. [ Google Scholar ] [ CrossRef ]
- Schalka, S.; Reis, V.M.S.d. Sun protection factor: Meaning and controversies. An. Bras. Dermatol. 2011 , 86 , 507–515. [ Google Scholar ] [ CrossRef ]
- Osterwalder, U.; Herzog, B. Sun protection factors: World wide confusion. Br. J. Dermatol. 2009 , 161 , 13–24. [ Google Scholar ] [ CrossRef ]
- Moyal, D. UVA protection labeling and in vitro testing methods. Photochem. Photobiol. Sci. 2010 , 9 , 516–523. [ Google Scholar ] [ CrossRef ]
- Wang, S.Q.; Stanfield, J.W.; Osterwalder, U. In vitro assessments of UVA protection by popular sunscreens available in the United States. J. Am. Acad. Dermatol. 2008 , 59 , 934–942. [ Google Scholar ] [ CrossRef ]
- Lademann, J.; Schanzer, S.; Jacobi, U.; Schaefer, H.; Pflucker, F.; Driller, H.; Beck, J.; Meinke, M.; Roggan, A.; Sterry, W. Synergy effects between organic and inorganic UV filters in sunscreens. J. Biomed. Opt. 2005 , 10 , 14008. [ Google Scholar ] [ CrossRef ]
- Vergou, T.; Patzelt, A.; Richter, H.; Schanzer, S.; Zastrow, L.; Golz, K.; Doucet, O.; Antoniou, C.; Sterry, W.; Lademann, J. Transfer of ultraviolet photon energy into fluorescent light in the visible path represents a new and efficient protection mechanism of sunscreens. J. Biomed. Opt. 2011 , 16 , 105001. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Latha, M.S.; Martis, J.; Shobha, V.; Shinde, R.S.; Banera, S.; Krishnankutty, B.; Bellary, S.; Varughese, S.; Rao, P.; Kumar, B.R.N. Sunscreening agents. J. Clin. Aesthet. Dermatol. 2013 , 6 , 16–26. [ Google Scholar ] [ PubMed ]
- Serpone, N.; Dondi, D.; Albini, A. Inorganic and organic UV filters: Their role and efficacy in sunscreens and sun care products. Inorganica Chim. Acta 2007 , 360 , 794–802. [ Google Scholar ] [ CrossRef ]
- Pathak, M.A. Sunscreens: Topical and systemic approaches for protection of human skin against harmful effects of solar radiation. J. Am. Acad. Dermatol. 1982 , 7 , 285–311. [ Google Scholar ] [ CrossRef ]
- Administration, U.F.A.D. Sunscreen drug products for over-the-counter human use. In Final Monograph ; Food and Drug Administration: Rockville, MD, USA, 2000. [ Google Scholar ]
- Siller, A.; Blaszark, S.C.; Lazar, M. Update about the effects of the sunscreen ingredients oxybenzone and octinoxate on humans and the environment. Plast. Surg. Nurs. 2018 , 38 , 158–161. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Paris, C.; Lhiaubet-Vallet, V.; Jiménez, O.; Trullas, C.; Miranda, M.A. A blocked diketo form of avobenzone: Photostability, photosensitizing properties and triplet quenching by a triazine-derived UVB-filter. Photochem. Photobiol. 2019 , 85 , 178–184. [ Google Scholar ] [ CrossRef ]
- Karlsson, I.; Hillerstrom, L.; Stenfeldt, A.-L.; Mårtensson, J.; Borje, A. Photodegradation of dibenzoylmethanes: Potential cause of photocontact allergy to sunscreens. Chem. Res. Toxicol. 2009 , 22 , 1881–1892. [ Google Scholar ] [ CrossRef ]
- Gaspar, L.R.; Maia Campos, P.M.B.G. Evaluation of the photostability of different UV filter combinations in a sunscreen. Int. J. Pharm. 2006 , 307 , 123–128. [ Google Scholar ] [ CrossRef ]
- Smijs, T.G.; Pavel, S. Titanium dioxide and zinc oxide nanoparticles in sunscreens: Focus on their safety and effectiveness. Nanotechnol. Sci. Appl. 2011 , 4 , 95–112. [ Google Scholar ] [ CrossRef ]
- Giacomoni, P.U. Sun Protection in Man ; Elsevier: Amsterdam, The Netherlands, 2001; Volume 3, pp. 495–519. [ Google Scholar ]
- Jacobs, J.F.; van de Poel, I.; Osseweijer, P. Sunscreens with titanium dioxide (TiO 2 ) nano-particles: A societal experiment. Nanoethics 2010 , 4 , 103–113. [ Google Scholar ] [ CrossRef ]
- Gonzalez, A.D.; Pechko, A.H.; Kalafsky, R.E. Photostable Sunscreen Compositions and Methods of Stabilizing. US6440402B1, 27 August 2002. [ Google Scholar ]
- Schroeder, P.; Lademann, J.; Darvin, M.E.; Stege, H.; Marks, C.; Bruhnke, S.; Krutmann, J. Infrared radiation-induced matrix metalloproteinase in human skin: Implications for protection. J. Investig. Dermatol. 2008 , 128 , 2491–2497. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Addor, F.A.S. Antioxidants in dermatology. An. Bra. Dermatol. 2017 , 92 , 356–362. [ Google Scholar ] [ CrossRef ] [ PubMed ] [ Green Version ]
- Masaki, H. Role of antioxidants in the skin: Anti-aging effects. J. Dermatol. Sci. 2010 , 58 , 85–90. [ Google Scholar ] [ CrossRef ]
- Pouillot, A.; Polla, L.L.; Tacchini, P.; Neequaye, A.; Polla, A.; Polla, B. Natural antioxidants and their effects on the skin. In Formulating, Packaging, and Marketing of Natural Cosmetic Products ; Dayan, N., Kromidas, L., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 2011; pp. 239–257. [ Google Scholar ]
- Anitha, D.; Reddy, K.Y.; Venkatesh, P.; Raani, M.J. A review-herbal sunscreen agents on skin protection. Eur. J. Pharm. Med. Res. 2016 , 3 , 308–313. [ Google Scholar ]
- Sopyan, I.; Gozali, D.; Tiassetiana, S. Formulation of tomato extracts ( Solanum lycopersicum L.) as a sunscreen lotion. Natl. J. Physiol. Pharm. Pharmacol. 2017 , 8 , 453–458. [ Google Scholar ] [ CrossRef ]
- Afaq, F.; Zaid, M.A.; Khan, N.; Dreher, M.; Mukhtar, H. Protective effect of pomegranate-derived products on UVB-mediated damage in human reconstituted skin. Exp. Dermatol. 2009 , 18 , 553–561. [ Google Scholar ] [ CrossRef ] [ Green Version ]
- Katiyar, S.K.; Elmets, C.A. Green tea polyphenolic antioxidants and skin photoprotection (review). Int. J. Oncol. 2001 , 18 , 1307–1313. [ Google Scholar ]
- Maheshwar, G.H.; Patil, B.S.; Prashant, D. Comparative sun protection factor determination fo fresh fruits extract of cucumber vs marketed cosmetic formulation. Res. J. Pharm. Biol. Chem. Sci. 2010 , 1 , 55–59. [ Google Scholar ]
- Shenoy, P.; Khot, S.; Chavan, M.; Takawale, J.; Singh, S. Study of sunscreen activity of aqueous, methanol and acetone extracts of leaves of Pongamia pinnata (L.) pierre, fabaceae. Int. J. Green Pharm. 2010 , 4 , 270. [ Google Scholar ] [ CrossRef ]
- Patil, V.; Patil, S.B.; Kondawar, M.S.; Naikwade, N.S.; Magdum, C.S. Study of methanolic extract of flower of Spathodea campanulata L. as an anti-solar. Int. J. Green Pharm. 2009 , 3 , 248. [ Google Scholar ] [ CrossRef ]
- Park, K.; Choi, H.S.; Hong, Y.H.; Jung, E.Y.; Suh, H.J. Cactus cladodes ( Opuntia humifusa ) extract minimizes the effects of UV irradiation on keratinocytes and hairless mice. Pharm.Biol. 2017 , 55 , 1032–1040. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Hyun, T.K.; Ko, Y.-J.; Kim, E.-H.; Chung, I.-M.; Kim, J.-S. Anti-inflammatory activity and phenolic composition of Dendropanax morbifera leaf extracts. Ind. Crop. Prod. 2015 , 74 , 263–270. [ Google Scholar ] [ CrossRef ]
- Chen, L.; Hu, J.Y.; Wang, S.Q. The role of antioxidants in photoprotection: A critical review. J. Am. Acad. Dermatol. 2012 , 67 , 1013–1024. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- McVean, M.; Liebler, D.C. Prevention of DNA photodamage by vitamin E compounds and sunscreens: Roles of ultraviolet absorbance and cellular uptake. Mol. Carcinog. 1999 , 24 , 169–176. [ Google Scholar ] [ CrossRef ]
- Dzialo, M.; Mierziak, J.; Korzun, U.; Preisner, M.; Szopa, J.; Kulma, A. The potential of plant phenolics in prevention and therapy of skin disorders. Int. J. Mol. Sci. 2016 , 17 , 160. [ Google Scholar ] [ CrossRef ]
- José, M.T.d.A.F.; Pedrita, A.S.; Emanuella, C.V.P.; Raimundo, G.d.O.J.; Fabrício, S.S.; Jackson, R.G.d.S.A.; Larissa, A.R.; Xirley, P.N.; Edigênia, C.d.C.A. Flavonoids as photoprotective agents: A systematic review. J. Med. Plants Res. 2016 , 10 , 848–864. [ Google Scholar ] [ CrossRef ] [ Green Version ]
- Stahl, W.; Sies, H. Beta-carotene and other carotenoids in protection from sunlight. Am. J. Clin. Nutr. 2012 , 96 , 1179S–1184S. [ Google Scholar ] [ CrossRef ]
- Food and Drug Administration (FDA). 127 newFDA Rules Regulations for Sunscreen. 2012. Available online: https://smartshield.com/news/reviews/54-resources/127-new-fda-rules-regulations-for-sunscreen (accessed on 20 April 2019).
- Kerr, A.; Ferguson, J. Photoallergic contact dermatitis. Photodermatol. Photoimmunol. Photomed. 2010 , 26 , 56–65. [ Google Scholar ] [ CrossRef ]
- Faurschou, A.; Beyer, D.M.; Schmedes, A.; Bogh, M.K.; Philipsen, P.A.; Wulf, H.C. The relation between sunscreen layer thickness and vitamin D production after ultraviolet B exposure: A randomized clinical trial. Brit. J. Dermatol. 2012 , 167 , 391–395. [ Google Scholar ] [ CrossRef ]
- Gorham, E.D.; Mohr, S.B.; Garland, C.F.; Chaplin, G.; Garland, F.C. Do sunscreens increase risk of melanoma in populations residing at higher latitudes? Annals Epidemiol. 2007 , 17 , 956–963. [ Google Scholar ] [ CrossRef ]
- Autier, P. Sunscreen abuse for intentional sun exposure. Brit. J. Dermatol. 2009 , 161 (Suppl. 3), 40–45. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Pan, Z.; Lee, W.; Slutsky, L.; Clark, R.A.; Permodet, N.; Rafailovich, M.H. Adverse effects of titanium dioxide nanoparticles on human dermal fibroblasts and how to protect cells. Small 2009 , 5 , 511–520. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Filipe, P.; Silva, J.N.; Silva, R.; Cirne de Castro, J.L.; Marques Gomes, M.; Alves, L.C.; Santus, R.; Pinheiro, T. Stratum corneum is an effective barrier to TiO 2 and ZnO nanoparticle percutaneous absorption. Skin Pharmacol. Phys. 2009 , 22 , 266–275. [ Google Scholar ] [ CrossRef ]
- Holick, M.F. Vitamin D: A millenium perspective. J. Cell Biochem. 2003 , 88 , 296–307. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Marks, R.; Foley, P.A.; Jolley, D.; Knight, K.R.; Thompson, S.C. The effect of regular sunscreen use on vitamin D levels in an Australian population. Arch. Dermatol. 1995 , 131 , 415–421. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Klein, K. Sunscreen products: Formulation and regulatory considerations. Cosmet. Sci. Technol. Ser. 1997 , 285–312. [ Google Scholar ]
- Schröder, B.; Ohrmann, R.; Issleib, M.; Endlein, E. O/W-Emulsifiers, O/W-Emulsions and Methods of Manufacture Thereof. US8961943B2, 24 February 2015. [ Google Scholar ]
- Smaoui, S.; Ben Hlima, H.; Ben Chobba, I.; Kadri, A. Development and stability studies of sunscreen cream formulations containing three photo-protective filters. Arab. J. Chem. 2017 , 10 , S1216–S1222. [ Google Scholar ] [ CrossRef ] [ Green Version ]
- Bara, I.; Mellul, M. New Cosmetic or Dermopharmaceutical Compositions in the Form of Aqueous Gels Modified by the Addition of Expanded Microspheres. U.S. Patent 5593680, 14 January 1997. [ Google Scholar ]
- Teng, J.; Lucas, J.M.; Stubits, M.C. Sunscreen Gel. U.S. Patent 4193989, 18 March 1980. [ Google Scholar ]
- Diec, K.H.; Gersbarlag, H.; Klier, M.; Schreiber, J.; Wolf, F. Cosmetic or Dermatological Gels Based on Microemulsions. U.S. Patent 6607733B1, 19 August 2003. [ Google Scholar ]
- Strobridge, J.R. Gel-Type Sunscreen Composition. U.S. Patent 4917882, 17 April 1990. [ Google Scholar ]
- Hougaz, L. Sunscreen Aerosol Spray. U.S. Patent 20090061001A1, 5 March 2009. [ Google Scholar ]
- Hanson, J.E.; Antonacci, C. Natural Sunscreen Composition. U.S. Patent 9056063, 16 June 2015. [ Google Scholar ]
- Nieuwenhuijsen, B. Composition of a Water-Soluble Sunscreen Preparation for Acne Rosacea. U.S. Patent 8216555, 10 July 2012. [ Google Scholar ]
- Rosenthal, A.; Stoddard, M.; Chipps, L.; Herrmann, J. Skin cancer prevention: A review of current topical options complementary to sunscreens. J. Eur. Acad. Dermatol. Venereol. 2019 , 33 , 1261–1267. [ Google Scholar ] [ CrossRef ]
- Megna, M.; Lembo, S.; Balato, N.; Monfrecola, G. “Active” photoprotection: Sunscreens with DNA repair enzymes. G. Ital. Dermatol. Venereol. 2017 , 152 , 302–307. [ Google Scholar ]
- Emanuele, E.; Altabas, V.; Altabas, K.; Berardesca, E. Topical application of preparations containing DNA repair enzymes prevents ultraviolet-induced telomere shortening and c-FOS proto-oncogene hyperexpression in human skin: An experimental pilot study. J. Drugs Dermatol. 2013 , 12 , 1017–1021. [ Google Scholar ]
- Carducci, M.; Pavone, P.S.; De, G.M.; Lovati, S.; Altabas, V.; Altabas, K.; Emanuele, E. Comparative effects of sunscreens alone vs sunscreens plus DNA repair enzymes in patients with actinic keratosis: Clinical and molecular findings from a 6-month, randomized, clinical study. J. Drugs Dermatol. 2015 , 14 , 986–990. [ Google Scholar ] [ PubMed ]
- Kuraoka, I. Diversity of endonuclease V: From DNA repair to RNA editing. Biomolecules 2015 , 5 , 2194–2206. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Stoddard, M.; Herrmann, J.; Moy, L.; Moy, R. Improvement of actinic keratoses using topical DNA repair enzymes: A randomized placebo-controlled trial. J. Drugs Dermatol. 2017 , 16 , 1030–1034. [ Google Scholar ] [ PubMed ]
- Liu, Z.; Tan, C.; Guo, X.; Kao, Y.-T.; Li, J.; Wang, L.; Sancar, A.; Zhong, D. Dynamics and mechanism of cyclobutane pyrimidine dimer repair by DNA photolyase. Proc. Natl. Acad. Sci. USA 2011 , 108 , 14831–14836. [ Google Scholar ] [ CrossRef ] [ PubMed ] [ Green Version ]
- Stege, H.; Roza, L.; Vink, A.A.; Grewe, M.; Ruzicka, T.; Grether-Beck, S.; Krutmann, J. Enzyme plus light therapy to repair DNA damage in ultraviolet-B-irradiated human skin. Proc. Natl. Acad. Sci. USA 2000 , 97 , 1790–1795. [ Google Scholar ] [ CrossRef ] [ PubMed ] [ Green Version ]
- Berardesca, E.; Bertona, M.; Altabas, K.; Altabas, V.; Emanuele, E. Reduced ultraviolet-induced DNA damage and apoptosis in human skin with topical application of a photolyase-containing DNA repair enzyme cream: Clues to skin cancer prevention. Mol. Med. Rep. 2012 , 5 , 570–574. [ Google Scholar ]
- Huang, X.X.; Scolyer, R.A.; Abubakar, A.; Halliday, G.M. Human 8-oxoguanine-DNA glycosylase-1 is downregulated in human basal cell carcinoma. Mol. Genet. Metab. 2012 , 106 , 127–130. [ Google Scholar ] [ CrossRef ]
- Wulff, B.C.; Schick, J.S.; Thomas-Ahner, J.M.; Kusewitt, D.F.; Yarosh, D.B.; Oberyszyn, T.M. Topical treatment with OGG1 enzyme affects UVB-induced skin carcinogenesis. Photochem. Photobiol. 2008 , 84 , 317–321. [ Google Scholar ] [ CrossRef ]
- Kuse, Y.; Ogawa, K.; Tsuruma, K.; Shimazawa, M.; Hara, H. Damage of photoreceptor-derived cells in culture induced by light emitting diode-derived blue light. Sci. Rep. 2014 , 4 , 5223. [ Google Scholar ] [ CrossRef ] [ Green Version ]
- Mark, J.R.; Gelpi-Hammerschmidt, F.; Trabulsi, E.J.; Gomella, L.G. Blue light cystoscopy for detection and treatment of non-muscle invasive bladder cancer. Can. J. Urol. 2012 , 19 , 6227–6231. [ Google Scholar ]
- Brown, S.B.; Brown, E.A.; Walker, I. The present and future role of photodynamic therapy in cancer treatment. Lancet Oncol. 2004 , 5 , 497–508. [ Google Scholar ] [ CrossRef ]
- Barolet, D.; Christiaens, F.; Hamblin, M.R. Infrared and skin: Friend or foe. J. Photochem. Photobiol. B 2016 , 155 , 78–85. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Kim, S.J.; Bae, J.; Lee, S.E.; Lee, J.B.; Park, C.H.; Lim, D.H.; Park, M.S. A novel in vivo test method for evaluating the infrared radiation protection provided by sunscreen products. Skin Res. Technol. 2019 , 0 , 1–6. [ Google Scholar ]
- Alan, K. FDA Regulations for Sunscreens. 2016. Available online: https://www.sansoleil.com/FDA-Regulations-for-Sunscreens_b_2.html (accessed on 14 February 2019).
Click here to enlarge figure
| Compounds | Protection Mechanism |
|---|---|
| Vitamin C | , ] ] , ] ] |
| Vitamin E | ] , ] |
| Phenolic compounds | ] ] ]. |
| Flavonoid compounds | ] ] |
| Carotenoids | O ) radicals generated in during photooxidation [ ] ] |
| DNA Repair Enzymes | Proposal Mechanism and Proven Effects |
|---|---|
| Topical T4 endonuclease | ] ] |
| Photolyase | , ] ] |
| 8-Oxoguanine glycosylase | ] ] |
Share and Cite
Ngoc, L.T.N.; Tran, V.V.; Moon, J.-Y.; Chae, M.; Park, D.; Lee, Y.-C. Recent Trends of Sunscreen Cosmetic: An Update Review. Cosmetics 2019 , 6 , 64. https://doi.org/10.3390/cosmetics6040064
Ngoc LTN, Tran VV, Moon J-Y, Chae M, Park D, Lee Y-C. Recent Trends of Sunscreen Cosmetic: An Update Review. Cosmetics . 2019; 6(4):64. https://doi.org/10.3390/cosmetics6040064
Ngoc, Le Thi Nhu, Vinh Van Tran, Ju-Young Moon, Minhe Chae, Duckshin Park, and Young-Chul Lee. 2019. "Recent Trends of Sunscreen Cosmetic: An Update Review" Cosmetics 6, no. 4: 64. https://doi.org/10.3390/cosmetics6040064
Article Metrics
Article access statistics, further information, mdpi initiatives, follow mdpi.

Subscribe to receive issue release notifications and newsletters from MDPI journals
- Advanced search

Advanced Search
The efficacy and safety of sunscreen use for the prevention of skin cancer
- Find this author on Google Scholar
- Find this author on PubMed
- Search for this author on this site
- Figures & Tables
- Related Content
Several well-conducted randomized controlled trials with long follow-up showed that sunscreen use reduces the risk of squamous cell and melanoma skin cancers.
Commercial sunscreens protect against the skin-damaging effects of ultraviolet radiation through either chemical or physical ingredients.
The Canadian Dermatology Association recommends the use of an adequate dose of a broad-spectrum sunscreen with a sun protection factor of at least 30 for most children and adults, as part of a comprehensive photoprotection strategy.
Emerging evidence suggests that some chemical sunscreen ingredients are systemically absorbed, but the clinical importance of this remains unclear; further research is required to establish whether this results in harm.
Ultraviolet filters found within chemical sunscreens may be harmful to the environment.
In Canada, more than 80 000 cases of skin cancer are diagnosed every year. 1 Because exposure to ultraviolet radiation is estimated to be associated with 80%–90% of skin cancers, the use of sunscreen — which blocks ultraviolet radiation — is promoted as an important means of preventing skin cancers, 2 , 3 as well as sunburn and skin photoaging (see definitions in Appendix 1, available at www.cmaj.ca/lookup/doi/10.1503/cmaj.201085/tab-related-content ). Use of sunscreen has been shown to reduce the incidence of both melanoma and nonmelanoma skin cancers. 4 , 5 Both the Canadian Dermatology Association and the American Academy of Dermatology recommend the use of sunscreen for the prevention of skin cancer. 6 , 7 Yet, since the development of the first commercial sunscreen in 1928, questions regarding the safety and efficacy of sunscreen have been raised, and more recently, the impact of sunscreens on the environment has become a cause for concern. We summarize evidence related to the effectiveness and harms of sunscreen to help physicians counsel their patients ( Box 1 ).
Evidence used in this review
We conducted a targeted search of MEDLINE using a combination of the search terms “sunscreen,” “skin cancer,” “melanoma,” “squamous cell carcinoma,” “basal cell carcinoma,” “photoaging,” “safety” and “environment” to identify studies published from 1984 to 2020. We particularly sought randomized controlled trials, systematic reviews and meta-analyses relevant to this article’s clinical questions. We also identified relevant review articles, basic science publications and institutional guidelines. We supplemented our search with literature from our own collections.
- How do sunscreens work?
Sunscreens contain chemical (organic) or physical (inorganic) compounds that act to block ultraviolet radiation, which is light with wavelengths shorter than visible light (subdivided into ultraviolet A [UVA]1, UVA2, ultraviolet B [UVB] and ultraviolet C [UVC]), as shown in Figure 1 . Generally, the shorter the wavelength, the greater the potential for light radiation to cause biological damage. Sunscreen filters are active against UVA1, UVA2 and UVB radiation. Chemical filters, such as oxybenzone, avobenzone, octocrylene and ecamsule, are aromatic compounds that absorb high-intensity ultraviolet radiation, resulting in excitation to higher energy states. When these molecules return to their ground states, the result is conversion of the absorbed energy into lower-energy wavelengths, such as infrared radiation (i.e., heat). 8
- Download figure
- Open in new tab
- Download powerpoint
Schematic representation of the electromagnetic spectrum of light, emphasizing ultraviolet radiation (UVR) frequencies and their effect on human skin. Generally, the shorter the wavelength of radiation, the greater the potential for biological damage. Note: UVA = ultraviolet A, UVB = ultraviolet B, UVC = ultraviolet C. Sunscreen filters are active against UVA1, UVA2 and UVB radiation.
Physical sunscreen filters, such as titanium dioxide and zinc oxide, reflect or refract ultraviolet radiation away from the skin; however, experimental studies have shown that when particle sizes are very small, as in micronized sunscreens, the mechanism of action is similar to that of chemical filters. More specifically, micronized zinc oxide and titanium dioxide behave as semiconductor metals, which absorb ultraviolet light throughout most of the electromagnetic spectrum. 9 The sunscreen ingredients that are currently approved by Health Canada are listed in Table 1 . 10
- View inline
Sunscreen ingredients approved by Health Canada 10
- What is the effectiveness of sunscreens in preventing photoaging and skin cancer?
Evidence from observational studies, 11 a large randomized controlled trial (RCT) 12 and smaller, nonrandomized experimental studies 13 – 15 support the effectiveness of sunscreens in preventing the signs of photoaging, including wrinkles, telangiectasia and pigmentary alterations induced by ultraviolet radiation. 11 – 15 Despite the challenges of studying skin cancer, owing to its multifactorial pathogenesis and long lead time, the following evidence supports the use of sunscreen in the prevention of skin cancer.
Experimental studies from the 1980s and 1990s showed that sunscreens protect against cell damage consistent with carcinogenesis in animal models. 16 , 17 A well-conducted community-based 4.5-year RCT of 1621 adult Australians, with follow-up for more than a decade, found a 40% lower incidence of squamous cell carcinomas among participants randomized to recommended daily sunscreen compared with participants assigned to use sunscreen on a discretionary basis (rate ratio 0.61, 95% confidence interval [CI] 0.46 to 0.81). 4 , 18 However, the incidence of basal cell carcinomas was not significantly reduced, possibly owing to the protracted pathogenesis of basal cell carcinomas. 18 Almost 15 years after the completion of the study, participants who used sunscreen daily throughout the 4.5-year study period showed a significantly reduced risk of invasive melanoma (hazard ratio [HR] 0.27, 95% CI 0.08 to −0.97), although very few invasive melanomas were noted, given the long lead time for this type of tumour. 5 A predefined subgroup analysis in this trial confirmed that regular use of sunscreen over a 4.5-year period can arrest signs of skin aging caused by photodamage. 12 Another large Australian RCT showed a significantly reduced rate of development of actinic keratoses (a precursor to squamous cell carcinoma) among participants randomized to regular use of sunscreen, compared with controls who used a nonactive base cream over 1 summer season (rate ratio 0.62, 95% CI 0.54 to −0.71). 19
In organ transplant recipients, a population at high risk of morbidity and death from skin cancer, a prospective single-centre study of 120 matched patients showed that the use of sun protection factor (SPF) 50 sunscreen over 24 months reduced the development of actinic keratoses, squamous cell carcinomas and, to a lesser extent, basal cell carcinomas. 20 Recent meta-analyses have not supported the findings of these RCTs, finding no significant effectiveness of sunscreen for preventing either melanoma or nonmelanoma skin cancers. 21 , 22 However, these meta-analyses included studies with retrospective designs with methodological inconsistencies among studies, and 1 included studies that used only UVB filters (rather than broad-spectrum sunscreens). 21 Overall, the highest-quality evidence available suggests that sunscreens do prevent skin cancer.
- Who should use sunscreen?
The American Academy of Dermatology recommends regular sunscreen use with an SPF of 30 or higher for people of all skin types, 23 although skin cancers are far more prevalent in White individuals than people with darker skin. 24 There have been no studies to assess the effectiveness of regular sunscreen use in reducing the risk of skin cancers among people who are not White.
For children older than 6 months, as well as adults, the Canadian Dermatology Association recommends the use of broad-spectrum sunscreens with an SPF of 30 or greater. 7 Split-face studies have shown that sunscreens with an SPF of 100 are superior to sunscreens with an SPF of 50 for preventing sunburns under actual use conditions, in both a beach setting 25 and a high-altitude skiing setting. 26
Health Canada does not recommend the use of sunscreen for children younger than 6 months because of the theoretical risk of increased absorption of sunscreen ingredients as a result of higher body surface-to-volume ratios and thinner epidermis. 27 The mainstays of sun safety in infants include sun avoidance and protective clothing. 28 If sunscreen is used in infants, experts suggest washing it off as soon as it is no longer needed, 29 and favouring physical sunscreens over chemical varieties.
- How should sunscreen be applied?
Observational studies have shown that consumers typically underapply sunscreen, with standard use ranging between 20% and 50% of the recommended application. 30 – 32 However, using sunscreens with higher SPFs may compensate for underapplication. 26 For example, when a sunscreen with an SPF of 50 is applied under real-world conditions, the sunscreen may provide an SPF of only 25.
A 2015 Canadian consensus meeting agreed that the wording “apply sunscreen generously” was most appropriate, given differences in body habitus of the public. 33 Figure 2 offers a rough estimate of the quantities of sunscreen that should be applied by a person of average height and build, based on advice from the Canadian Cancer Society and the American Academy of Dermatology.
Visual aid to guide the correct application of sunscreen for a person of average height and body habitus, based on advice from the Canadian Cancer Society and the American Academy of Dermatology.
Although product labelling often suggests that sunscreens should be applied 15 to 30 minutes before going outdoors, 34 in a recent study, immediate protection against ultraviolet radiation occurred after sunscreen application, although protection after water exposure was not examined. 35 Therefore, it may be prudent to wait 15 to 30 minutes if water resistance is required.
Recent experimental studies have shown that sunscreen remains on the skin at the desired SPF for as long as 8 hours after a single application, 35 – 38 suggesting that historical advice to reapply sunscreen every 2–3 hours need not be followed even when individuals are physically active. However, reapplication is suggested when the likelihood of sunscreen having been removed is high, such as after sweating, water immersion, friction from clothing and exfoliation from sand. 39 – 41 When swimming or sweating are anticipated, water-resistant sunscreens should be used. 40
Spray-on sunscreens are less desirable than cream-based ones, for several reasons. Wind can disperse the sunscreen, resulting in inadequate application. Moreover, because spray-on sunscreens are often fast drying, and sometimes not clearly visible once sprayed onto the skin, it is difficult to determine whether application was homogeneous. 42 Aerosolized sunscreens are also flammable, and several incidences of combustion on the skin have been reported after exposure to open flames, even after the sunscreen has been allowed to dry. Finally, the potential risks associated with inhalation of aerosolized sunscreens have not been adequately studied. 43
- What are the key safety concerns?
Skin reactions
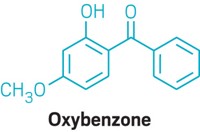
The most common reported adverse reactions to sunscreens include subjective irritation (e.g., stinging and burning) without a rash, irritant contact dermatitis and comedogenicity. Rarely, chemical sunscreen ingredients may also cause allergic contact dermatitis and photoallergic contact dermatitis, with the most commonly implicated allergenic ingredients being octocrylene, oxybenzone and octyl methoxycinnamate. 44
Absorption of sunscreen
In 2019, a small RCT with 24 participants, sponsored by the United States Food and Drug Administration, showed systemic absorption of 4 sunscreen ingredients: oxybenzone, avobenzone, octocrylene and ecamsule. 45 When applied under maximal use conditions, over 4 consecutive days, blood levels for these compounds exceeded those recommended by US Food and Drug Administration guidelines. 45 Moreover, the investigators noted long half-lives for each of these ingredients, suggesting that regular sunscreen use may lead to accumulation within the body. 46 A follow-up study confirmed these findings. 47 However, most people use far less than this volume of sunscreen and, despite their findings, the study investigators encouraged the use of sunscreen given its known protective effects, as the clinical importance of absorption of these ingredients is not yet known. Further research is needed to determine whether there are any potential health sequelae from absorption of sunscreen ingredients.
In contrast to chemical sunscreen ingredients, physical sunscreens are not systemically absorbed. An in-vitro study found that less than 0.03% of zinc nanoparticles penetrated the uppermost layer of the stratum corneum, and no particles were detected in the lower stratum corneum. 48 Physical sunscreens historically were less cosmetically appealing than chemical sunscreens, leaving a white residue on the skin, potentially leading to underapplication. Advances in formulation and micronization of physical ultraviolet radiation filters has led to more cosmetically acceptable physical sunscreens. 49
Endocrine effects
Low-quality evidence has led to concerns about possible estrogenic and antiandrogenic effects of chemical sunscreens. Although a recent meta-analysis found that oxybenzone is associated with reproductive adverse effects in fish, the summarized literature was nonuniform and the results therefore uninformative. 50 Among human research participants, a prospective study noted reduced fecundity when men were exposed to benzophenone-2 and 4-hydroxybenzophenone, but the findings could be explained by study confounding. 51 One systemic review, which evaluated both animal and human studies, found that high levels of oxybenzone exposure during pregnancy were associated with decreased gestational age in male neonates and decreased birthweight in female neonates. 50 However, high heterogeneity limited the usefulness of the study findings. 50
- How do sunscreens affect the environment?
Some recent studies have reported that chemical sunscreen ingredients are detectable in various water sources 52 , 53 and may persist despite waste-water treatment processing. 54 An additional recent concern is the detection of sunscreen filters in the tissues of various fish species, raising the possibility of bioaccumulation and biomagnification. 55
The effects of sunscreen ingredients on coral reefs are a current focus of scientific investigation. In-vitro studies have shown that oxybenzone affects coral reef larvae 56 and may be implicated in coral reef bleaching. However, possible confounding variables include increased ocean salinity and temperature associated with global warming. 55 These preliminary studies have prompted the banning of oxybenzone and octinoxate in some jurisdictions. 57
- What additional photoprotective measures may be used?
Sunscreen is only one part of a comprehensive photoprotection strategy. It is important to counsel patients regarding behaviours for avoiding ultraviolet radiation, including the use of wide-brimmed hats, eye protection (e.g., “wrap-around” sunglasses with ultraviolet radiation protection) and seeking shade when the ultraviolet index is above 3 (usually 11 am–3 pm, April to September in Canada). 33 Typically, thicker clothing with tighter weave fabrics — such as polyester and cotton, or nylon and elastane (i.e., Spandex, Lycra) — and darker colours offer greater protection. 58 , 59 Clothing has been designed for sun protection with an ultraviolet protection factor (UPF) up to 50. 28 All clothing will become less photoprotective if it is wet or stretched. 59
- Potential new sunscreen technologies
Topical photolyases and antioxidants (vitamin C, vitamin E, selenium and polyphenols found within green tea extracts) are emerging as potential agents of topical and nontopical photoprotection. Antioxidants cannot yet be stabilized within sunscreen formulations to remain biologically active. Studies have established that sunscreens that claim antioxidant activity have little to no actual antioxidant activity. 60 – 62
Photoprotective agents taken orally, such as niacinamide and Polypodium leucotomos extract, which is derived from a fern native to Central and South America, are used as agents for prevention of photodamage. There is evidence from small RCTs that P. leucotomos extract increases the minimal erythema dose of sun exposure without significant adverse effects, and is helpful for dermatologic diseases induced by ultraviolet radiation, such as polymorphous light eruption and solar urticaria. 63 – 65
Nicotinamide, also known as niacinamide, is the active amide form of niacin (vitamin B3). However, unlike niacin, it does not cause cutaneous flushing. Nicotinamide has been shown in early studies to enhance DNA repair and decrease the formation of cyclobutene pyrimidine dimers in human keratocytes. 62 In one phase III RCT, which has not been replicated, nicotinamide 500 mg twice daily was associated with a decreased rate of development of both actinic keratoses and nonmelanoma skin cancers over a 12-month period. 66 However, the skin cancers that did occur tended to be high-grade malignancies.
Exposure to ultraviolet radiation is directly harmful and has been associated with the development of skin cancers, which are common in Canada. High-quality evidence has shown that sunscreen reduces the risk of developing both melanoma and nonmelanoma skin cancer. Therefore, physicians should counsel patients on photoprotection strategies, including avoiding midday sun, seeking shade and wearing protective clothing, as well as using sunscreen if sun exposure cannot be avoided. Presently, the Canadian Dermatology Association recommends the use of a broad-spectrum sunscreen with an SPF of at least 30 for people older than 6 months, for photoprotection. Low-quality evidence has shown that some chemical sunscreen ingredients are systemically absorbed and may be contributing to environmental damage; people who are concerned may consider using physical sunscreens as an alternative. Research on the safety and efficacy of established sunscreens and novel agents is ongoing.
Competing interests: Toni Burbidge reports receiving honoraria from AbbVie, Celgene, Janssen, Leo Pharmaceuticals and Lilly. No other competing interests were declared.
This article has been peer reviewed.
Contributors: All of the authors contributed to the conception and design of the work, and the acquisition, analysis and interpretation of data. All of the authors drafted the manuscript, revised it critically for important intellectual content, gave final approval of the version to be published and agreed to be accountable for all aspects of the work.
- ↵ Canadian Cancer Society’s Advisory Committee on Cancer Statistics . Canadian cancer statistics 2014: special topic: skin cancers . Canadian Cancer Statistics . Toronto : Canadian Cancer Society ; 2014 : 1 – 132 . Available: www.cancer.ca/~/media/cancer.ca/CW/cancerinformation/cancer101/Canadiancancerstatistics/Canadian-Cancer-Statistics-2014-EN.pdf ( accessed 2020 Mar. 15 ).
- Geller AC ,
- Miller DR ,
- Parkin DM ,
- Williams G ,
- Williams GM ,
- ↵ Prevent skin cancer . Schaumburg (IL) : American Academy of Dermatology ; 2016 : 1 . Available: www.aad.org/public/diseases/skin-cancer/prevent/how ( accessed 2020 Mar. 15 ).
- ↵ Canadian Dermatology Association position statement sun protection and sunscreen use . Ottawa : Canadian Dermatology Association ; 2020 . Available: https://dermatology.ca/wp-content/uploads/2020/02/Sun-Protection-and-Sunscreen-Use-Position-Statement-EN.pdf ( accessed 2020 Mar. 15 ).
- Gasparro FP ,
- Mitchnick M ,
- Geoffrey K ,
- Mwangi AN ,
- ↵ Draft: guidance document — sunscreen monograph . Ottawa : Health Canada ; 2012 . Available: www.canada.ca/en/health-canada/services/drugs-health-products/public-involvement-consultations/natural-health-products/draft-guidance-document-sunscreen-monograph-consultation-document.html ( accessed 2020 Mar. 10 ).
- Cameron GS ,
- Hughes MCB ,
- Phillips TJ ,
- Reinhold K ,
- Jaenicke T ,
- Iannacone MR ,
- Sambuco CP ,
- Forbes PD ,
- Davies RE ,
- Ananthaswamy HN ,
- Loughlin SM ,
- van der Pols JC ,
- Pandeya N ,
- Thompson SC ,
- Jürgensen JS ,
- Tavares R ,
- da Paulitsch FS ,
- Rueegg CS ,
- Stenehjem JS ,
- ↵ Sunscreen FAQs . Des Plaines (IL) : American Academy of Dermatology Association ; 2020 . Available: www.aad.org/media/stats-sunscreen ( accessed 2020 Aug. 16 ).
- Gloster HM ,
- Nicholson CL ,
- Williams JD ,
- Atillasoy E ,
- ↵ Recalls and safety alerts: important sunscreen safety tips for Canadians . Ottawa : Health Canada ; 2018 . Available: https://healthycanadians.gc.ca/recall-alert-rappel-avis/hc-sc/2018/66966a-eng.php ( accessed 2020 Mar. 15 ).
- Colantonio S ,
- Cestari T ,
- Petersen B ,
- Philipsen PA ,
- Marrett LD ,
- Atkinson J ,
- ↵ Labeling and effectiveness testing: sunscreen drug products for over-the-counter human use — small entity compliance guide . Silver Spring (MD) : US Food and Drug Administration ; 2012 . Available: www.fda.gov/regulatory-information/search-fda-guidance-documents/labeling-and-effectiveness-testing-sunscreen-drug-products-over-counter-human-use-small-entity ( accessed 2020 Mar. 16 ).
- de Gálvez MV ,
- Aguilera J ,
- Buendía EA ,
- Bodekær M ,
- Akerström U ,
- Faurschou A ,
- Bodekaer M ,
- Stokes RP ,
- Goldsmith WT ,
- Greenwald R ,
- Rodríguez E ,
- Valbuena MC ,
- Zusterzeel R ,
- Califf RM ,
- Florian J ,
- Shanmuga SC ,
- Ghazipura M ,
- McGowan R ,
- Buck Louis GM ,
- Balmer ME ,
- Buser H-R ,
- Müller MD ,
- da Silva CP ,
- Emídio ES ,
- de Marchi MRR
- Schneider SL ,
- Kramarsky-Winter E ,
- Ouchene L ,
- Litvinov IV ,
- Netchiporouk E
- Osterwalder U ,
- Arellano-Mendoza M-I ,
- Choudhry SZ ,
- Ceilley R ,
- Nestor MS ,
- Middelkamp-Hup MA ,
- Pathak MA ,
- Parrado C ,
- Martin AJ ,
In this issue

- Table of Contents
- Index by author
Article tools
Thank you for your interest in spreading the word on CMAJ.
NOTE: We only request your email address so that the person you are recommending the page to knows that you wanted them to see it, and that it is not junk mail. We do not capture any email address.
Citation Manager Formats
- EndNote (tagged)
- EndNote 8 (xml)
- RefWorks Tagged
- Ref Manager

- Tweet Widget
- Facebook Like
Jump to section
Related articles.
- Efficacité et innocuité des écrans solaires pour la prévention du cancer de la peau
- Google Scholar
Cited By...
- Knowledge of Sunscreen Usage and Skin Cancer Among Malaysian Medical Students - A cross sectional study
More in this TOC Section
- Diagnosis and treatment of hyperemesis gravidarum
- Advances in the management of renal cell carcinoma
- Diagnosis and management of eosinophilic esophagitis
Similar Articles

An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
- My Bibliography
- Collections
- Citation manager
Save citation to file
Email citation, add to collections.
- Create a new collection
- Add to an existing collection
Add to My Bibliography
Your saved search, create a file for external citation management software, your rss feed.
- Search in PubMed
- Search in NLM Catalog
- Add to Search
Sunscreen and prevention of skin aging: a randomized trial
Affiliation.
- 1 Queensland Institute of Medical Research and University of Queensland, School of Population Health, Queensland, Australia.
- PMID: 23732711
- DOI: 10.7326/0003-4819-158-11-201306040-00002
Background: Sunscreen use and dietary antioxidants are advocated as preventives of skin aging, but supporting evidence is lacking.
Objective: To determine whether regular use of sunscreen compared with discretionary use or β-carotene supplements compared with placebo retard skin aging, measured by degree of photoaging.
Design: Randomized, controlled, community-based intervention. (Australian New Zealand Clinical Trials Registry: ACTRN12610000086066).
Setting: Nambour, Australia (latitude 26° S).
Patients: 903 adults younger than 55 years out of 1621 adults randomly selected from a community register.
Intervention: Random assignment into 4 groups: daily use of broad-spectrum sunscreen and 30 mg of β-carotene, daily use of sunscreen and placebo, discretionary use of sunscreen and 30 mg of β-carotene, and discretionary use of sunscreen and placebo.
Measurements: Change in microtopography between 1992 and 1996 in the sunscreen and β-carotene groups compared with controls, graded by assessors blinded to treatment allocation.
Results: The daily sunscreen group showed no detectable increase in skin aging after 4.5 years. Skin aging from baseline to the end of the trial was 24% less in the daily sunscreen group than in the discretionary sunscreen group (relative odds, 0.76 [95% CI, 0.59 to 0.98]). β-Carotene supplementation had no overall effect on skin aging, although contrasting associations were seen in subgroups with different severity of aging at baseline.
Limitation: Some outcome data were missing, and power to detect moderate treatment effects was modest.
Conclusion: Regular sunscreen use retards skin aging in healthy, middle-aged men and women. No overall effect of β-carotene on skin aging was identified, and further study is required to definitively exclude potential benefit or potential harm.
Primary funding source: National Health and Medical Research Council of Australia.
PubMed Disclaimer
Summary for patients in
- Summaries for patients. Sunscreen and prevention of skin aging. [No authors listed] [No authors listed] Ann Intern Med. 2013 Jun 4;158(11):I-28. doi: 10.7326/0003-4819-158-11-201306040-00001. Ann Intern Med. 2013. PMID: 23732729 No abstract available.
Similar articles
- A randomized controlled trial to assess sunscreen application and beta carotene supplementation in the prevention of solar keratoses. Darlington S, Williams G, Neale R, Frost C, Green A. Darlington S, et al. Arch Dermatol. 2003 Apr;139(4):451-5. doi: 10.1001/archderm.139.4.451. Arch Dermatol. 2003. PMID: 12707092 Clinical Trial.
- Does daily use of sunscreen or beta-carotene supplements prevent skin cancer in healthy adults? Del Mar C. Del Mar C. West J Med. 2000 Nov;173(5):332. West J Med. 2000. PMID: 11069872 Free PMC article. No abstract available.
- Effects of sunscreen on skin cancer and photoaging. Iannacone MR, Hughes MC, Green AC. Iannacone MR, et al. Photodermatol Photoimmunol Photomed. 2014 Apr-Jun;30(2-3):55-61. doi: 10.1111/phpp.12109. Epub 2014 Feb 19. Photodermatol Photoimmunol Photomed. 2014. PMID: 24417448 Review.
- Photoaging: prevention and topical treatments. Antoniou C, Kosmadaki MG, Stratigos AJ, Katsambas AD. Antoniou C, et al. Am J Clin Dermatol. 2010;11(2):95-102. doi: 10.2165/11530210-000000000-00000. Am J Clin Dermatol. 2010. PMID: 20141230 Review.
- Clinical Applications of Sunscreens and Formulation Advancements. Sunena, Tomar D, Jawla S. Sunena, et al. Curr Drug Res Rev. 2024;16(2):198-208. doi: 10.2174/2589977515666230718124841. Curr Drug Res Rev. 2024. PMID: 37464824 Review.
- Antioxidants in Sunscreens: Which and What For? Jesus A, Mota S, Torres A, Cruz MT, Sousa E, Almeida IF, Cidade H. Jesus A, et al. Antioxidants (Basel). 2023 Jan 6;12(1):138. doi: 10.3390/antiox12010138. Antioxidants (Basel). 2023. PMID: 36670999 Free PMC article.
- Review on photoprotection: a clinician's guide to the ingredients, characteristics, adverse effects, and disease-specific benefits of chemical and physical sunscreen compounds. McDonald KA, Lytvyn Y, Mufti A, Chan AW, Rosen CF. McDonald KA, et al. Arch Dermatol Res. 2023 May;315(4):735-749. doi: 10.1007/s00403-022-02483-4. Epub 2022 Nov 28. Arch Dermatol Res. 2023. PMID: 36443500 Review.
- Cost and quality in consumer sunscreen preferences. Hanna H, Patel S, Kundu RV. Hanna H, et al. Arch Dermatol Res. 2023 May;315(4):925-931. doi: 10.1007/s00403-022-02467-4. Epub 2022 Nov 22. Arch Dermatol Res. 2023. PMID: 36416977
- The Damaging Effects of Long UVA (UVA1) Rays: A Major Challenge to Preserve Skin Health and Integrity. Bernerd F, Passeron T, Castiel I, Marionnet C. Bernerd F, et al. Int J Mol Sci. 2022 Jul 26;23(15):8243. doi: 10.3390/ijms23158243. Int J Mol Sci. 2022. PMID: 35897826 Free PMC article. Review.
Publication types
- Search in MeSH
Related information
- Cited in Books
- PubChem Compound (MeSH Keyword)
LinkOut - more resources
Full text sources.
- Ovid Technologies, Inc.
Other Literature Sources
- The Lens - Patent Citations
- scite Smart Citations
- MedlinePlus Health Information
- Citation Manager
NCBI Literature Resources
MeSH PMC Bookshelf Disclaimer
The PubMed wordmark and PubMed logo are registered trademarks of the U.S. Department of Health and Human Services (HHS). Unauthorized use of these marks is strictly prohibited.
Advertisement
- Publications
This site uses cookies to enhance your user experience. By continuing to use this site you are agreeing to our COOKIE POLICY .
Grab your lab coat. Let's get started
Create an account below to get 6 c&en articles per month, receive newsletters and more - all free., it seems this is your first time logging in online. please enter the following information to continue., as an acs member you automatically get access to this site. all we need is few more details to create your reading experience., not you sign in with a different account..
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Already have an ACS ID? Log in here
The key to knowledge is in your (nitrile-gloved) hands
Access more articles now. choose the acs option that’s right for you..
Already an ACS Member? Log in here
$0 Community Associate
ACS’s Basic Package keeps you connected with C&EN and ACS.
- Access to 6 digital C&EN articles per month on cen.acs.org
- Weekly delivery of the C&EN Essential newsletter
$80 Regular Members & Society Affiliates
ACS’s Standard Package lets you stay up to date with C&EN, stay active in ACS, and save.
- Access to 10 digital C&EN articles per month on cen.acs.org
- Weekly delivery of the digital C&EN Magazine
- Access to our Chemistry News by C&EN mobile app
$160 Regular Members & Society Affiliates $55 Graduate Students $25 Undergraduate Students
ACS’s Premium Package gives you full access to C&EN and everything the ACS Community has to offer.
- Unlimited access to C&EN’s daily news coverage on cen.acs.org
- Weekly delivery of the C&EN Magazine in print or digital format
- Significant discounts on registration for most ACS-sponsored meetings

Your account has been created successfully, and a confirmation email is on the way.
Your username is now your ACS ID.
Consumer Safety
More evidence that sunscreens absorb through skin, us fda finds 6 active ingredients in blood plasma of study participants, by britt e. erickson, january 22, 2020 | a version of this story appeared in volume 98, issue 4.
- How researchers found arsenic and lead in tampons
- Accidental mix of bleach and acid kills Buffalo Wild Wings employee
- What is an allergy sensitizer, and how does a chemical become one?
- 10 years after Sheri Sangji’s death, are academic labs any safer?
The US Food and Drug Administration has confirmed that six active ingredients widely found in sunscreens penetrate through the skin and absorb into blood plasma. The agency’s findings, published on Jan. 21, put pressure on manufacturers to determine whether such exposure to sunscreen ingredients is safe ( JAMA 2020, DOI: 10.1001/jama.2019.20747 ).

“The fact that an ingredient is absorbed through the skin and into the body does not mean that the ingredient is unsafe,” Janet Woodcock, director of the FDA’s Center for Drug Evaluation and Research, says in a statement . “Rather, this finding calls for further industry testing to determine the safety and effect of systemic exposure of sunscreen ingredients, especially with chronic use.”
The FDA has yet to finalize a rule proposed in February 2019 that would require sunscreen manufacturers to provide safety data if their products contain certain ingredients, so that the agency can evaluate whether the chemicals are generally recognized as safe and effective. Those ingredients include the six chemicals—avobenzone, homosalate, octinoxate, octisalate, octocrylene, and oxybenzone—the FDA tested in its new study. The FDA wants the additional data because of increased use of sunscreens and potential risks.
The latest study follows up on earlier FDA work that found that four sunscreen active ingredients absorb through the skin ( JAMA 2019, DOI: 10.1001/jama.2019.5586 ). Of the six compounds evaluated in the new study, three had been assessed previously and three were new. The FDA tested various formulations—lotion, aerosol spray, nonaerosol spray, and pump spray—on more people than the first study. After a single application, all six active ingredients in all tested formulations produced levels of the active ingredient in participants’ blood plasma greater than 0.5 ng/mL, the FDA’s threshold for potentially waiving safety studies.
Sign up for C&EN's must-read weekly newsletter
Contact us to opt out anytime
The Personal Care Products Council and the Consumer Healthcare Products Association, which represent sunscreen manufacturers, noted in a statement that “there were no serious drug-related adverse events reported in the trial, consistent with the excellent safety record associated with sunscreen active ingredients over decades of real-world use.”
While the industry conducts further testing, the FDA is advising consumers to continue using sunscreens in conjunction with other measures, such as wearing protective clothing, to reduce the risk of skin cancer.
You might also like...
- Share on Facebook
- Share on Linkedin
- Share on Reddit
This article has been sent to the following recipient:
Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter
The power is now in your (nitrile gloved) hands
Sign up for a free account to get more articles. or choose the acs option that’s right for you..
Already have an ACS ID? Log in
Create a free account To read 6 articles each month from
Join acs to get even more access to.
Sunscreens and Photoaging: A Review of Current Literature
- Review Article
- Published: 13 August 2021
- Volume 22 , pages 819–828, ( 2021 )
Cite this article

- Linna L. Guan 1 ,
- Henry W. Lim 1 &
- Tasneem F. Mohammad 1
21k Accesses
106 Citations
121 Altmetric
15 Mentions
Explore all metrics
Sunscreens have been on the market for many decades as a means of protection against ultraviolet-induced erythema. Over the years, evidence has also shown their efficacy in the prevention of photoaging, dyspigmentation, DNA damage, and photocarcinogenesis. In the USA, most broad-spectrum sunscreens provide protection against ultraviolet B (UVB) radiation and short-wavelength ultraviolet A (UVA) radiation. Evidence suggests that visible light and infrared light may play a role in photoaging and should be considered when choosing a sunscreen. Currently, there is a paucity of US FDA-approved filters that provide protection against long UVA (> 370 nm) and none against visible light. Additionally, various sunscreen additives such as antioxidants and photolyases have also been reported to protect against and possibly reverse signs of photoaging. This literature review evaluates the utility of sunscreen in protecting against photoaging and further explores the requirements for an ideal sunscreen.
Similar content being viewed by others

Sunscreens: An Update

Review on photoprotection: a clinician’s guide to the ingredients, characteristics, adverse effects, and disease-specific benefits of chemical and physical sunscreen compounds


Photoprotection: Concept, Classification, and Mechanism of Action
Avoid common mistakes on your manuscript.
The perception of sunscreen use has shifted from purely protecting against ultraviolet (UV)-induced erythema to broad-spectrum protection against not only erythema but also photoaging, dyspigmentation, DNA damage, and photocarcinogenesis. |
Evidence suggests that visible light and infrared light may play a role in photoaging and should be considered when choosing a sunscreen. A broad-spectrum tinted sunscreen with sun protection factor (SPF) ≥ 30 used daily will offer protection against UV radiation and visible light to reduce their effects on photoaging. |
Sunscreen additives such as antioxidants, photolyases, and more have not only opened the door to improved photoprotection against skin aging but also the exploration of newer theories in the reversal of skin aging, but larger-scale and replicable studies are needed before clinical guidelines can be issued. |
1 Introduction
Chronic sun exposure has long been known to cause photoaging, a process where the skin undergoes changes in epidermal thickness, increases in pigment heterogeneity and dermal elastosis, degradation of collagen in the dermis, development of ectatic vessels, and increases in mutagenesis of keratinocytes and melanocytes in the skin [ 1 ]. Clinically, this is characterized by an increase in rhytides, telangiectasias, dyspigmentation including lentigines and ephelides, volume loss, and cutaneous malignancies [ 1 ]. A recent observational study further characterized skin aging as hypertrophic and atrophic variants, with atrophic photoaging presenting with erythema and increased risk of skin cancers and hypertrophic photoaging with increased skin thickness and sallowness [ 2 ].
In today’s society, the value placed on a youthful appearance is reflected in the multibillion-dollar industry centered around anti-aging products [ 3 , 4 ]. It has been reported that approximately 80% of skin aging on the face can be attributed to ultraviolet (UV) exposure [ 5 ]. Therefore, despite the emphasis of the market on the reversal of skin aging, the best defense against cutaneous age-related changes is through prevention with rigorous photoprotection [ 4 ]. It should be noted that proper photoprotection consists of seeking shade when outdoors; wearing a wide-brimmed hat, photoprotective clothing, and sunglasses; and applying sun protection factor (SPF) ≥ 30 broad-spectrum tinted sunscreen on exposed sites.
In the USA, most broad-spectrum sunscreens provide protection against UVB radiation and short wavelength UVA radiation. However, there is a paucity of US FDA-approved filters that provide protection against long UVA (> 370 nm) and none against visible light (VL), making the ideal sunscreen a product that requires further innovation and research. Notable exceptions are pigmentary grade zinc oxide and titanium dioxide, which reflect VL; however, the whitish discoloration they leave on the surface of the skin makes them cosmetically unappealing to consumers. This review evaluates the utility of sunscreen in protecting against photoaging and further explores the requirements of an ideal sunscreen.
2 Electromagnetic Radiation and Photoaging
Solar UV radiation (UVR) consists of UVA (320–400 nm), UVB (280–320 nm), and UVC (100–280 nm). UVA is further categorized as UVA1 (340–400 nm) and UVA2 (320–340 nm). UVC is the shortest wavelength and considered the most damaging type of UVR. However, it is completely absorbed by the ozone and does not reach the earth’s surface [ 6 ].
UVB is the major portion of UVR that induces sunburns or UV-induced erythema. It is known to be significantly more erythemogenic than UVA [ 6 ]. For example, for skin phototype I, the minimal erythema dose for UVB is 20–40 mJ/cm 2 , whereas that for UVA is 20–40 J/cm 2 . Although UVB accounts for approximately 6% of all UVR that reaches the earth’s surface, it is more cytotoxic than UVA, causing direct DNA damage through photon absorption in the form of cyclobutane pyrimidine dimers (CPDs) or 6,4-photoproducts that eventually induce mutagenesis and skin cancers [ 7 , 8 ]. UVB has been shown to be highly associated with the development of squamous cell carcinomas [ 9 ]. Additionally, even suberythemal doses of UVB have been shown to induce CPD formation and therefore increased p53 expression as cells undergo apoptosis or repair [ 10 ]. UVB has also been shown to induce matrix metalloproteinases (MMPs), reactive oxygen species (ROS), and elastases involved in photoaging [ 11 ].
UVB is predominantly absorbed by the skin’s epidermis, whereas UVA has a longer wavelength and therefore deeper dermal penetration, making it the primary driver of photoaging [ 12 ]. Although UVA is lower in energy than UVB, it is approximately 20 times more abundant in the earth’s atmosphere and is not blocked by glass [ 13 ]. The ratio of UVB/UVA varies by season [ 14 ]. Studies of UVA on skin models demonstrated that UVA caused the induction of apoptosis in dermal fibroblasts and increased MMP levels, which are enzymes involved in collagen degradation [ 12 , 15 ]. Additionally, repeat exposure to UVA on in vivo human skin induced elevated markers of photoaging, such as ferritin and lysozyme, which are involved in the oxidative stress response and elastin degradation, respectively [ 16 ]. In a study looking at asymmetric UVA exposure of the face, chronic exposure to UVA significantly affected the clinical level of wrinkling and roughness of the skin [ 17 ]. Furthermore, in a study of 22 participants exposed to multiple sessions of low-dose UVA1, increasing levels of MMP-1 and MMP-3 were observed in a dose-dependent response in the dermis, further highlighting the role of UVA in collagen breakdown and photoaging [ 13 ]. In skin of color, UVA has been shown to induce irregular spotty pigmentation associated with photoaging [ 12 ].
However, the effects of UVA and UVB are not always distinct, as overlapping cutaneous biologic effects have been observed. UVA has been shown to induce CPDs through ROS generated by photo-activation of UVA-absorbing molecules (chromophores) in the skin such as riboflavin, porphyrins, and heme-containing proteins [ 18 ]. Similarly, UVB has also been shown to induce dermal fibroblast senescence [ 19 ].
There is increasing evidence that infrared light (IR; 700 nm–1 mm) and VL (400–700 nm), predominantly in the blue light range (380–455 nm), play a role in photodamage and photoaging. Studies have demonstrated that VL can independently generate ROS, proinflammatory cytokines, and MMP-1 expression and potentiate the effects of UVR [ 20 , 21 , 22 , 23 ]. Effects of photoaging have also been observed with irradiation of skin within the UV/VL boundary region (385–405 nm), demonstrating differential expression of genes involved in inflammation, oxidative stress, and photoaging when compared with nonirradiated skin [ 24 ]. Likewise, in vivo skin irradiated with IR and VL has shown significantly increased MMP-1 and MMP-9 expression and decreased type I procollagen expression, implicating IR and VL light in the degradation of dermal collagen [ 25 ]. Moreover, studies have demonstrated that there is a synergistic relationship between even small amounts of UVA1 and VL in the induction of increased and prolonged pigmentation [ 21 , 26 ]. This suggests that VL and IR may play a significant but underreported role in photoaging and dyspigmentation.
Although the exact mechanisms are not yet fully understood, increasing literature indicates a need for photoprotection against the broad spectrum of electromagnetic radiation (UV, VL, and IR) to prevent photoaging.
3 Role of Sunscreens in Photoaging
The concept of a topical photoprotective product has been around since the times of the ancient Egyptians in 4000 BC, but the first commercial sunscreens were not available until the 1920–1930s [ 27 , 28 ]. At that time, understanding of UV radiation was limited and focused mainly on UVB protection. With the increasing popularity of sunscreen over the years, the concept of standardization of photoprotection against UVB was introduced [ 27 ]. SPF was recognized by the FDA in 1978 as the standard for measuring sun protection [ 27 ].
UV-induced erythema is mostly attributed to UVB, with a minor contribution by UVA2. The concept of SPF, an assessment using UV-induced erythema as an endpoint, as a sole measurement of sun protection persisted for many decades despite advances in the study of UVR suggesting that UVA may play a significant role in photoaging [ 27 , 29 , 30 ]. In 1992, the UVA star rating system was created by The Boots Company in the UK but was not widely implemented [ 27 ]. Although other methods of evaluating the efficacy of UVA filters have been proposed, the FDA currently uses critical wavelength (CW) determination. With this method, sunscreen products whose 90% UV absorbance occurs at ≥ 370 nm are allowed to be labeled as “broad spectrum” [ 31 ]. In Europe, the International Organization Standardization 24443 guidelines use a minimum ratio of UVA protection factor to SPF of 1:3 for all marketed sunscreens [ 32 ]. In a study of 20 sunscreens tested against the FDA guidelines and the ISO 24443 guidelines, 19 of 20 sunscreens met the CW requirements set by the FDA, whereas only 11 of 20 sunscreens met the ISO 24443 standard [ 31 ]. To address this disparity, the FDA proposed a new rule on sunscreens in 2019 that specifically highlighted a requirement for a UVA1 (340–400 nm) to UVA and UVB (290–400 nm) ratio of ≥ 0.7; however, the FDA has not yet made a final decision [ 33 ]. Clearly, there exists further need for global standardization to help protect and guide consumers.
In recent years, tinted sunscreens have become more prevalent as a means of protection against VL. Most FDA-approved compounds for UV protection do not adequately protect against VL because compounds must be opaque to filter VL [ 34 ]. Zinc oxide and titanium dioxide can protect against VL but only when they are pigmentary grade and not micronized. Tinted sunscreens incorporate combinations of iron oxides and pigmentary titanium dioxide to offer VL protection and utilize the different colors of iron oxides and pigmentary titanium dioxide to improve color match on people of all Fitzpatrick skin types [ 34 , 35 ]. It should be noted that iron oxides are not considered to be UV filters so are listed under “inactive ingredients” on sunscreen product packages, whereas pigmentary-grade titanium dioxide and zinc oxide are FDA-approved inorganic filters. However, the exact efficacy of specific tinted sunscreens for VL protection has been largely unregulated as no standards or guidelines for VL protection yet exist. A method for VL protection factor has been recently suggested using in vivo assessment in melano-competent subjects [ 22 , 36 ].
There is good evidence that daily photoprotection and daily sunscreen use plays an important role in the prevention of photoaging [ 37 , 38 ]. In a study of 46 patients randomly selected to use vehicle or sunscreens with UVA and UVB protection daily for 24 months, a significant histological difference in solar elastosis was observed in the vehicle versus treatment group [ 38 ]. Furthermore, in a study of 12 subjects in which each subject was exposed to one minimal erythemal dose of simulated solar radiation to three areas of buttock skin (unprotected skin, vehicle, and day cream with UVA and UVB protection) and control (no exposure), the unprotected skin demonstrated significant melanization, increased stratum corneum and stratum granulosum thickness, elevated expression of tenascin, reduced type I procollagen, and slightly increased lysozyme and alpha-1 antitrypsin, which were all mitigated by the day cream–sunscreen combination [ 39 ]. Not only have sunscreens been shown to prevent photoaging but evidence also suggests that they may play a role in the reversal of extrinsic aging. In a prospective study, 32 subjects were asked to apply daily broad-spectrum photostable sunscreen (SPF 30) for 52 weeks. At the end of the study, significant improvements in skin texture, clarity, and mottled and discrete pigmentation were observed, with 100% of subjects showing improvement in skin clarity and texture [ 40 ]. However, further research into the molecular mechanism of sunscreen’s effects on the reversal of chronologic aging must be performed.
4 Challenges and Limitations of Current Sunscreens
Sunscreen technology has made great advancements in accessibility, consumer acceptability, and overall safety and efficacy over the years. However, the challenges and limitations of current sunscreens leave room for further research and innovation. In the evaluation of sunscreens available for US consumers today, FDA regulations, safety in humans, and safety for the environment must be carefully considered.
In the 2019 proposed rule on sunscreens, the FDA proposed to categorize sunscreen filters as category I—“GRASE” (Generally Recognized as Safe and Effective), category II—non GRASE, or category III—requires further evaluation (Table 1 ) [ 41 ]. Currently, only two UV filters are category I: titanium dioxide and zinc oxide [ 42 ]. Both of these inorganic filters work by scattering, reflecting, and absorbing UV. The aggregation of these particles on the skin means they tend to leave a whitish hue on the skin that is unacceptable for many consumers, especially those with skin of color [ 43 , 44 ].
In the 2019 FDA-proposed rule, two ingredients, para-aminobenzoic acid (PABA) and trolamine salicylate, were classified as category II and banned from products marketed in the USA given their safety concerns. PABA has been linked to cases of allergic and photoallergic dermatitis and is a cross-sensitizer to sulfonamide antibiotics, thiazide diuretics, local anesthetics, and dyes [ 42 ]. Trolamine salicylate is a salicylate class of UV filters and has been linked to systemic absorption and increased risk of bleeding and salicylate toxicity [ 42 ]. It should be noted that neither of these has been used in the US market for years, so this categorization does not affect the US market.
Organic UV filters, dioxybenzone, sulisobenzone, oxybenzone, avobenzone, cinoxate, octinoxate, octisalate, homosalate, padimate O, ensulizole, meradimate, and octocrylene have now been categorized as category III, which means that additional data to determine the general recognition of safety is needed [ 42 ]. Organic UV filters absorb the higher energy of UV rays and emit a lower thermal energy [ 41 , 45 ]. It should be noted that the FDA is only requesting safety data for these 12 filters and did not question the efficacy of UV filters. None of the 12 category III UV filters offer effective visible light protection, and only meradimate and avobenzone offer partial UVA1 protection [ 41 ].
The organic UV filters can be categorized into cinnamates, benzophenones, salicylates, PABA derivatives, and others. Octinoxate, a cinnamate, is the most common sunscreen ingredient in the USA. It is photolabile and is often combined with other UVB absorbers to increase both its final SPF and its photostability [ 46 ].
The benzophenones include dioxybenzone, sulisobenzone, oxybenzone, and avobenzone, with oxybenzone the most commonly used agent in the group [ 46 ]. Although benzophenones have been shown to be effective UVA filters, their lack of photostability requires them to be compounded with other filters such as octocrylene, salicylates, micronized zinc oxide, and titanium dioxide to improve their photostability [ 44 , 46 , 47 ]. Additionally, oxybenzone is the most common photoallergen of the UV filters.
The salicylates octisalate and homosalate are only weak UVB absorbers and are mainly used in sunscreens as photostabilizers in combination with other organic filters [ 46 ]. Padimate O is a PABA derivative; like its predecessor, it has potent UVB filtration but is rarely used [ 44 , 46 ]. Ensulizole is primarily a UVB filter with minimal UVA2 activity [ 48 ]. Meradimate is a weak UVA blocker and has no activity against UVB [ 41 , 46 ]. Octocrylene is a photostable UVB and UVA2 filter primarily used as a photostabilizer in conjunction with other filters [ 46 ]. Ecamsule (Mexoryl SX) is an effective UVA filter that has been shown to be effective against photoaging when combined with UVB filters [ 49 ]. It has been approved via the new drug application process, with its use as an active ingredient permitted only in certain products under specific concentrations [ 41 , 43 , 44 ].
Although other photostable and more effective broad-spectrum UV filters, including bemotrizinol, bisoctrizole, and drometrizole trisiloxane, are available in other countries, these agents—along with many other UV filters available in other countries—are still pending FDA approval in the USA [ 27 , 41 ]. In over a decade, no new UV filters have been approved by the FDA to be added to the 16 currently approved filters. In contrast, the European Commission currently has 27 approved UV filters [ 27 ]. However, with the Coronavirus Aid, Relief, and Economic Security (CARES) Act signed into law in March 2020, the FDA has been mandated to move from a laborious rulemaking process to an administrative order process, which means it should not take as long to implement a monograph. The FDA is to issue a new proposed administrative order by 27 September 2021. Once the final administrative order has been enacted, industry has 12 months to comply. In addition, the CARES Act also incentivizes innovation by providing an 18-month exclusivity period to the requesting manufacturer of a new filter [ 50 ].
Controversy regarding organic sunscreen safety in humans has increasingly been a topic of discussion after studies showed systemic absorption of six commonly used sunscreen active ingredients [ 51 , 52 ]. This 2020 study of 48 randomized participants applying 2 mg/cm 2 of sunscreen product to 75% of body surface areas between one and four times per day for 4 days demonstrated systemic absorption of avobenzone, oxybenzone, octocrylene, homosalate, octisalate, and octinoxate [ 51 ]. However, a systematic review of 29 studies looking at the effects of two of the most commonly studied sunscreen ingredients—oxybenzone and octinoxate—demonstrated that oxybenzone had no adverse effects on male and female fertility, female reproductive hormone levels, adiposity, fetal growth, childhood neurodevelopment, or sexual maturation, and octinoxate had no effect on thyroid and reproductive hormone levels [ 53 ]. Although the review recommended further research into the effects of oxybenzone levels on thyroid hormone, testosterone level, kidney function, and pubertal timing, the evidence is not yet sufficient to support a causal relationship between the elevated systemic levels of oxybenzone or octinoxate and adverse health outcomes. Further longitudinal randomized controlled studies should be performed before factoring the biological effects of systemically absorbed agents into clinical and practical guidelines [ 54 , 55 ]. A recent report by Valisure LLC, an independent laboratory, also raised safety concerns regarding benzene in sunscreen products. After testing multiple batches of 69 brands of sunscreen and after-sun skincare products, they found that 78 batches contained elevated levels of benzene, a carcinogen known to cause leukemia and lymphoma [ 56 ]. It is important to note that both organic and inorganic sunscreens and some cosmetic products that did not contain any UV filters were among the contaminated products. In addition, many sunscreen products tested did not contain benzene. The report concluded that the contamination was due to supply chain issues in the manufacturing process rather than degradation of sunscreen filters. These findings led to an FDA citizen petition for the recall of identified batches of sunscreen with elevated levels of benzene and further investigation into these products and their manufacturing processes. A full report, including a list of products tested, can be found on the Valisure website [ 57 ].
Additionally, the National Oceanic and Atmospheric Administration identified ten sunscreen ingredients as being toxic to coral and marine life: oxybenzone, benzophenone-1, benzophenone-8, PABA, 4-methylbenzylidene camphor, 3-benzylidene camphor, nano-titanium dioxide, nano-zinc oxide, octinoxate, and octocrylene [ 58 ]. Studies that demonstrated marine toxicity were performed in vitro with high concentrations of sunscreen ingredients [ 44 , 55 , 59 ]. In a review looking at all 32 published studies until June 2020, 14 different organic UV filters in seawater near coral reefs were detected in the nanograms per liter range, in contrast to toxic levels in the micrograms per liter to milligrams per liter range reported in nine papers [ 60 ]. This puts the toxic levels of organic UV filters at 1000- to 1 million-fold higher concentrations than currently reported. Although 27 of the 32 reviewed studies showed no risk of UV filters to coral reefs, three studies of oxybenzone and octinoxate demonstrated a few data points where some risk was present [ 54 ]. This reflects the major data gaps that immediately need to be addressed with high-quality monitoring and toxicity studies applicable to the real world. To address this issue, on 9 February 2021, the National Academies formed a committee sponsored by the Environmental Protection Agency to study the environmental and health impacts of sunscreens. Although data supporting that the coral reefs are adversely impacted by environmental exposure to UV filters are limited, the state of Hawaii banned sunscreens containing oxybenzone and octinoxate in 2018, and Key West, Florida, USA, did the same in 2019 [ 59 ].
Although FDA guidelines aim to protect US consumers from harm, it has also greatly diminished the variety of UV filters available to consumers. Newer and more effective broad-spectrum UV filters are available in other countries but are not currently FDA approved [ 41 ]. With the new proposed administrative order under the CARES Act and careful consideration of human safety, environmental safety, photostability, and consumer cosmesis, the development and approval of new sunscreens that are effective against UVA, UVB, and VL must be considered for protection against photoaging.
5 Additives in Sunscreens
With the rise of cosmeceuticals and additives in sunscreens, it is important to evaluate the safety and efficacy of these substances. Although the exact mechanism of UVR- and VL-induced photoaging is still being explored, the downstream effects of increased ROS, MMPs, and DNA damage have been widely reported [ 8 , 11 ]. To combat the deleterious effects of sunlight on the skin, additives have been used or proposed in sunscreens to enhance photoprotection and help prevent photoaging.
Antioxidants play an important role in preventing, ameliorating, and dampening free radicals and oxidative stress. Although our bodies produce natural antioxidants, UVR and other stressors can often overwhelm our endogenous supply [ 61 ]. Topical antioxidants have been formulated into sunscreens to replenish depleted antioxidant supplies and diminish oxidative stress on the skin. Yet the exact role and efficacy of antioxidants in sunscreens remains controversial. A 2011 ex vivo study by Wang et al. [ 62 ] evaluated the radical skin protection factor (RSF) and antioxidant power (AP) of 12 sunscreen products containing vitamin C, vitamin E, or other antioxidant substances against simulated UVA- and UVB-induced ROS. RSF was defined as the ratio of free radicals in unprotected skin to protected skin, and AP evaluates the capacity and reaction time of antioxidants by measuring free electron spin [ 62 ]. They demonstrated that the RSF correlated with the UVA RSF rather than any antioxidant ingredients [ 62 ]. However, the study was performed ex vivo and may not correlate to in vivo responses in humans. More recent reviews and studies have demonstrated positive effects of the addition of antioxidants into sunscreen formulations. For example, a study looking at skin irradiated with UVB found that sunscreens with SPF 25 and a mixture of caffeine, vitamin E, vitamin C, Echinacea pallida extract, gorgonian extract, and chamomile essential oil demonstrated less MMP-1 expression than those with only SPF 25 [ 63 ]. The variability in the efficacy of antioxidants in sunscreens may depend on the formulation of the sunscreen. It has been proposed that, for antioxidants to be efficacious, they must have high antioxidative capacities, be present in high concentrations, be stable in the final formulation, and be able to penetrate the stratum corneum and still exist at high enough concentrations in the epidermis and dermis to be effective [ 61 ].
In terms of antioxidants that have been explored in topical formulations, vitamin C ( l -ascorbic acid) is the predominant antioxidant in the skin and plays an important role in the skin’s aqueous compartments because of its water solubility [ 61 ]. It also helps replenish vitamin E, acts as a cofactor in collagen synthesis, and reduces elastin accumulation [ 61 ]. It is not synthesized by the human body and must be replenished via oral intake [ 64 ]. Additionally, because of its ionic charge at physiologic pH, it cannot penetrate the stratum corneum without becoming unstable. Fortunately, a stable formulation can be made by compounding it with other antioxidants: vitamin E (alpha-tocopherol) and ferulic acid [ 61 , 64 ]. Murray et al. [ 65 ] demonstrated that skin irradiated with solar-simulated UVR after application of a topical formulation of 15% l -ascorbic acid, 1% alpha-tocopherol, and 0.5% ferulic acid (CEFer) for 4 days significantly decreased UV-induced erythema, sunburn cells, thymine dimers, and p53 induction when compared with untreated skin. Furthermore, vitamin E has been shown to be effective in the reduction of lipid peroxidation, photoaging, immunosuppression, and photocarcinogenesis in multiple animal and human studies [ 61 ]. This suggests a role for topical CEFer in protecting against photoaging and skin cancers [ 64 , 65 ].
Vitamin A and its derivatives, mainly retinoids and carotenoids, have been well studied in the realm of antiaging and have shown benefit in the prevention and reversal of photoaging [ 66 ]. They bind to cytoplasmic receptors such as cellular retinoic acid-binding protein types I and II and cellular retinol-binding protein as well as nuclear receptors such as nuclear retinoic acid receptors and retinoid X receptors to inhibit activation of protein-1 and MMP-1 expression [ 61 ]. This leads to increased epidermal proliferation, leading to epidermal thickening, compaction of the stratum corneum, synthesis and deposition of glycosaminoglycans, and increased collagen production [ 61 , 67 ]. Furthermore, there is evidence that topical retinoids may play a role in chemoprevention of nonmelanoma skin cancers through initiating growth arrest of tumor cells and normal cellular differentiation [ 68 ]. However, given the relative instability of retinol and retinoids when exposed to UV and visible light, their use as a sunscreen additive is predominantly for their anti-aging effects and not for increased photoprotection. They are rarely found in recreational sunscreens, and their stability is highly dependent on their formulation and chemical structure. For example, when tretinoin is compounded in ethanol, it undergoes isomerization within just a few seconds when irradiated with light of 300–800 nm [ 69 ]. The stability of tretinoin is improved when incorporated into liposomes [ 69 ]. Retinyl palmitate is an ester of retinol that is widely used in cosmetic products because of their high thermal stability when compared with retinol [ 70 ]. A study of 11 healthy volunteers using two formulations of retinyl palmitate for 60 days reported significant improvements in skin smoothness, skin roughness, scaliness, and wrinkles with both formulations [ 71 ]. Retinyl palmitate can be compounded with photostabilizers and UV filters and loaded onto nanotechnology-based drug-delivery systems to improve stability and drug penetration, but large-scale randomized controlled trials are needed to study the antiaging properties of these formulations [ 70 , 72 ]. Additionally, concerns have been raised regarding an increase in cutaneous malignancy with simultaneous use of topical retinyl palmitate and UVR exposure. A recent study looking at SKH-1 hairless mice treated with control cream or creams containing retinyl palmitate and subsequently irradiated with simulated solar light demonstrated an increased risk of photo-co-carcinogenesis in the group using cream containing retinyl palmitate [ 73 ]. However, these claims have not been largely substantiated or reported in humans and need to be further studied.
Other antioxidants that have been reported in the literature include soy extracts, polyphenols, melatonin, algae extract, and Polypodium leucotomos extract [ 30 ]. A study of 68 participants observed that soy moisturizer containing soybean-derived serine protease inhibitors (soybean trypsin inhibitor and Bowman–Birk protease inhibitor) significantly improved mottled pigmentation, blotchiness, dullness, fine lines, overall texture, overall skin tone, and overall appearance when compared with vehicle [ 74 ]. This positive clinical effect may be related to the role of soybean-derived serine protease inhibitors on the regulation of keratinocytes through keratinocyte protease-activated receptor 2, but additional studies must be performed to further elucidate its mechanism [ 74 ].
Polyphenols are found in many botanicals, including tea leaves, grape seeds ( Vitis vinifera ), blueberries, almond seeds, and pomegranate extract [ 75 ]. In a study of five participants, sunscreen compounded with tea extracts containing polyphenols such as epigallocatechin-3-gallate better protected human skin against solar-simulated UVR over sunscreen alone in regards to decreasing MMP-1 [ 63 ]. Additionally, green tea extract compounded with resveratrol, another polyphenol, provided SPF protection independent of physical and chemical UV filters, but additional in vivo studies must be performed to fully assess its effectiveness [ 76 ].
Melatonin acts as an antioxidant in three different but complementary ways. It can act as a free radical scavenger, decrease free radical generation, and upregulate antioxidant enzymes [ 77 ]. It has shown promise against both UVB- and UVA-induced oxidative stress. In studies of human melanocytes and keratinocytes, cells pretreated with melatonin decreased p53 expression, improved DNA repair, and decreased CPD generation [ 78 , 79 ]. An in vitro study of mouse fibroblast cells (NIH3T3) pretreated with melatonin and irradiated with UVA demonstrated increased heme-degrading enzymes and suppression of UVA-induced photodamage when compared with untreated irradiated cells [ 77 ]. Additionally, melatonin protected against UV-induced erythema and activated endogenous enzymes to act against oxidative stress [ 75 ]. This suggests a potential role of melatonin as an additive to protect keratinocytes, melanocytes, and fibroblasts against UV-induced photoaging.
Many studies have shown that multicellular algae not only have UV-absorbing properties but also provide benefits against oxidative stress [ 75 ]. Mycosporine-like amino acids (MAAs) produced by algae are potent UV filters with maximum absorption between 310 and 362 nm [ 80 ]. Shinorine is a commercialized MAA extracted from a type of red algae, Porphyra umbilicalis, and has already been used in sunscreens produced by two European companies [ 81 ]. Furthermore, the algae and algae products have also demonstrated protective properties against photoaging. Alga Corallina pilulifera methanol extract reduced MMP-2 and MMP-9 in UV-irradiated human dermal fibroblasts [ 82 ]. Additionally, many species of brown algae are protective against photo-oxidative stress [ 75 ]. With controversies around chemical sunscreens and their effects on marine life, algae-derived sunscreens may provide a future solution for eco-friendly photoprotection; however, most formulations of sunscreens with MAAs currently contain only a very small percentage of this active ingredient, and it functions as an adjuvant to UV filters and other sources of photoprotection [ 83 ].
Polypodium leucotomos extract (PLE) is derived from a tropical fern found in Central and South America and has antioxidative, chemoprotective, immunomodulatory, and anti-inflammatory effects [ 84 , 85 ]. In a recent study of 22 individuals irradiated with UVB, UVA, and VL, oral PLE demonstrated suppressive effects on UVB-induced erythema within 2 h of administration [ 84 ]. Oral PLE demonstrated similar photoprotective effects against VL. In a cross-over study, subjects taking PLE 480 mg daily demonstrated a significant decrease in persistent pigment darkening, delayed tanning, and cyclooxygenase-2 compared with pre-PLE [ 86 , 87 ]. Oral PLE should be taken daily to receive benefit and is meant to be an adjuvant to sunscreen, not a replacement. Topical formulations of PLE were also effective in reducing sunburn cells and reducing CPD in an in vitro reconstructed human epidermis model [ 87 ]. However, future in vivo studies must be performed to better assess the feasibility of topical PLE as a sunscreen additive.
In addition to antioxidants, photolyases are also beneficial additives in sunscreens. Photolyases are enzymes with a unique ability to repair DNA damage, specifically CPDs. They are flavoproteins and require flavonoids as cofactors to absorb UV radiation. The absorbed energy from UV radiation is then transferred to damaged DNA to break CPD bonds in both in vivo and in vitro studies [ 30 ]. It also significantly reduced markers of photoaging when added to SPF 50 sunscreen and antioxidants compared with sunscreen alone or sunscreen and antioxidants [ 88 ]. This suggests that photolyases may synergistically enhance the photoprotective effects of sunscreens and antioxidants [ 30 ].
The perception of sunscreen use has shifted from purely protecting against UV-induced erythema to broad-spectrum protection against not only erythema but also photoaging, dyspigmentation, DNA damage, and photocarcinogenesis. The impact of visible light and IR light in photoaging is still being explored, but better methods of protection against these wavelengths are needed. Sunscreens continue to be adapted to provide the broadest coverage while being cosmetically appealing. However, with the increased scrutiny of UV filters in the 2019 FDA proposed rule, new UV filters that are safe for humans and the environment, photostable, and consumer friendly must be developed and approved to offer continued sun protection for US consumers. When choosing a sunscreen, a broad-spectrum tinted sunscreen with SPF ≥ 30 used daily will offer protection against UVR and VL to reduce their effects on photoaging. Additionally, sunscreen additives such as antioxidants, photolyases, and more have opened the door for not only improved photoprotection against but also the reversal of skin aging. However, larger-scale and replicable studies must be performed before clinical guidelines can be issued.
Yaar M, Gilchrest BA. Photoageing: mechanism, prevention and therapy. Br J Dermatol. 2007;157:874–87.
Article CAS PubMed Google Scholar
Sachs DL, Varani J, Chubb H, Fligiel SEG, Cui Y, Calderone K, et al. Atrophic and hypertrophic photoaging: clinical, histologic, and molecular features of 2 distinct phenotypes of photoaged skin. J Am Acad Dermatol. 2019;81:480–8.
Anti-Aging Products-Market Study by Global Industry Analysts, Inc. [Internet]. [cited 2021 Mar 10]. Available from: https://www.strategyr.com/market-report-anti-aging-products-forecasts-global-industry-analysts-inc.asp .
Poon F, Kang S, Chien AL. Mechanisms and treatments of photoaging. Photodermatol Photoimmunol Photomed. 2015;31:65–74.
Flament F, Bazin R, Laquieze S, Rubert V, Simonpietri E, Piot B. Effect of the sun on visible clinical signs of aging in Caucasian skin. Clin Cosmet Investig Dermatol. 2013;6:221–32.
Article PubMed PubMed Central Google Scholar
Young AR, Claveau J, Rossi AB. Ultraviolet radiation and the skin: photobiology and sunscreen photoprotection. J Am Acad Dermatol. 2017;76:S100–9.
Miyamura Y, Coelho SG, Schlenz K, Batzer J, Smuda C, Choi W, et al. The deceptive nature of UVA-tanning versus the modest protective effects of UVB-tanning on human skin. Pigment Cell Melanoma Res. 2011;24:136–47.
Article PubMed Google Scholar
Marrot L, Meunier J-R. Skin DNA photodamage and its biological consequences. J Am Acad Dermatol (Elsevier). 2008;58:S139–48.
Article Google Scholar
de Gruijl FR, Sterenborg HJ, Forbes PD, Davies RE, Cole C, Kelfkens G, et al. Wavelength dependence of skin cancer induction by ultraviolet irradiation of albino hairless mice. Cancer Res. 1993;53:53–60.
PubMed Google Scholar
Seité S, Fourtanier A, Moyal D, Young AR. Photodamage to human skin by suberythemal exposure to solar ultraviolet radiation can be attenuated by sunscreens: a review. Br J Dermatol. 2010;163:903–14.
Article PubMed CAS Google Scholar
Pillai S, Oresajo C, Hayward J. Ultraviolet radiation and skin aging: roles of reactive oxygen species, inflammation and protease activation, and strategies for prevention of inflammation-induced matrix degradation—a review. Int J Cosmet Sci. 2005;27:17–34.
Battie C, Jitsukawa S, Bernerd F, Bino SD, Marionnet C, Verschoore M. New insights in photoaging, UVA induced damage and skin types. Exp Dermatol. 2014;23:7–12.
Wang F, Smith NR, Tran BAP, Kang S, Voorhees JJ, Fisher GJ. Dermal damage promoted by repeated low-level UV-A1 exposure despite tanning response in human skin. JAMA Dermatol. 2014;150:401.
Cole C, VanFossen R. Measurement of sunscreen UVA protection: an unsensitized human model. J Am Acad Dermatol. 1992;26:178–84.
Lee YK, Cha HJ, Hong M, Yoon Y, Lee H, An S. Role of NF-κB-p53 crosstalk in ultraviolet A-induced cell death and G1 arrest in human dermal fibroblasts. Arch Dermatol Res. 2012;304:73–9.
Séite S, Moyal D, Richard S, de Rigal J, Lévêque JL, Hourseau C, et al. Mexoryl SX: a broad absorption UVA filter protects human skin from the effects of repeated suberythemal doses of UVA. J Photochem Photobiol B. 1998;44:69–76.
Mac-Mary S, Sainthillier J-M, Jeudy A, Sladen C, Williams C, Bell M, et al. Assessment of cumulative exposure to UVA through the study of asymmetrical facial skin aging. Clin Interv Aging. 2010;5:277–84.
PubMed PubMed Central Google Scholar
McMillan TJ, Leatherman E, Ridley A, Shorrocks J, Tobi SE, Whiteside JR. Cellular effects of long wavelength UV light (UVA) in mammalian cells. J Pharm Pharmacol. 2008;60:969–76.
Cavinato M, Jansen-Dürr P. Molecular mechanisms of UVB-induced senescence of dermal fibroblasts and its relevance for photoaging of the human skin. Exp Gerontol. 2017;94:78–82.
Liebel F, Kaur S, Ruvolo E, Kollias N, Southall MD. Irradiation of skin with visible light induces reactive oxygen species and matrix-degrading enzymes. J Invest Dermatol. 2012;132:1901–7.
Kohli I, Chaowattanapanit S, Mohammad TF, Nicholson CL, Fatima S, Jacobsen G, et al. Synergistic effects of long-wavelength ultraviolet al and visible light on pigmentation and erythema. Br J Dermatol. 2018;178:1173–80.
Kohli I, Nahhas AF, Braunberger TL, Chaowattanapanit S, Mohammad TF, Nicholson CL, et al. Spectral characteristics of visible light-induced pigmentation and visible light protection factor. Photodermatol Photoimmunol Photomed. 2019;35:393–9.
Mahmoud BH, Hexsel CL, Hamzavi IH, Lim HW. Effects of visible light on the skin. Photochem Photobiol. 2008;84:450–62.
Lawrence KP, Douki T, Sarkany RPE, Acker S, Herzog B, Young AR. The UV/Visible Radiation Boundary Region (385–405 nm) damages skin cells and induces “dark” cyclobutane pyrimidine dimers in human skin in vivo. Sci Rep. 2018;8:12722.
Article PubMed PubMed Central CAS Google Scholar
Cho S, Lee MJ, Kim MS, Lee S, Kim YK, Lee DH, et al. Infrared plus visible light and heat from natural sunlight participate in the expression of MMPs and type I procollagen as well as infiltration of inflammatory cell in human skin in vivo. J Dermatol Sci. 2008;50:123–33.
Ruvolo E, Fair M, Hutson A, Liebel F. Photoprotection against visible light-induced pigmentation. Int J Cosmet Sci. 2018;40:589–95.
Ma Y, Yoo J. History of sunscreen: an updated view. J Cosmet Dermatol. 2021;20:1044–9.
Aldahan AS, Shah VV, Mlacker S, Nouri K. The history of sunscreen. JAMA Dermatol. 2015;151:1316.
Kligman AM. Early destructive effect of sunlight on human skin. JAMA. 1969;210:2377–80.
Yeager DG, Lim HW. What’s new in photoprotection: a review of new concepts and controversies. Dermatol Clin. 2019;37:149–57.
Wang SQ, Xu H, Stanfield JW, Osterwalder U, Herzog B. Comparison of ultraviolet A light protection standards in the United States and European Union through in vitro measurements of commercially available sunscreens. J Am Acad Dermatol (Elsevier). 2017;77:42–7.
Article CAS Google Scholar
14:00-17:00. ISO 24443:2012 [Internet]. ISO. [cited 2021 Mar 21]. Available from: https://www.iso.org/cms/render/live/en/sites/isoorg/contents/data/standard/04/65/46522.html .
Wang SQ, Lim HW. Highlights and implications of the 2019 proposed rule on sunscreens by the US Food and Drug Administration. J Am Acad Dermatol. 2019;81:650–1.
Lyons AB, Trullas C, Kohli I, Hamzavi IH, Lim HW. Photoprotection beyond ultraviolet radiation: a review of tinted sunscreens. J Am Acad Dermatol. 2021;84:1393–7.
Geisler AN, Austin E, Nguyen J, Hamzavi I, Jagdeo J, Lim HW. Visible light Part II. Photoprotection against visible and ultraviolet light. J Am Acad Dermatol. 2021;84:1233–44.
Article CAS PubMed PubMed Central Google Scholar
Lim HW, Kohli I, Granger C, Trullàs C, Piquero-Casals J, Narda M, et al. Photoprotection of the skin from visible light‒induced pigmentation: current testing methods and proposed harmonization. J Invest Dermatol. 2021;S0022-202X(21)01123–4.
Hughes MCB, Williams GM, Baker P, Green AC. Sunscreen and prevention of skin aging: a randomized trial. Ann Intern Med. 2013;158:781–90.
Boyd AS, Naylor M, Cameron GS, Pearse AD, Gaskell SA, Neldner KH. The effects of chronic sunscreen use on the histologic changes of dermatoheliosis. J Am Acad Dermatol. 1995;33:941–6.
Seité S, Fourtanier AMA. The benefit of daily photoprotection. J Am Acad Dermatol. 2008;58:S160-166.
Randhawa M, Wang S, Leyden JJ, Cula GO, Pagnoni A, Southall MD. Daily use of a facial broad spectrum sunscreen over one-year significantly improves clinical evaluation of photoaging. Dermatol Surg Off Publ Am Soc Dermatol Surg Al. 2016;42:1354–61.
CAS Google Scholar
Sabzevari N, Qiblawi S, Norton SA, Fivenson D. Sunscreens: UV filters to protect us: Part 1: changing regulations and choices for optimal sun protection. Int J Womens Dermatol. 2021;7:28–44.
Sunscreen Drug Products for Over-the-Counter Human Use [Internet]. Fed. Regist. 2019 [cited 2021 Mar 22]. Available from: https://www.federalregister.gov/documents/2019/02/26/2019-03019/sunscreen-drug-products-for-over-the-counter-human-use .
Forestier S. Rationale for sunscreen development. J Am Acad Dermatol (Elsevier). 2008;58:S133–8.
Mancuso JB, Maruthi R, Wang SQ, Lim HW. Sunscreens: an update. Am J Clin Dermatol. 2017;18:643–50.
Gabros S, Nessel TA, Zito PM. Sunscreens and photoprotection. StatPearls [Internet]. Treasure Island: StatPearls Publishing; 2021 [cited 2021 Mar 27]. Available from: http://www.ncbi.nlm.nih.gov/books/NBK537164/ .
Kullavanijaya P, Lim HW. Photoprotection. J Am Acad Dermatol. 2005;52:937–58.
Kim EJ, Kim MJ, Im NR, Park SN. Photolysis of the organic UV filter, avobenzone, combined with octyl methoxycinnamate by nano-TiO2 composites. J Photochem Photobiol B. 2015;149:196–203.
Nedorost S. Ensulizole (phenylbenzimidazole-5-sulfonic acid) as a cause of facial dermatitis: two cases. Dermatitis. 2005;16:148.
Seité S, Colige A, Piquemal-Vivenot P, Montastier C, Fourtanier A, Lapière C, et al. A full-UV spectrum absorbing daily use cream protects human skin against biological changes occurring in photoaging. Photodermatol Photoimmunol Photomed. 2000;16:147–55.
Mohammad TF, Lim HW. The important role of dermatologists in public education on sunscreens. JAMA Dermatol. 2021;157:509.
Matta MK, Florian J, Zusterzeel R, Pilli NR, Patel V, Volpe DA, et al. Effect of sunscreen application on plasma concentration of sunscreen active ingredients: a randomized clinical trial. JAMA. 2020;323:256.
Matta MK, Zusterzeel R, Pilli NR, Patel V, Volpe DA, Florian J, et al. Effect of sunscreen application under maximal use conditions on plasma concentration of sunscreen active ingredients: a randomized clinical trial. JAMA. 2019;321:2082.
Suh S, Pham C, Smith J, Mesinkovska NA. The banned sunscreen ingredients and their impact on human health: a systematic review. Int J Dermatol. 2020;59:1033–42.
Abbasi J. FDA trials find sunscreen ingredients in blood, but risk is uncertain. JAMA. 2020;323:1431–2.
Fivenson D, Sabzevari N, Qiblawi S, Blitz J, Norton BB, Norton SA. Sunscreens: UV filters to protect us: Part 2—increasing awareness of UV filters and their potential toxicities to us and our environment. Int J Womens Dermatol. 2021;7:45–69.
Valisure Detects Benzene in Sunscreen [Internet]. Valisure. 2021 [cited 2021 Jul 6]. Available from: https://www.valisure.com/blog/valisure-news/valisure-detects-benzene-in-sunscreen/ .
Valisure. Re: Valisure Citizen Petition on Benzene in Sunscreen and After-sun Care Products [Internet]. [cited 2021 Jul 26]. Available from: https://www.valisure.com/wp-content/uploads/Valisure-Citizen-Petition-on-Benzene-in-Sunscreen-and-After-sun-Care-Products-v9.7.pdf .
US Department of Commerce NO and AA. Sunscreen chemicals and marine life [Internet]. [cited 2021 Mar 27]. Available from: https://oceanservice.noaa.gov/news/sunscreen-corals.html .
Tsatalis J, Burroway B, Bray F. Evaluation of “reef safe” sunscreens: labeling and cost implications for consumers. J Am Acad Dermatol (Elsevier). 2020;82:1015–7.
Mitchelmore CL, Burns EE, Conway A, Heyes A, Davies IA. A critical review of organic ultraviolet filter exposure, hazard, and risk to corals. Environ Toxicol Chem. 2021;40:967–88.
Chen L, Hu JY, Wang SQ. The role of antioxidants in photoprotection: a critical review. J Am Acad Dermatol. 2012;67:1013–24.
Wang SQ, Osterwalder U, Jung K. Ex vivo evaluation of radical sun protection factor in popular sunscreens with antioxidants. J Am Acad Dermatol. 2011;65:525–30.
Matsui MS, Hsia A, Miller JD, Hanneman K, Scull H, Cooper KD, et al. Non-sunscreen photoprotection: antioxidants add value to a sunscreen. J Investig Dermatol Symp Proc. 2009;14:56–9.
Lin F-H, Lin J-Y, Gupta RD, Tournas JA, Burch JA, Selim MA, et al. Ferulic acid stabilizes a solution of vitamins C and E and doubles its photoprotection of skin. J Invest Dermatol (Elsevier). 2005;125:826–32.
Murray JC, Burch JA, Streilein RD, Iannacchione MA, Hall RP, Pinnell SR. A topical antioxidant solution containing vitamins C and E stabilized by ferulic acid provides protection for human skin against damage caused by ultraviolet irradiation. J Am Acad Dermatol. 2008;59:418–25.
Kligman AM, Grove GL, Hirose R, Leyden JJ. Topical tretinoin for photoaged skin. J Am Acad Dermatol (Elsevier). 1986;15:836–59.
Mukherjee S, Date A, Patravale V, Korting HC, Roeder A, Weindl G. Retinoids in the treatment of skin aging: an overview of clinical efficacy and safety. Clin Interv Aging. 2006;1:327–48.
Rosenthal A, Stoddard M, Chipps L, Herrmann J. Skin cancer prevention: a review of current topical options complementary to sunscreens. J Eur Acad Dermatol Venereol. 2019;33:1261–7.
Kryczyk-Poprawa A, Kwiecień A, Opoka W. Photostability of topical agents applied to the skin: a review. Pharmaceutics. 2019;12:10.
Article PubMed Central CAS Google Scholar
Benevenuto CG, Matteo MASD, Campos PMBGM, Gaspar LR. Influence of the photostabilizer in the photoprotective effects of a formulation containing UV-filters and vitamin A. Photochem Photobiol. 2010;86:1390–6.
Farooq U, Mahmood T, Shahzad Y, Yousaf AM, Akhtar N. Comparative efficacy of two anti-aging products containing retinyl palmitate in healthy human volunteers. J Cosmet Dermatol. 2018;17:454–60.
Oliveira MB, do Prado AH, Bernegossi J, Sato CS, Lourenço Brunetti I, Scarpa MV, et al. Topical application of retinyl palmitate-loaded nanotechnology-based drug delivery systems for the treatment of skin aging. BioMed Res Int. 2014;2014:632570.
Boudreau MD, Beland FA, Felton RP, Fu PP, Howard PC, Mellick PW, et al. Photo-co-carcinogenesis of topically applied retinyl palmitate in SKH-1 hairless mice. Photochem Photobiol. 2017;93:1096–114.
Wallo W, Nebus J, Leyden JJ. Efficacy of a soy moisturizer in photoaging: a double-blind, vehicle-controlled, 12-week study. J Drugs Dermatol JDD. 2007;6:917–22.
Dunaway S, Odin R, Zhou L, Ji L, Zhang Y, Kadekaro AL. Natural antioxidants: multiple mechanisms to protect skin from solar radiation. Front Pharmacol. 2018;9:392.
Bhattacharya S, Sherje AP. Development of resveratrol and green tea sunscreen formulation for combined photoprotective and antioxidant properties. J Drug Deliv Sci Technol. 2020;60:102000.
Rezzani R, Rodella LF, Favero G, Damiani G, Paganelli C, Reiter RJ. Attenuation of ultraviolet A-induced alterations in NIH3T3 dermal fibroblasts by melatonin. Br J Dermatol. 2014;170:382–91.
Janjetovic Z, Jarrett SG, Lee EF, Duprey C, Reiter RJ, Slominski AT. Melatonin and its metabolites protect human melanocytes against UVB-induced damage: Involvement of NRF2-mediated pathways. Sci Rep. 2017;7:1274.
Janjetovic Z, Nahmias ZP, Hanna S, Jarrett SG, Kim T-K, Reiter RJ, et al. Melatonin and its metabolites ameliorate ultraviolet B-induced damage in human epidermal keratinocytes. J Pineal Res. 2014;57:90–102.
Yang G, Cozad MA, Holland DA, Zhang Y, Luesch H, Ding Y. Photosynthetic production of sunscreen shinorine using an engineered cyanobacterium. ACS Synth Biol. 2018;7:664–71.
Pandika M. Looking to nature for new sunscreens. ACS Cent Sci. 2018;4:788–90.
Ryu B, Qian Z-J, Kim M-M, Nam KW, Kim S-K. Anti-photoaging activity and inhibition of matrix metalloproteinase (MMP) by marine red alga, Corallina pilulifera methanol extract. Radiat Phys Chem. 2009;78:98–105.
Lawrence KP, Long PF, Young AR. Mycosporine-like amino acids for skin photoprotection. Curr Med Chem. 2018;25:5512–27.
Kohli I, Shafi R, Isedeh P, Griffith JL, Al-Jamal MS, Silpa-archa N, et al. The impact of oral Polypodium leucotomos extract on ultraviolet B response: a human clinical study. J Am Acad Dermatol. 2017;77:33-41.e1.
Delgado-Wicke P, Rodríguez-Luna A, Ikeyama Y, Honma Y, Kume T, Gutierrez M, et al. Fernblock ® upregulates NRF2 antioxidant pathway and protects keratinocytes from PM2.5-Induced xenotoxic stress. Oxid Med Cell Longev. 2020;2020:2908108.
Mohammad TF, Kohli I, Nicholson CL, Treyger G, Chaowattanapanit S, Nahhas AF, et al. Oral polypodium leucotomos extract and its impact on visible light-induced pigmentation in human subjects. J Drugs Dermatol JDD. 2019;18:1198–203.
CAS PubMed Google Scholar
Torricelli P, Fini M, Fanti PA, Dika E, Milani M. Protective effects of Polypodium leucotomos extract against UVB-induced damage in a model of reconstructed human epidermis. Photodermatol Photoimmunol Photomed. 2017;33:156–63.
Emanuele E, Spencer JM, Braun M. An experimental double-blind irradiation study of a novel topical product (TPF 50) compared to other topical products with DNA repair enzymes, antioxidants, and growth factors with sunscreens: implications for preventing skin aging and cancer. J Drugs Dermatol. 2014;13:309–14.
Download references
Author information
Authors and affiliations.
Department of Dermatology, Henry Ford Health Systems, Henry Ford Medical Center-New Center One, 3031 W. Grand Boulevard, Suite 800, Detroit, MI, 48202, USA
Linna L. Guan, Henry W. Lim & Tasneem F. Mohammad
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to Tasneem F. Mohammad .
Ethics declarations
No sources of funding were used to conduct this study or prepare this manuscript.
Conflict of interest
LG has no conflicts of interest that are directly relevant to the content of this article. HWL has served as coinvestigator for studies sponsored by Incyte, L'Oreal, Pfizer, and PCOI; as consultant for Pierre Fabre, ISDIN, Ferndale, La Roche-Posay, and Beiersdorf; and as a speaker on general educational sessions for La Roche-Posay and Cantabria Labs. TFM has served as subinvestigator for Allergan and Ferndale Laboratories.
Availability of data and material
Not applicable.
Ethics approval
Author contributions.
All authors were significant contributors to the concept and planning, drafting, and revision of the manuscript. All authors approved the final submitted version of the manuscript.
Rights and permissions
Reprints and permissions
About this article
Guan, L.L., Lim, H.W. & Mohammad, T.F. Sunscreens and Photoaging: A Review of Current Literature. Am J Clin Dermatol 22 , 819–828 (2021). https://doi.org/10.1007/s40257-021-00632-5
Download citation
Accepted : 29 July 2021
Published : 13 August 2021
Issue Date : November 2021
DOI : https://doi.org/10.1007/s40257-021-00632-5
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
Advertisement
- Find a journal
- Publish with us
- Track your research
- Share full article

Spray Sunscreen Is Convenient. But Does It Work?
Dermatologists explain the benefits — and drawbacks — of this popular option.
Credit... Joyce Lee for The New York Times
Supported by
By Erica Sweeney
- Published June 4, 2024 Updated June 10, 2024
Q: I use spray sunscreens all summer because they’re so easy to put on. But are they as good as lotions?
There are many things to love about spray sunscreens: They often feel lighter on the skin and are easier to apply than their lotion counterparts. But if you think just a few haphazard spritzes will provide adequate protection against the sun’s harmful rays, think again, dermatologists say.
Daily sunscreen use is vital, said Dr. Maral Kibarian Skelsey, a clinical associate professor of dermatology at Georgetown University Medical School. It prevents sunburn and protects your skin from long-term sun damage, which can cause wrinkles and sun spots and raise your risk of skin cancer — the most common form of cancer in the United States.
Yet many Americans don’t apply sunscreen often enough . In a 2020 survey from the Centers for Disease Control and Prevention, just 12 percent of men and 29 percent of women said they always used sunscreen when they were outside for more than one hour on a sunny day.
Here’s what to know about the pros and cons of spray sunscreens, and how to use them effectively.
The Benefits and Drawbacks of Spray Sunscreen
Spray sunscreens are popular because they’re so easy to use, said Dr. Jean Charles, a dermatologist in Cedar Park, Texas. Spraying lets you target large swaths of the body, including hard-to-reach spots like your back.
Sprays tend to be lighter, less “sticky, oily and heavy,” and less likely to leave a white residue than lotions, said Dr. David Kim, a cosmetic dermatologist at Idriss Dermatology in New York City.
We are having trouble retrieving the article content.
Please enable JavaScript in your browser settings.
Thank you for your patience while we verify access. If you are in Reader mode please exit and log into your Times account, or subscribe for all of The Times.
Thank you for your patience while we verify access.
Already a subscriber? Log in .
Want all of The Times? Subscribe .
Advertisement
Expert consensus on the use of sunscreen agents: Indian perspective
- December 2021
- International Journal of Research in Dermatology 8(1):168
- This person is not on ResearchGate, or hasn't claimed this research yet.

- Apollo Institute of Medical Sciences and Research
Abstract and Figures

Discover the world's research
- 25+ million members
- 160+ million publication pages
- 2.3+ billion citations
- Malvika Kohli
- Anchala Parthasaradhi

- Indian J Dermatol
- Sakeena Fatima

- IltefatH Hamzavi

- Shreya Shanbhag

- Shital Mahindra Maru

- Victoria P. Werth

- R. Bouillon
- V. Callender

- J AM ACAD DERMATOL

- Henry W. Lim

- Andrea Dawson

- Recruit researchers
- Join for free
- Login Email Tip: Most researchers use their institutional email address as their ResearchGate login Password Forgot password? Keep me logged in Log in or Continue with Google Welcome back! Please log in. Email · Hint Tip: Most researchers use their institutional email address as their ResearchGate login Password Forgot password? Keep me logged in Log in or Continue with Google No account? Sign up
- Guest Posts
- Fisheries Management
- Fishing Gear
- Collaborate
- Communication Fellowship
Did you leave some of your sunscreen behind?

A glimpse into a tourist-free environment reveals how everyday choices shape environmental health .
Research Need
Recreational and commercial fishers agree that healthy water quality is essential for sustaining healthy fisheries. Yet, pinpointing the specific threats to water quality can sometimes be challenging. Often, these threats are not immediately obvious.
One such overlooked threat is sunscreen pollution, which introduces harmful chemicals into coastal waters. These chemicals can have devasting effects on marine life like fish, sea turtles, and migratory birds. In fish, for instance, ingredients in sunscreens, like oxybenzone and octocrylene, cause DNA damage, developmental issues, and reduced reproductive health across generations.
In response, some areas have introduced legislation and policies to reduce sunscreen pollution from tourism. But is tourism the main culprit behind this pollution in coastal waters?
The COVID-19 lockdowns provided a rare chance to study the impact of tourism by nearly eliminating human activity. A research team used this opportunity to see if sunscreen pollution decreased in 2020 and how severely it returned as tourism resumed.
What did they study?
The study focused on two key U.S. coastal national parks — Cape Lookout National Seashore in North Carolina and Kaloko-Honokōhau National Historical Park in Hawaiʻi — which both face significant concerns about the effects of mass tourism.
Researchers collected sand and water samples in May 2020 and August 2021 from each national park to analyze contaminant levels during and after the pandemic lockdowns. They tested the samples for active ingredients in sunscreen products, including: oxybenzone, octinoxate, octocrylene, octisalate, and homosalate, as well as relevant products that occur when they break down.
The research team then conducted risk assessments for oxybenzone and octocrylene to evaluate the threat these chemicals pose to coastal ecosystems.
What did they find?
Both national parks experienced a significant decrease in sunscreen pollution during the pandemic lockdowns. Sand samples at Cape Lookout showed no measurable levels of sunscreen chemicals, except for one sample containing a relatively low concentration of 10 nanograms of octinoxate per gram of sand. At Kaloko-Honokōhau NHP, only octocrylene was detected, with an average concentration of 57 nanograms per gram of sand across all five beach sites. This is significantly lower than the 2021 average of over 27,000 nanograms per gram of sand.
As tourism recovered, there was a sudden and drastic increase in sunscreen pollution. By 2021, Cape Lookout sand had measurable levels of 6 out of 7 tested chemicals, while Kaloko-Honokōhau NHP sand had 5 out of 7.
Risk assessments revealed that octocrylene levels in both sand and water samples from 2021 pose a severe threat to wildlife at both national park sites. The same applied to oxybenzone levels at Cape Lookout, but no measurable concentrations of oxybenzone were found in samples from Kaloko-Honokōhau NHP in 2021. This is likely due to legislation banning the sale and distribution of sunscreen containing this chemical in Hawaiʻi starting in January 2021.
Anything else?
Both national parks support many marine organisms, including sea turtles. Kaloko-Honokōhau NHP is essential for juvenile green turtles, while Cape Lookout provides crucial nesting sites for several turtle species. Nevertheless, researchers observed that aerosol sunscreen sprays contribute significantly to pollution at both locations. Strong onshore winds can blow away up to 95% of the sunscreen, leading to environmental contamination. Swimming immediately after application worsens this issue.
The research team recommends limiting the use of aerosol sunscreen sprays and implementing educational campaigns and park-specific regulations in order to protect these important habitats while preserving the visitor experience.
Consumers can help by choosing sunscreen lotions over sprays to reduce pollution from overspray, especially in windy environments. Additionally, waiting the recommended 15 minutes after application before entering the water allows the sunscreen to properly bind to the skin. This not only maximizes UV protection but also minimizes environmental contamination.
Downs, C. A., Akerlof, K. L., Stien, D., Rodrigues, A. M. S., Diaz-Cruz, M. S., Quintana, G., & Fulton, D. (2024). Sunscreen pollution is abated during the COVID-19 “Anthropause” of 2020 in two U.S. National Parks: Cape Lookout National Seashore and Kaloko-Honokōhau National Historical Park. Journal of Sea Research , 200 , 102510. https://doi.org/10.1016/j.seares.2024.102510
This project was funded by National Park Service, Grant/Award Number: P14PC00630/140P2119F0193; Cooperative Agreement, Grant/Award Number: P20AC00961/P17AC00691.
BY MADELINE PAYNE
Lead photo by Seb7157 | CC BY-SA 4.0
The text from Hook, Line & Science is available to reprint and republish at no cost, but only in its entirety and with this attribution: Hook, Line & Science , courtesy of Scott Baker and Sara Mirabilio, North Carolina Sea Grant.

- contaminants
More From Hook, Line and Science
Are fish shrinking, how does seagrass restoration affect coastal fishes , can boaters stop the spread of harmful aquatic hitchhikers.
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- J Clin Aesthet Dermatol
- v.6(1); 2013 Jan

Sunscreening Agents
a Global Medical Affairs, Dr. Reddy's Laboratories Ltd., Hyderabad, India;
Jacintha Martis
b Department of Dermatology, Fr. Muller Medical College, Mangalore, India;
Rutuja Sham Shinde
c Department of Dermatology, Dr. Reddy's Laboratories Ltd., Hyderabad, India;
Sudhakar Bangera
d Clinical Research Consultant, Hyderabad, India;
Binny Krishnankutty
e Department of Pharmacology, Azeezia Medical College, Kollam, India
Shantala Bellary
Sunoj varughese, prabhakar rao, b.r. naveen kumar.
The increasing incidence of skin cancers and photodamaging effects caused by ultraviolet radiation has increased the use of sunscreening agents, which have shown beneficial effects in reducing the symptoms and reoccurrence of these problems. Many sunscreen compounds are in use, but their safety and efficacy are still in question. Efficacy is measured through indices, such as sun protection factor, persistent pigment darkening protection factor, and COLIPA guidelines. The United States Food and Drug Administration and European Union have incorporated changes in their guidelines to help consumers select products based on their sun protection factor and protection against ultraviolet radiation, whereas the Indian regulatory agency has not yet issued any special guidance on sunscreening agents, as they are classified under cosmetics. In this article, the authors discuss the pharmacological actions of sunscreening agents as well as the available formulations, their benefits, possible health hazards, safety, challenges, and proper application technique. New technologies and scope for the development of sunscreening agents are also discussed as well as the role of the physician in patient education about the use of these agents.
Photoprotective agents protect the skin by preventing and minimizing the damaging effects of ultraviolet (UV) rays of natural light. They can be used as sunblock, which is opaque when applied over the skin and blocks a higher percentage of light as compared to sunscreens, which are translucent and require frequent reapplication for optimum efficacy. Photoaging—manifested as sagging, wrinkling, and photocarcinogenesis—is caused by damage to cells and deoxyribonucleic acid (DNA). It has been observed that sunscreens increase skin's tolerability to UV rays. 1
UV radiation has broad spectrum, ranging from 40 to 400nm (30–3eV), which is divided into Vacuum UV (40–190nm), Far UV (190–220nm), UVC (220–290nm), UVB (290–320), and UVA (320–400nm), of which the latter two are medically important. There are two distinct subtypes of UVA radiation. Short-wave UVA (320–340nm) and long-wave UVA (340–400nm), the latter constituting most of UVA radiation. The amount of exposure to UVA usually remains constant, whereas UVB exposure occurs more in the summer. 2
Effects of UVA manifest usually after a long duration of exposure, even if doses are low. It has been postulated that UVA up regulates the formation of matrix metalloproteinase (MMPs), enzymes that degrade the matrix protein's elastin and collagen, which, if not prevented, can result in marked reduction in skin elasticity and increased wrinkling. UVA radiation damages skin by penetrating into the layers of skin and producing reactive oxygen resulting in acute and chronic changes. 2 UVA radiation can induce polymorphous light eruptions (PMLE) in sensitive skin, 3 but in some it has also shown to reduce PMLE. 4 UVA can also cause exacerbation of cutaneous lupus erythematoses, whereas solar urticaria can be caused by both UVA and UVB radiation. 5
Studies have shown that UVA impairs the antigen presenting cell (APC) activity of the epidermal cells and thereby causes immune suppression, thus contributing to the growth of skin cancer. Sunscreening agents have shown to provide significant protection against epidermal APC activity induced by high UVA dose. 6 Mutation occurring in human melanocyte due to damage caused to DNA by UVA radiation is one of the proposed reasons. 7 In summary, UVA radiation can cause nuclear and mitochondrial DNA damage, gene mutations and skin cancer, dysregulation of enzymatic chain reactions, immune suppression, lipid peroxidation (membrane damage), and photoallergic and phototoxic effects.
UVB radiation can also cause acute changes, such as pigmentation and sunburn, and chronic changes, such as immune-suppression and photocarcinogenesis. Both UVA and UVB radiation can cause sunburn, photoaging reactions, erythema, and inflammation. 2
Sunburn is the most commonly encountered skin damage caused by natural light. Improper sunscreen usage and inadequate application also contribute to the increased prevalence of sunburn, despite the frequent use of sunscreening agents. Available evidence indicates that sunburn is more commonly seen in white-skinned people and young people with sensitive skin. Sunburn is common in the United States with 34.4 percent of adults affected. 8 In Sweden, children are frequently affected, and use of sunscreen among children has been found to be protective. 9
With the increased incidence in skin cancer cases, such as squamous and basal cell carcinomas, reported worldwide, use of photoprotective agents has increased over the years. 10 , 11 There has been symptomatic improvement and inhibition of reoccurrence of these conditions when photoprotective agents are used either therapeutically or prophylactically, indicating the need to promote and regularize their application.
The authors intend to spread awareness among physicians regarding the amount of sunscreening agents needed, method of application, reapplication, and the importance of patient education in all populations in order to reduce the damaging solar effects on skin.
Composition and Mechanism of Action
Sunscreening agents contain titanium dioxide (TiO 2 ), kaolin, talc, zinc oxide (ZnO), calcium carbonate, and magnesium oxide. Newer chemical compounds, such as bemotrizinol, avobenzone, bisoctizole, benzophenone-3 (BZ-3, oxybenzone), and octocrylene, are broad-spectrum agents and are effective against a broad range of solar spectrum both in experimental models and outdoor settings. Ecamsule (terephthalylidene dicamphor sulphonic acid), dometrizole trisiloxane, bemotrizinol, and bisoctrizole are considered organic UVA sunscreening agents. Classification 12 of sunscreening agents is shown in Figure 1 . Commercial preparations available in the market include a combination of these agents to cover a wide range of UV rays.

Classification of sunscreening agents
Composition and mechanism of action of sunscreening agents vary from exerting their action through blocking, reflecting, and scattering sunlight. Chemical sunscreens absorb high-energy UV rays, and physical blockers reflect or scatter light. Multiple organic compounds are usually incorporated into chemical sunscreening agents to achieve protection against a range of the UV spectrum. Inorganic particulates may scatter the microparticles in the upper layers of skin, thereby increasing the optical pathway of photons, leading to absorption of more photons and enhancing the sun protection factor (SPF), resulting in high efficiency of the compound. 13 , 14
Researchers are postulating that the generation of sunlight-induced free radicals causes changes in skin; use of sunscreens reduces these free radicals on the skin, suggesting the antioxidant property. 15 Broad-spectrum agents have been found to prevent UVA radiation-induced gene expression in vitro in reconstructed skin and in human skin in vivo . 16
Insect repellents, such as picaridin and N, N-diethyl-3-methylbenzamide (DEET), have been incorporated into sunscreening agents to minimize the risk of developing insect-borne infections. Picardin was found to be a more suitable component than DEET when used along with BZ-3, as it minimizes the penetration of chemicals. 17
Ideal sunscreening agents should be safe, chemically inert, nonirritating, nontoxic, photostable, and able to provide complete protection to the skin against damage from solar radiation. They should be formulated in a cosmetically acceptable form and ingredients should remain on the upper layers of the skin even after sweating and swimming. Sunscreening agents should provide efficient scavenging activities against singlet oxygen and other reactive oxygen species. 18 They should also effectively block both UVB and UVA rays, which is possible with an agent that has an SPF of 30 or greater. Sunscreens with an SPF of 30 or greater that incorporate photostable or photostabilized UVA filters (labeled as “broad spectrum” in the US) are usually ideal. 19 Sunscreens should not only protect the skin from the sun, but also minimize the cumulative health hazards from sun damage caused over time.
Factors Determining Efficacy
SPF and substantivity (the property of continuing therapeutic action despite removal of the vehicle ) are the factors that contribute to the effectiveness of sunscreening agents. 20 UVB protection is measured by a product's SPF, which theoretically indicates that products with high SPFs provide more protection against hazardous effects of sunlight than those with low SPFs. 21 SPF is measured as the ratio of the amount of UV radiation required to burn the protected skin (with sunscreen) to that required to burn the same unprotected skin (without sunscreen), all other factors being constant. SPF is measured using the following formula:
SPF = MED of protected skin/MED of unprotected skin (MED = minimal erythemal dose) .
This means when a product with SPF 50 is applied, it will protect the skin until it is exposed to 50 times more UVB radiation than that is required to burn the unprotected skin.
SPF level, efficacy against a wavelength of UV radiation, and UVA/UVB ratio can be calculated using a computer program or software, such as sunscreen simulators, and can determine if the product meets the regulatory standards.
Bodekaer et al 22 studied the reduction in SPF of organic and inorganic sunscreening agents in participants who, over the course of eight hours, performed physical activities, were then exposed to a hot environment, and finally bathed. There was a reduction in SPF of 38 and 41 percent after four hours and of 55 and 58 percent after eight hours of application of organic and inorganic sunscreen, respectively. 22 Hence, it is necessary to apply the adequate and recommended amount of sunscreening agent to obtain the claimed benefit (i.e., 2mg/cm 2 , which is shown to be effective on Asian skin as well). Studies have shown that people apply about a quarter of the recommended dose of sunscreen, which is an inadequate amount. 23 , 24
Protection offered by a sunscreening agent against UVA radiation is measured by the Persistent Pigment Darkening (PPD) Protection Factor. This technique was developed in Japan and has been routinely used by manufacturers. Stanfield 25 has discussed its disadvantages. PPD is not done in skin type 1, which is the skin type more prone to solar damage. For wavelengths less than 320nm, action sprectrum is not defined; moreover, clinical significance of PPD is not very clear.
Immediate pigment darkening response is calculated as the dose of UVA required to produce the effect with the sunsceening agent to that produced without an agent. 26 Although this test gives rapid results for low doses of UVA, responses have been found to be highly variable and an accurate reproduction of results is difficult. This is usually performed in skin types III and IV and its clinical significance is unknown. 27
COLIPA guideline is a new standardized, reproducible, and in-vitro method to measure UVA protection offered by sunscreening products and was developed by “ In-Vitro Sun Protection Methods” group. This has been in use by European Union (EU) countries for testing and labeling sunscreen products as it is in line with regulatory recommendation. 28
Immunoprotection factor is a measure of a sunscreening agent to prevent UV-induced immunosuppression. 12
Regulatory Guidelines
Misguiding information on sunscreen labels has compelled regulatory agencies to make changes in the international regulatory guideline on sunscreens to avoid confusion among the general public, to assist them in selecting a suitable agent, to provide adequate sun protection, and to minimize health hazards of solar damage including the occurrence of skin cancers.
United States Food and Drug Administration (FDA) guidelines. Previously, FDA guidelines 29 , 30 aimed at protection against UVB radiation and sunburns, not toward protection against UVA radiation and prevention of skin cancers. Inappropriate and misguiding labeling with false claims has made the FDA revise its guidelines on sunscreening agents. New, improvised guidelines address such issues as “broad-spectrum designation, use claims, waterproof, sweat proof, sun proof, and water resistance claims and drug facts”. According to the new guidelines, claims about UVA and UVB protection should be made only after the specific tests have proved the same. It is mandatory to test both UVA and UVB radiation. A product can be classified as a broad-spectrum sunscreening agent if it passes the required test, but the reduction in the risk of skin cancer and early skin aging when used as per direction can be stated by only those with SPF 15 or higher. Those with SPF 2 to 14 cannot state the latter.
Labels claiming sunscreens are “waterproof,” “sweat proof,” or “sun blocks” are not legally permitted as these claims overemphasize the product's efficacy. If a product claims to be water resistant, the label should clearly indicate the duration of effectiveness (e.g., 40 minutes or 80 minutes) during activities such as swimming. If the product does not claim to be water resistant, consumers should be instructed to use a water-resistant sunscreen during swimming and those activities that produce sweat. Reapplication for better efficacy has to be mentioned on the label, and manufacturers are not allowed to claim sun protection lasting more than two hours without reapplication. Claims, such as instant protection, are also not permitted. If any such claims are made, supporting data should be submitted to obtain FDA approval.
Labels should also include standard drug facts. Products containing an SPF of more than 50 should mention in the label that there is a lack of evidence to support that sunscreens with an SPF of more than 50 have better efficacy than those containing SPF 50 or below. Manufacturers have to submit supporting data if the formulation is a spray or another dosage form of which comparison with the regular dosage form, such as cream or lotion, is not possible. These new rules became effective June 18, 2012.
EU guidelines. Revised EU guidelines 31 , 32 mandate a minimum level of UVA protection in terms of SPF. The UVA protection factor measured by PPD ( in vivo ) or COLIPA ( in vitro ) must be at least one-third of the SPF in-vivo value. Products with SPF 6, 10, 15, 20, 25, 30, 50, 50+ are permitted for consumer use and are categorized as low (SPF 6, 10), medium (SPF 15, 20, 25), high (SPF 30, 50), and very high (SPF 50+). Compounds should provide protection against a minimum critical wavelength of 37nm, which is also under consideration. Products that meet the regulatory standard will have the UVA seal.
Actual protection against UVA is represented by a star system for easy understanding by consumers. This measure was developed by Boots Company in Nottingham, United Kingdom, and was based on Diffey's UVA/UVB ratio. The star system ranges from one to five stars where 1=minimum sun protection, 2=moderate, 3=good, 4=superior, and 5=ultra.
Guidelines from other countries. Japan, Australia, and New Zealand have their own indices on UV protection factor. 12 Australian standards define UVA protection in a compound when the transmission of sunlight between a wavelength of 320 and 360nm (at a path length of 8µm) is less than 10% (of the incoming light that is passing through).
New Australian guidelines have set SPF 50+ as a benchmark for sunscreening agents. It has also endorsed the revisions by international standards on terminology, such as “water resistant,” “waterproof,” “sun block,” and “sweat resistant,” as these terms are misleading to consumers. High requirements have been set by the guideline regarding water resistance as per their lifestyle requirement. 33
Japanese regulatory guidelines 34 describe the method of testing the photoprotection factor of UVA (PFA) as the amount of product to be applied, dose of radiation, and radiation field. These guidelines define minimal persistent pigment darkening (MPPD) dose as the minimum dose of UV rays required to produce slight darkening over the whole radiation area within 2 to 4 hours after exposure. The guidelines also define the time to measure MPPD and who should measure it. PFA is calculated using the following formula:
PFA = MPPD of protected skin/MPPD of unprotected skin. Products are graded based on the PFA value ( Table 1 ) .
Photoprotection grades according to Japanese cosmetic industry association guidelines
| PHOTOPROTECTION FACTOR OF UVA VALUE | PROTECTION GRADE OF UVA (PA) | PROTECTION LEVEL |
|---|---|---|
| 2 or more, but less than 4 | PA+ | Low |
| 4 or more, but less than 8 | PA++ | Moderate |
| 8 or more | PA+++ | High |
Source: JCIA/persistent pigment darkening protocol
Korea follows Korean measurement standards for UV protection effects (KFDA) and has standards for UVB protection (SPF measurement) and protection grade of UVA (PA). On labels, SPF should be listed for UVB and PA for UVA. 35
India guidelines. In India, there are no industry guidelines for standardizing sunscreen agents and there is no detailed list of approved products. The Indian regulatory agency's official website lists only two combination products as approved drugs ( Table 2 ). Many products are classified as cosmetics and are not listed in this section. Apart from routinely used agents, such as BZ-3, ZNO, and TiO 2 , other agents, such as camphor benzalkonium methosulfate (6%), octyl salicylate (5%), camphor derivatives, and broad-spectrum UV filters (i.e., bis-ethylhexyloxyphenol mcthoxyphcnyl triazine [10%] and methylene bis-benzotriazolyl tetramethylbutylphenol [10%]) are widely used. Table 2 lists some of these agents, which are manufactured by pharmaceutical companies and are available in India. Most of the products available are combination products.
Approved sunscreeening agents (combination products) and available preparations in India
| COMBINATION APPROVED BY INDIAN REGULATORY AUTHORITY | PROTECTION GRADE OF UVA (PA) APPROVED CONCENTRATION (%) | |
|---|---|---|
| Octinoxate + Avobenzone + Oxybenzone + Octocrylene + Zinc Oxide lotion (approved on March 19, 2009) | 7.5+2+3+3+2 | |
| Octinoxate + Avobenzone + Oxybenzone + Titanium dioxide lotion (approved on March 23, 2009) | 7.5+3+3+2 | |
| Bis-Ethylhexyloxyphenol Methoxyphenyl Triazine ((Tinosorb S, Bemotrizinol)+ Butyl Methoxydibenzoylmethane ((Tinosorb M- active, Avobenzone)+ Methylene Bis-Benzotriazolyl Tetramethylbutylphenol ( Bisoctrizole )+ Benzophenone-3 (Uvinul M40)+ Octocrylene (Uvinul N539T) | 2+2+5+2+7 | 30 |
| Bis-Ethylhexyloxyphenol Methoxyphenyl Triazine + Butyl Methoxydibenzoylmethane Methylene Bis-Benzotriazolyl Tetramethylbutylphenol ((Tinosorb M, active) + Benzophenone-3 (Uvinul M40)+ Octocrylene (Uvinul N539T) | 5+2+7+3+10 | 50 |
| Tinosorb M; Octinoxate; Octyl triazone [EHT/Uvinul T150] - S3 Complex | 60 | |
| Octinoxate +Avobenzone + Oxybenzone + Octocrylene + Zinc Oxide | 7.5 +2+3+3+2 | 50+ |
| Oxybenzone + OMC + Tinosorb M | 3+5+8 | 30 |
| Tinosorb M +Octinoxate | 30 | |
| Octinoxate +Avobenzone +Oxybenzone + Zinc Oxide | 7.5 +2+3+2 | 26 |
| Octinoxate +Avobenzone +Oxybenzone + Zinc Oxide (micronized) | 7.5 +2+3+6 | NA |
| OMC + Oxybenzone + Titanium Dioxide | 7.5 +3+3 | 20.47 |
| OMC + Oxybenzone + Titanium Dioxide | 8+3+6 | 50+ |
| Zinc Oxide + Octinoxate | 15.5+7.5 | 30 |
| Octylmethoxycinnamate+oxybenzene+Titanium dioxide | 8.5+3+6.5% | 50+ |
| OMC+Avobenzone+Phenyl benzimidazole suphomived | 7.5+2+2 | 30+ |
Pharmacokinetics
It was observed that lipid microparticles loaded with ethylhexyl methoxycinnamate (EHMC), which filters UVB, and butyl methoxydibenzoylmethane (BMDBM), which filters UVA, had reduced skin penetration, thus preserving the UV filter efficacy and limiting potential toxicological risks. 36 Gonzalez et al 37 studied the percutaneous absorption of BZ-3 after repeated whole-body applications, with and without UV irradiation in 25 volunteers. They observed that large amounts of BZ-3 is absorbed, accumulated in the body, and excreted, even after five days after the last application. 37 In another study, pharmacokinetics of BZ-3 was studied in 11 healthy volunteers after topical application. After 48 hours, the average amount of BZ-3 excreted in urine was 11mg (median=9.8mg). In some volunteers, BZ-3 was excreted even after 48 hours. This study showed that BZ-3 undergoes conjugation and converts to a water-soluble compound. The age at which liver attains maturity and is able to metabolize these chemicals and conjugate is unknown. Therefore, it is recommended that physical filters (i.e., zinc oxide, titanium dioxide, ferrous oxide) be used in children. 38 BZ-3 is FDA approved for use in children above six months of age.
Pharmacokinetics of the following three chemical UV absorbers—benzophenone-3 (BZ-3), octyl-methoxy-cinnamate (OMC), and 3-(4-methylbenzylidene) camphor (4-MBC)—were studied in 32 healthy volunteers, 15 of whom were young male volunteers and 17 of whom were postmenopausal women. The volunteers were exposed to daily whole-body topical application of 2mg/cm 2 of sunscreen formulation at 10% (weight/weight) for four days. Blood and urine concentrations were measured at regular intervals as specified in the protocol. Before the first application of these agents, their concentration was undetectable in plasma and urine, but was detectable 1 to 2 hours after the first application. In female volunteers, the maximum median plasma concentrations of 187ng/mL BP-3, 16ng/mL 4-MBC, and 7ng/mL OMC were seen. In male volunteers, maximum median plasma concentrations were 238ng/mL (BZ-3), 18ng/mL (4-MBC), and 16ng/mL OMC.
The urinary concentration level of BZ-3 was higher in men (81ng/mL) than women (44ng/mL). However, no significant changes were seen with other agents (female volunteers = 4ng/mL of 4-MBC and 6ng/mL OMC; male volunteers = 4ng/mL of 4-MBC and OMC). Men showed a higher concentration of 4-MBC and OMC, whereas women showed a similar pattern in BZ-3 and 4-MBC when 96-hour median concentrations were compared to 24-hour concentrations. 39
Janjua et al 40 studied the absorption of sunscreens BZ-3, octyl-methoxycinnamate (OMC), and 3-(4-methyl-benzylidene) camphor (4-MBC) from topical application and their effects on the endogenous reproductive hormones in 32 healthy volunteers. After two-week, whole-body application, there was no change in follicle-stimulating hormone (FSH) levels or luteinizing hormone (LH) levels, but there was a minor difference in testosterone levels. In men, a minor difference in serum estradiol and inhibin B levels were observed. 40
Formulation also plays a role in the penetration of the compound to the skin. Skin penetration of BZ-3 is faster and greater if formulated as emulsion. However, the rate of penetration is dependent on the concentration of BZ-3 in the formulation. 41
Filipe et al 42 studied the localization of TiO 2 and ZnO nanoparticles and their skin penetration levels and concluded that drug concentration was either undetectable or insufficient under the stratum corneum, indicating minimal systemic absorption with no or minimal penetration into keratinocytes and good skin retention. 42 – 44 Lacatusu et al 45 showed that coupling UV absorbers and lipid nanoparticles makes the combination photostable and provides better photoprotection. 45
Efficacy of a sunscreen is tested in vitro and in vivo for SPF, UVA indices, and UV protection profile. Figures 2 and and3 3 show the SPF and UV indices for a product with SPF 30 and Figures 4 and and5 5 show that for a compound with SPF 50. The ability of a sunscreen to absorb UV radiation is measured in terms of extinction coefficient value. Figure 6 shows the optimal UV protection across the full UV spectrum of various UV filters.

SPF profile of a product with SPF 30

UV protection profile of a product with SPF 30. Profiles before (initial) and after (final) irradiation dose of SPF × MED (1 Minimal Erythema Dose passes through sunscreen onto skin).

SPF profile of a product with SPF 50

UV protection profile of a product with SPF 50

Optimal UV protection across the full UV spectrum of various UV filters
Studies are suggesting the use of broad-spectrum sunscreening agents for greater protection. 46 Daily application of these agents helps in minimizing solar UV-induced skin changes. 47 Results of a study by Diffey 11 indicated that regular use of topical photoprotective agents significantly reduces lifetime UV exposure to the face compared to nonuse. The study also emphasized that it is very important to begin regular daily use of topical sunscreens early in life. Sunscreens are used more during the summer season than throughout the year in some regions. Consumers consider SPF, action of sunscreens against UV range, and the usage pattern as less important. 11 Green et al 48 observed reduction in the incidence of squamous cell carcinoma (40%) and basal cell carcinoma with regular use of sunscreens, supporting their role in the prevention of these skin cancers.
Kuhn et al 49 assessed the exclusive use of a broad-spectrum sunscreen in preventing the skin lesions in patients with different subtypes of cutaneous lupus erythematosus (CLE) induced by UV irradiation under standardized conditions in 25 patients. They concluded that use of broad-spectrum sunscreening agents prevents skin lesions in these patients. Efficacy of sunscreens depends upon skin type, amount and frequency of application, exposure to sunlight and time of day, environmental factors, and the amount of product absorbed by the skin.
The safety of sunscreening agents is determined by toxicity studies, ability to cause irritation, sensitization, phototoxicity, and its impact on environment. Hayden et al 50 studied the safety of five commonly used sunscreen agents (avobenzone, octinoxate, octocrylene, BZ-3 [oxybenzone] and padimate O) by determining the penetration of topical agents and found that BZ-3 penetrated the epidermis the most after 24 hours of exposure; however, the concentration in the stratum corneum was too low to cause toxicity. Toxicities have been reported with BZ-3, which has been associated with anaphylaxis. 51 The inhalation of spray sunscreens can pose a danger as well. McKinney et al observed pulmonary and cardiovascular changes in rats on inhalation of a product containing TiO 2 nanoparticles.
Use of sunscreening agents in Asian skin
Asian skin is classified as type IV, 26 which is darker in color, rarely burns, and is more prone to rapid tanning. Asian skin is comparatively smoother, with a slight yellowish tinge and is more prone to pigmentation. Presence of protein melanin in the skin of Asians differentiates it from the skin of Caucasians. It has been observed that melanin equally filters all wavelengths of light, thereby receiving five times less UV radiation. This protein provides photoprotection to a certain extent, minimizing phototoxicity and making the skin less vulnerable to the acute and chronic phototoxic effects. 52 Nevertheless, this population shows the effects of photodamage in terms of pigmentation, wrinkling, and sunburn. The formation of freckles in the Asian population is encountered much less frequently. However, overexposure to sunlight can cause photodamaging effects, including skin cancers. Hence, it is advisable for Asians to use sunscreening agents regularly as a preventive measure just as it is in other parts of the world. However, since Asian skin is more prone to hypersensitivity reactions, cosmetic products should be used with care.
Sunscreen Use in Special Populations
Studies have shown that dialysis and organ transplant patients, including renal transplantation patients, should follow photoprotective measures, as they are more prone to develop skin cancers. Use of sunscreening agents have prevented the development of premalignant skin changes in these patients. 53 , 54 Hence, physicians should educate these patients regarding the regular use of preventive measures against sun damage, including the regular use of appropriate sunscreens.
Formulations
Generally, sunscreens are available in the form of creams, lotion, gels, ointments, pastes, oils, butters, sticks, and sprays, which are considered over-the-counter (OTC) products. Less frequently used products include wipes, towelettes, powders, body washes, and shampoos, which are considered non-OTC products by the FDA. Of late, these types of products have been marketed as multifunctional cosmetic formulations incorporated into other cosmetics, such as moisturizers, facial foundations, and foam foundations (mousse). Spray or gel-based sunscreens are preferred in oily skin and acne. New sunscreens with microfine particles are found to be safe and effective in patients with acne and rosacea. Sunscreen filters are also added to hair care products, such as shampoo, to minimize sun damage to hair.
Sunscreen-containing moisturizers usually have SPFs between 15 and 30. Coverage foundations are transparent formulations containing titanium dioxide with an SPF of 2 while moderate coverage foundations are usually translucent with an SPF of 4 to 5.
Gogna et al 55 observed that the use of polymethyl-methacrylate (PMMA) microspheres of ethylhexyl methoxycinnamate (EHM) increases the efficacy of the latter by four times and also improves photostability of the preparation. Sprays containing sunscreening agents with high concentrations have been found to retain the medicaments on the top layers of skin, minimizing deeper penetration. 56
Studies have shown that microspheres increase the efficacy of sunscreening agents. 55 Incorporation of nanoparticles has shown to increase the efficacy of sunscreen agents in terms of superior UV protection and reduced whitening on the skin in comparison with the older generations of sunscreens. 57 Currently, formulations containing nanoparticles of TiO 2 and ZnO are available. However, studies have shown that nanoparticles of these two compounds cause cytotoxicity, genotoxicity, 58 , 59 and potential photocarcinogenecity. 60 In addition, nanoparticles of ZnO, even at a much lower concentration, may induce inflammation by releasing inflammatory mediators, such as cytokines interleukin (IL)-6 and tumor necrosis factor (TNF)-α. 61 Sunspheres and microencapsulations are newer technologies in the preparation of sunscreen formulation. 12
Health Hazards of Sunscreening Agents
Although considered safe, sunscreening agents are not free from adverse effects. Sensitivity, though rare, can occur in the form of photoallergic reactions, including contact dermatitis. Photopatch testing helps to identify sensitivities. 62
There have been reports of increased incidence of melanoma as a result of sunscreen use. Gorham et al 63 reviewed the risk of developing melanoma as a result of sunscreen use and opined that those who live in latitudes greater than 40 degress may have an increased risk of melanoma. The reason for this may be because sunscreens absorb UVB almost completely, but transmit large quantities of UVA. 63 Sunscreen use may prolong the duration of intentional exposure giving people a false sense of security, especially when using products that have high SPF ratings, thereby increasing the risk of skin cancer. 64 A similar trend was observed in European countries as well. 65
A product assessment in the United States in January 2011 revealed that retinyl palmitate, a form of vitamin A, which is a widely used compound in cosmetics and sunscreens (as an antioxidant against the aging effects of UV radiation), is thought to increase the rate of the development of skin tumors and lesions. However, Wang et al 66 opined that its role in human carcinogenesis is doubtful as there is a lack of evidence. Fourschou et al 67 noted an exponential increase in vitamin D levels with the application of thinner layers of sunscreen after UVB exposure, indicating that application of thicker layers can cause a decrease in vitamin D levels resulting in its deficiency.
It is postulated that BZ-3 can disrupt the hormones in the body. The Centers for Disease Control and Prevention has detected BZ-3 in the 97 percent of Americans tested during biomonitoring surveys. Although there have been reports of adverse events with this agent, studies have shown that products formulated with 1 to 6% of BZ-3 do not possess a significant sensitization or irritation potential for the general public. 68
Exacerbation of acne and rosacea can also occur with the use of sunscreen agents that contain physical blockers, such as ZnO and TiO 2 , that are greasy and have large particle sizes, thereby blocking skin pores.
Effective Practice
According to the Environmental Working Group's 2010 Annual Sunscreen Guide, zinc and titanium-based sunscreens are considered more safe and effective than other products available in the United States. 69 Powder and spray sunscreens should be avoided, as they may lead to inhalation of particles, which is hazardous. The FDA recommends applying 2mg/cm 2 of sunscreen to achieve maximum benefits. Sunscreen should be reapplied every two hours as well as after sweating, toweling off, bathing, and swimming. It is recommended that sunscreen labels highlight the importance of reapplication. 70
Avoiding exposure to sunlight during the time of day when UV radiation is at its highest—between 10 am and 3 pm—is recommended. When sun exposure during this time is unavoidable, it is advisable to use sun protection (i.e., umbrella and sun protection clothing).
Causes of Sunscreen Failure
Underapplication and failure to reapply sunscreen every two hours are the main reasons sunscreens fail. Additionally, these agents are unaffordable by many in developing and underdeveloped countries. Sunscreen use year round is expensive, 71 which is why some people do not use sunscreen regularly. Another contributing factor to sunscreen failure is the mismatch between the labeled SPF and that delivered on application to skin and exposure to sunlight.
Promoting the Use of Sunscreen Agents
As the rate of sunscreen use is low, education and awareness about the hazards of sun exposure and the benefits of regularly applying sunscreening agents to reduce these effects must be spread. 23 , 72 , 73 Outdoor activities should be performed before 10 am or after 3 pm. 74 Education should target preadolescents so they develop the habit of using sunscreening agents at a young age, particularly as adolescents are more prone to seek sun exposure for intentional tanning purposes. 75 Organ transplant patients need to be educated regarding the regular use of sunscreening agents as well.
Lack of awareness among the public regarding the use of sunscreening agents is more evident in the United States, where only about 3 in 10 adults routinely practice sun-protection behaviors. Women and older adults have been found to practice sun protection more than others. 8
Role of Physician/Dermatologist
Physicians should be aware of the composition of sunscreen agents and the UVA protection factors of formulations. They should also instruct their patients about the proper application technique and insist on reapplication. Additionally, they should counsel preteens and adolescents regarding the regular and proper use of broad-spectrum sunscreens.
Recent Developments
Newer broad-spectrum chemical agents, such as bis-ethylhexyloxyphenol methoxyphenyl triazine (BEMT), methylene bis-benzotriazolyl tetramethylbutylphenol (MBBT), and butyl methoxy-dibenzoyl methane (BMDBM), have been found to be effective against UVA and UVB rays ranging from 280 to 400nm. These new agents have been formulated to be more fat soluble (oil soluble in cosmetic oils) to aid in efficacy and broad-spectrum activity. They are known to prevent the formation of free radicals induced by UV radiation to a significant level. These agents claim to be photostable, minimize erythema, and provide excellent anti-aging effects as well as protect the skin's antioxidant defense system. In studies, these new agents have shown to provide protection against intentional self tanning. Further, they also claim that there is no bioaccumulation, thereby exhibiting a good safety profile. Figure 6 shows the comparison of photoprotection by these newer compounds. 76
These new broad-spectrum sunscreen agents have been found to be compliant with regulatory guidelines in terms of PPD, SPF, COLIPA, and Boots star rating. They also claim to have the following advantages: instant action, longer duration of protection, improved cosmetic appearance of the skin in the form of less wrinkles, suitability for sensitive skin, and suitability for chikdren.
Future Developments/Scope
Bacterial-derived melanin has been shown to provide significant protection to fibroblast cells against UVA radiation. It is a promising product that helps to keep UVA-irradiated skin from pigment darkening, especially in those with photosensitivity. 77
The use of antioxidants has shown to minimize the release of oxidants after excessive sun exposure on unprotected skin. 15 A compound that is effective in protecting against complete UV spectrum and infrared radiation is welcome.
Despite the efforts of physicians and regulatory authorities to spread awareness regarding sunburn, skin cancer, and the benefits of regularly using sunscreening agents, treatment adherence is low. 78 Providing cosmetically acceptable preparations and educating people about following the application instructions, is a challenge often faced by treating physicians. Pricing sunscreens reasonably and making them water resistant and non-sticky are a few of the challenges faced by manufacturers. Manufacturers must also consider sensitization reactions, especially in those having eczema or photodermatoses. 79 Narrow-spectrum sunscreening agents, especially those that absorb only UVB rays, may contribute to the development of melanoma at latitudes over 40 degrees due to transmission of UVA rays in large amounts. 63
Use of sunscreening agents is beneficial in minimizing the occurrence of skin cancers in people with fair skin. However, the same effect on Asian skin is debatable, as this skin type is considered to be resistant to skin cancers. Sunscreen use is advisable in young adults to prevent and minimize other photodamaging effects. Affordability and proper application techniques are the challenges that must be addressed in order to achieve regular sunscreen usage. The authors recommend further comparative studies on sunscreens as well as studies on the Indian population, as there is insufficient data in this population.
DISCLOSURE: Dr. Latha, Ms. Sham Shinde, Dr. Bellary, and Mr. Rao are employed by Dr. Reddy's Laboratories Ltd. and are stakeholders in Dr. Reddy's Laboratories Ltd. Dr. Krishnankutty, Dr. Kumar, and Mr. Varughese were former employees of Dr. Reddy's Laboratories Ltd. and are presently not stakeholders in Dr. Reddy's Laboratories Ltd. Dr. Martis, Dr. Bangera, and Dr. Shobha report no relevant conflicts of interest.

IMAGES
COMMENTS
In Canada, more than 80 000 cases of skin cancer are diagnosed every year. 1 Because exposure to ultraviolet radiation is estimated to be associated with 80%-90% of skin cancers, the use of sunscreen — which blocks ultraviolet radiation — is promoted as an important means of preventing skin cancers, 2, 3 as well as sunburn and skin photoaging (see definitions in Appendix 1, available at ...
Abstract. Sunscreens have been on the market for many decades as a means of protection against ultraviolet-induced erythema. Over the years, evidence has also shown their efficacy in the prevention of photoaging, dyspigmentation, DNA damage, and photocarcinogenesis. In the USA, most broad-spectrum sunscreens provide protection against ...
Formulation of sunscreen involves four critical steps; selection of the target product design, choice of active ingredients and the delivery vehicle followed by product optimization as shown in Fig. 4. The primary objective of the formulation expert is to develop a product that forms a continuous film on the skin.
The article reviews evaluation methods for sunscreens, such as SPF, UVA protection, and water resistance. SPF measures the level of UVB protection, while UVA protection indicates defense against UVA radiation. Water resistance assesses the sunscreen's durability after exposure to water or sweat.
However, the use of sunscreen has faced many challenges, including inducing photoallergic dermatitis, environment pollution, and deficiency of vitamin D production. ... provides an outlook for future research directions and describes possible research applications. Feature papers are submitted upon individual invitation or recommendation by the ...
Background: Although chemical sunscreens have traditionally been at the forefront of sun protection, safety concerns and increasing awareness of the environmental impact of personal-care products have led to greater interest in the use of mineral blockers as photoprotective agents. Objective: To examine the safety and efficacy of mineral-based sunscreens to allow patients to make informed ...
Sunscreens indeed impair vitamin D synthesis if they are used in the recommended amount of 2 mg/cm2, but not in lesser thickness below 1.5 mg/cm2 that corresponds better to what users apply in real life conditions. Large molecular last generation UVB-UVA broad spectrum sunscreens have a better benefit-risk ratio than former organic filters ...
The use of sunscreen products has been advocated by many health care practitioners as a means to reduce skin damage produced by ultraviolet radiation (UVR) from sunlight. There is a need to better ...
Sunscreen efficacy and safety is of paramount importance for both human health [] and the environment [].The limited list [3, 4] of chemicals available for use as sun protecting active ingredients is concerning, especially considering the emerging public scrutiny [2, 5,6,7] of ingredients.Within the past few years, there have been multiple highly publicized studies regarding the potential ...
KEY POINTS. Several well-conducted randomized controlled trials with long follow-up showed that sunscreen use reduces the risk of squamous cell and melanoma skin cancers. Commercial sunscreens protect against the skin-damaging effects of ultraviolet radiation through either chemical or physical ingredients. The Canadian Dermatology Association ...
The sunscreen innovation act is the latest guide guiding the production of sunscreens and established the framework for approval of the next generation of sunscreens. ( FDA, 2016 ) In the European Union (EU), cosmetic products are regulated under the Cosmetic Regulation (EC) No 1223/2009 which came into implementation in July 2013.
Photoprotection is indicated for the reduction of ultraviolet (UV) radiation-induced skin damage and skin cancers. Photoprotection includes sunscreens, clothing, hats, makeup, sunglasses, and windshields. The damaging effects of UV radiation include photoaging and photocarcinogenesis. Photoaging can manifest as sagging and wrinkling, while photocarcinogenesis is due to the damage of cells and ...
Background: Sunscreen use and dietary antioxidants are advocated as preventives of skin aging, but supporting evidence is lacking. Objective: To determine whether regular use of sunscreen compared with discretionary use or β-carotene supplements compared with placebo retard skin aging, measured by degree of photoaging. Design: Randomized, controlled, community-based intervention.
The US Food and Drug Administration has confirmed that six active ingredients widely found in sunscreens penetrate through the skin and absorb into blood plasma. The agency's findings, published ...
This paper also provides a reference for adopting novel technical measures (extracting high content components, changing the type of solution, optimizing formulation, applying Nano technology, et al) to design and prepare nature sunscreen formulations equated with commercial sunscreen formulations. ... In a related research, Ntohogian, found ...
A. There are two types of sunscreens: Physical blockers reflect ultraviolet rays from the sun and contain one of two active ingredients, zinc oxide or titanium dioxide. Chemical blockers contain chemicals that absorb the sun's ultraviolet rays. In the United States these typically include aminobenzoic acid, avobenzone, octisalate, octocrylene ...
Awareness of the risks associated with sunrays has been increasing in the last century, and as a result, the science, technologies, and formulation have advanced significantly. The use of ...
Sunscreen Agents protect the skin from Ultraviol et (UV) rays by absorption, scattering and by blocking. phenomena. Ultravi olet (UV) rays are divided into three wavelengths UV-A,UV-B and UV-C in ...
Sunscreens have been on the market for many decades as a means of protection against ultraviolet-induced erythema. Over the years, evidence has also shown their efficacy in the prevention of photoaging, dyspigmentation, DNA damage, and photocarcinogenesis. In the USA, most broad-spectrum sunscreens provide protection against ultraviolet B (UVB) radiation and short-wavelength ultraviolet A (UVA ...
Introduction. Over a decade ago, an article discussing sunscreens and SPF (Sun-Protection Factor) noted that "sunscreen with an SPF of 30 may provide an effective SPF-rating of only 2 against UV- A" ().Until then UV-B rays (280-320 nm) were considered the main cause of skin cancer—for example, the FDA Panel on sunscreens noted "the lower wavelength limit of cancer-producing radiation ...
Mineral sunscreens form a barrier between the skin and the sun's rays, while chemical sunscreens bind to the top layer of skin and transform UV rays into heat that disperses, said researcher Dr ...
Spray sunscreens are popular because they're so easy to use, said Dr. Jean Charles, a dermatologist in Cedar Park, Texas. Spraying lets you target large swaths of the body, including hard-to ...
short-term as well as long-term changes in the structure. of the skin.1 In short ter m effects, repeated exposure. leads to erythema, whereas repeated exposure in the long. term co uld cause ...
2.1. Overview of the Use of Natural Ingredients in Sunscreens from Terrestrial and Marine Sources. The preliminary analysis of the presence of natural ingredients in all of the studied 444 sunscreens, in a total of 43 brands, indicates that 211 (48%) contain ingredients from terrestrial organisms while marine ingredients are present in 57 (13%) of the studied formulations (Figure 1).
Vandenberg has a different approach. "Based on what I know and in my own practice, I lean towards a physical sunscreen, and then use it correctly: more than you think you need, and reapplying ...
A research team used this opportunity to see if sunscreen pollution decreased in 2020 and how severely it returned as tourism resumed. What did they study? The study focused on two key U.S. coastal national parks — Cape Lookout National Seashore in North Carolina and Kaloko-Honokōhau National Historical Park in Hawaiʻi — which both face ...
Spray or gel-based sunscreens are preferred in oily skin and acne. New sunscreens with microfine particles are found to be safe and effective in patients with acne and rosacea. Sunscreen filters are also added to hair care products, such as shampoo, to minimize sun damage to hair. Sunscreen-containing moisturizers usually have SPFs between 15 ...