Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Open access
- Published: 17 August 2023

Intelligent diagnostic model for malaria parasite detection and classification using imperative inception-based capsule neural networks
- Golla Madhu ORCID: orcid.org/0000-0002-4170-3146 1 ,
- Ali Wagdy Mohamed ORCID: orcid.org/0000-0002-5895-2632 2 , 3 ,
- Sandeep Kautish ORCID: orcid.org/0000-0001-5120-5741 4 ,
- Mohd Asif Shah ORCID: orcid.org/0000-0002-0351-9559 5 , 6 , 7 &
- Irfan Ali ORCID: orcid.org/0000-0002-1790-5450 8
Scientific Reports volume 13 , Article number: 13377 ( 2023 ) Cite this article
4245 Accesses
10 Citations
1 Altmetric
Metrics details
- Epidemiology
Malaria is an acute fever sickness caused by the Plasmodium parasite and spread by infected Anopheles female mosquitoes. It causes catastrophic illness if left untreated for an extended period, and delaying exact treatment might result in the development of further complications. The most prevalent method now available for detecting malaria is the microscope. Under a microscope, blood smears are typically examined for malaria diagnosis. Despite its advantages, this method is time-consuming, subjective, and requires highly skilled personnel. Therefore, an automated malaria diagnosis system is imperative for ensuring accurate and efficient treatment. This research develops an innovative approach utilizing an urgent, inception-based capsule network to distinguish parasitized and uninfected cells from microscopic images. This diagnostic model incorporates neural networks based on Inception and Imperative Capsule networks. The inception block extracts rich characteristics from images of malaria cells using a pre-trained model, such as Inception V3, which facilitates efficient representation learning. Subsequently, the dynamic imperative capsule neural network detects malaria parasites in microscopic images by classifying them into parasitized and healthy cells, enabling the detection of malaria parasites. The experiment results demonstrate a significant improvement in malaria parasite recognition. Compared to traditional manual microscopy, the proposed system is more accurate and faster. Finally, this study demonstrates the need to provide robust and efficient diagnostic solutions by leveraging state-of-the-art technologies to combat malaria.
Similar content being viewed by others

Reducing data dimension boosts neural network-based stage-specific malaria detection
Efficient deep learning-based approach for malaria detection using red blood cell smears
Enhancing parasitic organism detection in microscopy images through deep learning and fine-tuned optimizer
Introduction.
Malaria is a life-threatening disease that involves the Plasmodium parasite, which poses a high death rate. It is transmitted to humans by biting an infected female mosquito with the parasite. Malaria is predominantly a tropical disease since mosquitoes thrive in tropical areas, and it is both preventable and treated. According to the latest Global Malaria Report, there are projected to be around 241 million malaria cases and 627 thousand fatalities worldwide by 2022 1 . Moreover, research by the World Health Organization (WHO) suggests that concerns related to COVID-19 could triple the number of malaria cases 2 , 3 . In response to this global epidemic, the WHO has enacted policies to prevent, treat, eradicate, and monitor malaria 4 . Malaria, a preventable disease, can be controlled and prevented if adequate processes and protocols are used, including early diagnosis of the malarial parasite 4 . Several laboratory techniques, including polymerase chain reaction (PCR), microscopy, and rapid diagnostic test (RDT) are commonly used for investigating malaria using thick or thin blood smears 5 , 6 , 7 , 8 . However, conventional methods tend to rely heavily on manually examining blood smears under a microscope. These methods are time-consuming, subjective, and require highly trained personnel. Additionally, the reliance on clinical experts raises concerns about the consistency and accuracy of the diagnosis. To address these deficiencies, computer-aided diagnostic (CAD) methods for malaria evaluation are being developed to reduce mortality rate 9 . Therefore, automated and accurate diagnostic systems are needed to improve malaria detection. Artificial intelligence has gained more and more attention in the scientific community. It has contributed to improving detection through various diagnostic processes. Most medical imaging analyses now incorporate CAD procedures that leverage deep learning techniques for effective model learning.
However, despite advancements, malaria remains endemic in some areas where the disease is common. Early screening plays a crucial role in detecting malaria and saving lives. Consequently, this motivates us to create faster and more accurate malaria diagnosis procedures. Recently, deep learning architectures have received much attention in terms of research and are the most important method to detect disease automatically and more accurately. These generic deep networks have played a vital role in image classification, detection, and recognition 10 , 11 . In a similar vein, data-driven deep learning (DL) algorithms have surpassed manually constructed feature extraction techniques 12 . A convolutional neural network (CNN) is a type of deep learning model that employs different mechanisms, such as local receptive fields, shared weights, and clustering layers, to leverage information. Its purpose is not limited to extracting features but also extends to generating predictive targets and furnishing actionable predictive models that can effectively aid physicians 10 , 13 . Deep neural networks have shown outstanding performance in computer vision tasks in recent years. This is done using methods like the ResNet-32 network model to identify ductal carcinomas 14 precisely. Despite their effectiveness, CNN suffers from limitations in the modeling of spatial relationships and the lack of an internal representation of the geometrical restrictions on the image data. When these flaws are applied to microscopic cell images, the diagnostic model may be misclassified. The need for a more precise and efficient model arises to improve the performance of detecting and classifying malaria parasites. These challenges have prompted us to develop a rapid and more accurate diagnosis procedure for malaria. The specific hypotheses tested in this study include:
Hypothesis 1
Using the inception neural network will enable the extraction of rich and discriminative features from microscopic images of malaria cells, improving parasite detection and classification accuracy.
Hypothesis 2
The incorporation of the imperative capsule neural network will enhance the modeling of spatial relationships within the images, allowing for a more precise classification of malaria parasites.
By testing these hypotheses, the study aims to demonstrate the superiority of the proposed approach over traditional manual microscopy and other existing methods for malaria diagnosis.
This paper is organized as follows: The relevant research is presented in Section “ Related works ”, and the proposed inception-based imperative capsule neural network is discussed in Section “ Materials and methods ”. Part “ Experimental results ” summarizes and describes the outcomes of this network. Part “ Conclusions ” concludes with the article's conclusions and suggested recommendations for further study.
Related works
Several researchers have demonstrated promising results in medical applications by using data-driven machine learning (ML) and deep learning (DL) models. This study examines contemporary deep-learning applications that elicit key decision-making factors in the diagnosis process. Liang et al. 15 presented a 16-layer CNN to classify the parasitized and uninfected cells in thin blood smears. Features are extracted using a pre-trained AlexNet 16 , and a support vector machine (SVM) is trained on these features, and the model has an average accuracy of 97.37%. However, the transfer learning method achieves only 91.99% accuracy. Bibin et al. 17 proposed and tested a six-layer deep belief network to detect malaria parasites in cell images. Based on their findings, the study achieved 96.4% classification accuracy on a custom dataset using training or test randomization. Dong et al. 18 presented SVM and CNN-based approaches for classifying malaria parasites from cell images. This study attained an accuracy of more than 95% using pre-trained deep learning models such as those used in LeNet 19 , AlexNet 16 , and GoogLeNet 20 . Rajaraman et al. 21 proposed a deep-learning model for malaria parasite detection and classification. The method visualizes the activation maps of each layer and understands the probabilities of the different layers to understand the modeling process. As a result, it obtains an accuracy of 98.61%. Mahdi Postchi et al. 22 surveyed the latest advancements in image analysis and machine-learning techniques for diagnosing malaria through microscopy. Although many machine learning models using traditional features have been developed for image classification and decision-making, these models may lack generalization ability. Sivaramakrishnan et al. 23 suggested a customized CNN model and evaluated the effectiveness of pre-trained and deep-learning CNN models as feature extractors for microscopic images to differentiate between healthy and parasitic blood cells. The model uses surface features to achieve more outstanding results than deep features and applies a level-set-based algorithm to detect and segment red blood cells. This model achieved 98.6% (cell-level) accuracy. Yang et al. 24 presented a fivefold cross-validation for two-step CNN models. In the first step, the model uses an intensity-based iterative Global Mini-mum Screening method to recognize parasites, and then a CNN uses a custom CNN to classify the presence of parasites. The success rate of this method is 93.46%. Vijayalakshmi et al. 25 presented a transfer learning method with a classification accuracy of 93.13% to discriminate between illustrations of malaria-diseased cells and healthy using the VGG16 model and a support vector machine. Madhu et al. 26 proposed an improved dynamic routing process to classify malaria-infected cells from healthy cells using a fully trained capsule network, and the model achieved an accuracy of 98.82%. Loddo et al. 27 used the DenseNet-201 neural network to categorize Plasmodium falciparum life stages into four groups and used two different datasets to assess the robustness of the model. The binary classification accuracy rate was 97.68%, and the multi-classification accuracy rate was 99.40%. Meng et al. 28 proposed a neighborhood correlation graph convolutional network to identify multistage malaria parasites. The model has excellent recognition ability for multistage malaria parasites, outperforming the comparison method by at least 8.67%. Madhu et al. 29 proposed an automated diagnostic model based on deep Siamese capsule arrays for uniquely detecting and classifying malaria parasites. When simplified on the largest test sample (test = 40%), the model achieved an accuracy of 96.61% and 98%, respectively. Ha et al. 30 presented a semi-supervised graph learning framework to solve the problem of identifying apicomplexan parasites. Hybrid graph learning is also used in this approach to explore the relationships between different parasites with and without labels.
In malaria, the Plasmodium parasite causes an acute fever that is carried by female Anopheles mosquitoes. It produces life-threatening sickness if left untreated for a long time, and delaying exact treatment might lead to the development of additional comorbidities. A microscope is currently the most prevalent method for detecting malaria. Consequently, an automated approach to diagnosing malaria is required. This study proposes the development of an urgent, inception-based capsule network for classifying parasitized and uninfected cells from micrographs. These diagnostic models contain neural networks based on the Inception and Imperative Capsule architectures. Using a trained model, such as Inception V3, the first block collects rich characteristics from images of malaria cells. In the second block, a dynamic imperative capsule neural network classifies malaria cells into infected and uninfected red blood cells. The experiment's findings indicate a considerable improvement in recognizing malaria parasites, which contributes to better illness diagnosis and prevention.
By observing the existing challenges, this study aims to develop an automatic diagnostic prototype for classifying malaria parasites from microscopic cell images using the Inception neural network with the Imperative Capsule neural network. The preliminary results of this study are presented as follows:
To develop an innovative approach employing an urgent, inception-based capsule network to recognize parasitized and uninfected cells from microscopic images.
The Inception block extracts rich features from malaria cell images using a pre-trained model, such as Inception V3, which facilitates efficient representation learning to recognize the parasites.
The dynamic imperative capsule neural network is utilized to classify microscopic images into parasitized and healthy cells, enabling the detection of malaria parasites.
To compute routing by agreement among low-level and higher-level capsules that can be used to predict malaria cells and classify them into parasitized and uninfected cells using L2-Norm.
This study underscores the importance of leveraging state-of-the-art technologies to combat malaria by providing a robust and efficient diagnostic solution.
Materials and methods
Dataset collection.
Images of thin blood smears containing two distinct strains of malaria—one infected and the other not—were used in the study. These samples were gathered from patients and healthy controls who had Plasmodium falciparum infections, and they were stored at the National Institutes of Health (NIH) repository, which is open to the public for study 23 . The collection includes 13,779 images of parasites and 13,779 images of uninfected cells, totaling 27,558 images of labeled and segmented cells from thin Giemsa-stained blood smear slides. Figure 1 offers some parasitic and uninfected cell images to visualize their physical traits.
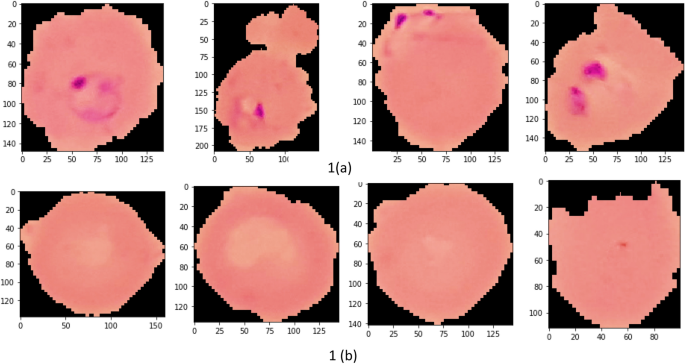
Illustration of sample malaria cell images: ( a ) Infected images; ( b ) Uninfected images (without parasites).
k-fold cross-validation (CV) test
The dataset contains 27,558 blood cell images with malaria-positive and negative samples, which were evaluated in our study for data sample training and testing, and used k-folds (k = 10, 20, 30, 40, 50) Cross-validation to evaluate the proposed model. As shown in Table 1 , the dataset is split into training and testing subsets.
Inception neural network and the imperative capsule neural network
Geoffrey Hinton et al. 31 motivated this research by addressing the limitations of traditional CNNs by proposing inception-based capsule neural networks, which require small data but have higher computational complexity.
This research develops an inception-based imperative capsule neural network for malaria detection, and its basic architecture is shown in Fig. 2 , which is similar to the architecture advocated for image classification problems by Sabour et al. 31 . According to Fig. 2 , input is first routed through fully connected inception blocks, which receive the parasitized and uninfected portions of the cell images as input and extract features on the parasitized and uninfected portions of the cell images. The inception block's output is used as the primary capsule layer's input. The primary and higher capsule layers utilize an imperative routing mechanism to learn the captured features by discerning the spatial orientation of the parasites on the extracted features. After multiple iterations, the resulting output is a feature vector with a length equivalent to the probability of the interval [0, 1], which preserves the object's pose information, minimizing the information loss caused by the feature vector extraction. This feature vector is then used to classify a test sample as infected or healthy cells, aiding in its classification.
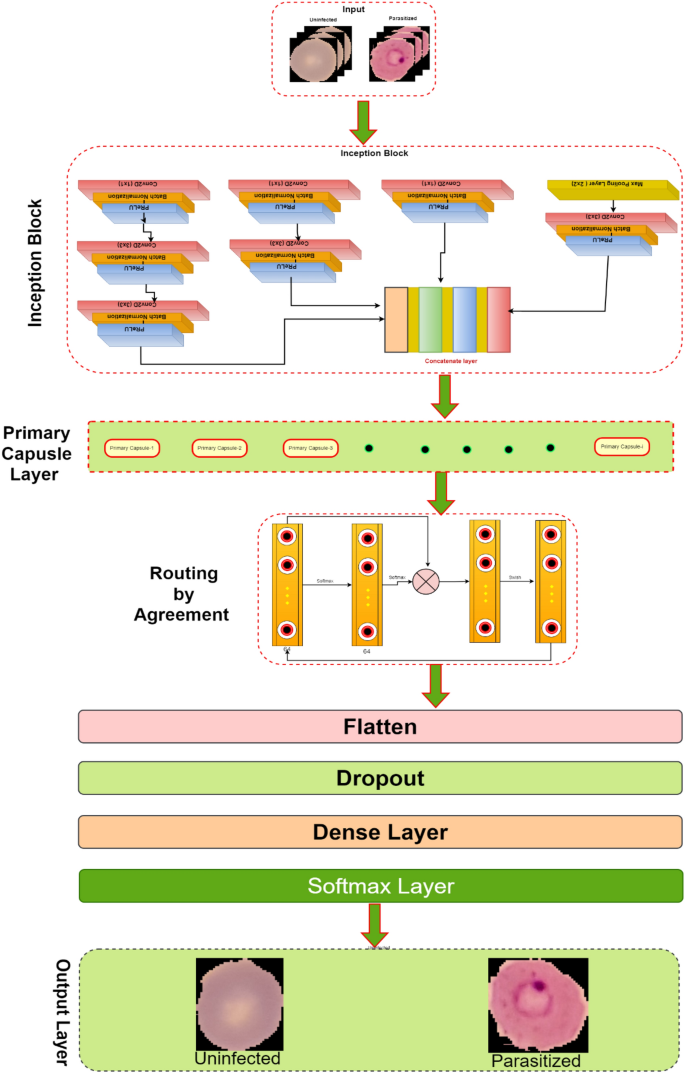
The proposed architecture of Inception-based capsule neural network.
Inception neural network block
In 2015, Google introduced a module for GoogleNet 32 , also known as Inception V3, a convolutional neural network that helps us with image analysis and object detection.
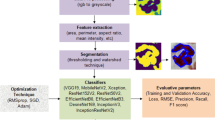
Convolutional layers are frequently employed in convolutional neural networks (CNNs) to extract information from images of malaria blood cells. The CNN's initialization block, which is made up of parallel convolutional layers with filters and kernels of various sizes, extracts feature from various scales to obtain multi-view information on parasites and healthy cells. The structure of the inception block, which is used to extract characteristics at various scales, is shown in Fig. 3 . To extract features at various sizes, this block has four parallel convolutional layers with various kernels (1 × 1, 3 × 3, and 3 × 3). A max-pooling layer with a kernel size of 2 × 2, a convolution layer with a kernel size of 1 × 1, and a batch normalizing layer make up the final parallel convolutional layer. Each parallel layer's computational cost and channel count can be decreased by using a 1 × 1 convolutional layer, and the model's computational cost can be decreased by employing a 3 × 3 max-pooling layer. The output feature maps of each of the four simultaneous convolutional layers are combined after computation to produce new feature maps that are used as the input for the capsule network.
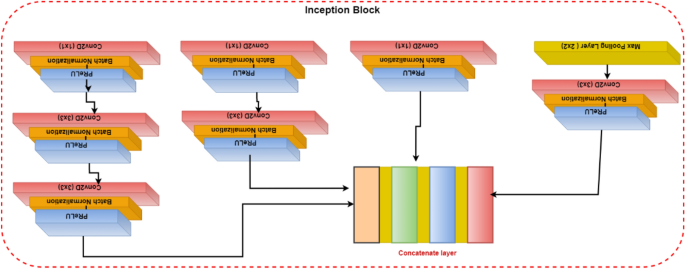
Illustration of the inception block.
Capsule networks block
To classify the items in the MNIST dataset, Sabour et al. 31 presented a capsule network (CapsNet). It uses a neural network to produce an output vector that includes both a scalar and a vector encoding the features of the objects in the image. In our experiment, these capsule networks are trained by carefully adjusting the number of rounds in the dynamic routing algorithm. Using Parametric ReLU (PReLU), it is possible to investigate the behavior of nonlinear activations during dynamic routing 33 . The presence of features in the form of vectors containing low-level entity instantiation parameters is estimated using the principal capsule layer. CapsNet transforms the scalar output using feature detectors in this layer, then passes the vector output of the capsules to the following layer using a modified routing method 31 . Because parameter tuning is critical for better network learning and faster convergence, proper initialization is used to start the routing procedure with kernel initializer before the primary capsule layer; the dynamic routing algorithm is activated with Glorot-normalization 34 . Each capsule, \(i\) has an activity vector \({u}_{i}\in R\) in the layer of \(l,\) which captures information about the features extracted from an entity (i.e., blood cell image). The output of the activity vector \({u}_{i}\) of the \(i\) th level capsule is fed as data into the next level layer, i.e., \(l+1\) layer. The \({j}{\text{th}}\) layer capsules of layer \(l+1\) will get data from \({u}_{i}\) and compute the product weight matrix \({W}_{ij}^{T}\) . The results are stored in the form of \({\widehat{u}}_{(j|i)}.\) This vector is the layer of capsules \(i\) at level \(l\) layer, which is the transformation of the entity represented by capsule \(j\) at the level of \(l+1\) . Then apply the transformation matrix \({W}_{ij}^{T}\) to capsule output \({u}_{i}\) of the previous layer, as shown in Eq. ( 1 ).
In Eq. ( 1 ), capsule \(i\) is the primary capsule layer, \(j\) is the higher-level capsule layer, and \({u}_{i}\) is the output of the capsule network of the upper layer and \({W}_{ij}^{T}\) is the learnable weighted matrix between the \({i}{\text{th}}\) capsule to \({j}{\text{th}}\) capsule. Which is multiplied by each output vector and the coupling coefficient \({C}_{ij}\) is added to the linear sum stage. Then the capsules are in the higher level, which is filled with the sum of the output vector in the lower-level layer, and we add it with a coupling coefficient \({C}_{ij}\) which is computed during the routing method shown in Eq. ( 2 ).
In dynamic routing, the coupling coefficient is determined by Eq. ( 2 ). In the process of calculating \({S}_{j}\) in forward propagation, \({W}_{ij}^{T}\) is set to a random value, \({a}_{ij}\) is initialized to zero, \({u}_{i}\) is the output of the previous layer, and then compute a weighted sum \({S}_{j}\) with weights \({C}_{ij}\) (the sum of these coefficients is equal to one) and it is denoted as follows:
The squashing function map of \({S}_{j}\) yields the output vector \({v}_{j},\) which is obtained is defined as follows:
The squashing function, defined by Eq. ( 4 ), ensures that short vectors are reduced to fewer dimensions near zero while long vectors are scaled to unit length, thus introducing nonlinearity to the capsule network. The total input Sj processed by the jth dimensional capsule array contributes to the coupling coefficient Cij. An activation function PReLU is applied to update the coupling coefficients, instead of the squashing function, by operating on Sj. During the iterative learning phase, these coupling coefficients are updated using Eq. ( 5 ), which proceeds as follows:
In Eq. ( 5 ), \({a}_{ij}\) is a parameter used as a weighted proxy, which means that it gives higher weights to appropriate predictions, and it starts at zero and is modified as the training progress.
However, it is initialized with the current input weights to improve the learning method by reducing the computational cost and improving the predictive ability. The number of routing iterations (n = 3) is used as a hyperparameter allowing one to choose a specific number of iterations during the training (here, epochs = 100) period, and the details of this network parameters are shown in Table 2 . The learning period is evaluated by evaluating the convergence, and our model is repeated for only three iterations. Figure 4 depicts the comprehensive learning curves for iterations over 100 epochs.
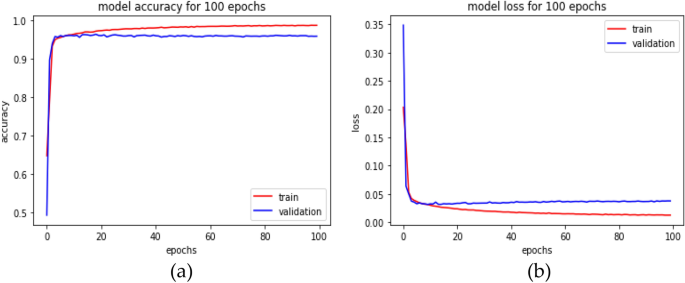
An inception-based capsule network with a router in 3 iterations, depicted as ( a ) accuracy curves and ( b ) loss decay curves.
PReLU activations are utilized during the routing by agreement process to improve the understanding of feature invariance in the captured images of malaria cells. In a conventional capsule network, the squash activation function is typically used as a non-linearity. However, using PReLU as a non-linearity is believed to lead to better generalization and convergence over time. The last layer of the network comprises two capsules (parasitized and uninfected cells) reflecting the probability of the interval [0, 1] and the position information of the object, preserving the pose information to reduce information loss caused by the extracted feature vector. This enables the classification of test samples into either parasitized or uninfected cells, thus aiding in cell feeding.
Loss function
Our current loss function 31 also includes the mean squared error rate (MSE) alongside the marginal loss. Change the settings for faster convergence and add proper model regularization and noise addition when training the classification model with a value set to 0.45.
In Eq. ( 6 ), \({m}^{+}\) and \({m}^{-}\) are the category prediction values, \(\sigma \) is the balance coefficient, \({T}_{x} \mathrm{is \, the \, label \, of \, category}, \) and classification probability vector \(\Vert {v}_{x}\Vert \) is the size. For this study, the default values are set as \({m}^{+}=0.85 \& {m}^{-}=0.15\) , \(\sigma =0.45\) . The total loss function, in this case, refers to the loss of capsules representing both malaria-parasitized and uninfected classes.
Experimental results
This section describes the proposed model's implementation in-depth and thoroughly analyses how well it performs under various restrictions. The proposed network was evaluated against front-line classification models created by several authors, which were pre-trained using NIH malaria datasets 23 and other private datasets to assess whether red blood cells are parasitized or not. According to Table 3 , the proposed model for malaria parasite identification and classification performed well on the NIH malaria dataset, along with the comparison findings. It is important to note that most models typically exhibit low performance on this dataset. Although their weights can handle common classification datasets, they frequently fall short because of ineffective feature extraction brought on by too much depth. Instead, the Inception-based capsule network model classifies parasitized and uninfected cells accurately during the diagnostic process by utilizing external knowledge to produce rich characteristics. On international benchmarks, the suggested model performs noticeably better.
As stated in the Table 4 , our model is assessed for layer-wise testing cell images, varying from training to 80% and testing to 20%.
In this analysis, experiments are conducted on various distributions, and the suggested network's implementation, as shown in Table 4 , achieves an accuracy of 99.35% and an AUC score of at least 99.73% at a test ratio of 20%. Table 4 shows the models' overall generality as measured by various standard classification metrics, including accuracy score, AUC–ROC, sensitivity, and specificity. Limiting diagnostic power does not assess the likelihood that a certain patient will acquire a disease, but it does affect diagnostic accuracy, even though they choose sensitivity and specificity. Table 5 displays the effectiveness of the suggested capsule array at various nonlinearity levels. Compared to the performance of cutting-edge pre-trained models, the generalization distribution for the training and test samples is 80% to 20%.
The performance metrics for every deep learning architecture are compiled in Table 5 . The proposed malaria detection algorithm outperforms the compared deep learning models in terms of performance. The results showed an accuracy of more than 99.35%, an AUC score of 99.73%, and an F1 score of 99.36%. The accuracy score is a well-known metric with a domain that is invariant to general utility; hence it is imperative to note. As a result, the effectiveness of the suggested model is assessed using various measuring techniques. The model was created to be assessed by segregating partition samples that vary from 10 to 50%, ensuring that the model is adequately generalized. Figure 5 displays the predicted results of the suggested model on images of malarial cells. The true value is shown on the x-axis, and the model forecast is shown on the y-axis.
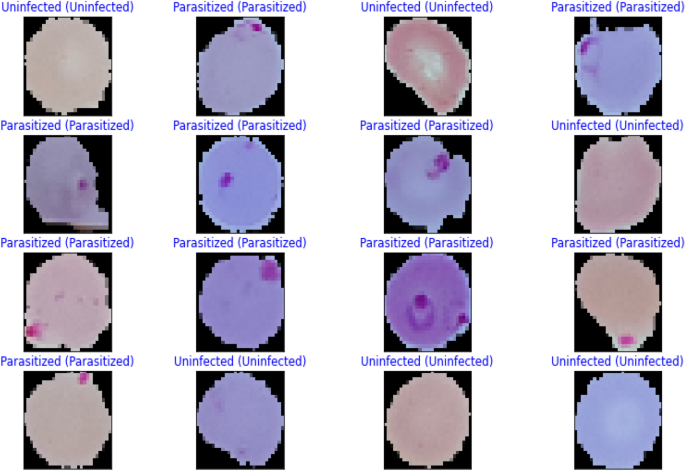
Illustration of some prediction results of the proposed model.
Time complexity analysis
According to our study, the learning model was trained for 100 epochs to assess the time complexity of the model. The results show that our model takes around 33.8667 min for training and 3 s for complete testing, which is less than all the compared models. This study addresses the urgent need for automated malaria detection and classification. It proposes a novel approach based on integrating inception and imperative capsule neural networks. This research has the potential to significantly improve malaria diagnosis, contributing to more effective disease management and prevention. Additionally, the study contributes to the growing field of deep learning in medical image analysis. It showcases the applicability of advanced neural network architectures to address critical healthcare challenges.
Conclusions
This research develops a deep-learning approach by combining the imperative capsule neural network with the inception neural network to distinguish between malaria-parasitized and uninfected cells. This enhances the classification accuracy of identifying malaria parasites from photographs of blood cells. With well-chosen parameters, the capsule model can efficiently finish the procedure for classifying uninfected cells or parasites into different categories. Models with different loss parameters are compared to the proposed model, and the results show that the model's performance can be increased by adjusting the loss parameters. The proposed network achieves higher classification accuracy while analyzing blood cell images for malaria than competing deep learning methods. Under the worst-case scenario (50/50 split), the model obtains an accuracy of 98.10% on the test, while on the 20% split, it achieves an accuracy of 99.355%. These experimental results are helpful since the developed model is robust and flexible and has outperformed competing models. In the work's future scope, the model may be utilized to recognize parasite species and stages in thin blood smears. This research opens opportunities for future advancements in malaria diagnosis and surveillance, including using mobile and portable imaging devices for point-of-care testing.
Data availability
The data that support the findings of this study are openly available in the National Library of Medicine (NLM)—Malaria Data: https://lhncbc.nlm.nih.gov/LHC-research/LHC-projects/image-processing/malaria-datasheet.html and reference number Ref. 23 .
https://www.who.int/news-room/fact-sheets/detail/malaria .
Alnussairi, M. H. D. & İbrahim, A. A. Malaria parasite detection using deep learning algorithms based on (CNNs) technique. Comput. Electr. Eng. 103 , 108316 (2022).
Article Google Scholar
Chakradeo, K., Delves, M. & Titarenko, S. Malaria parasite detection using deep learning methods. Int. J. Comput. Inf. Eng. 15 (2), 175–182 (2021).
Google Scholar
Fact Sheet about MALARIA. https://www.who.int/news-room/fact-sheets/detail/malaria . Accessed 26 Nov 2022.
Devi, S. S., Roy, A., Singha, J., Sheikh, S. A. & Laskar, R. H. Malaria infected erythrocyte classification based on a hybrid classifier using microscopic images of thin blood smear. Multimed. Tools Appl. 77 (1), 631–660 (2018).
Mfuh, K. O. et al. A comparison of thick-film microscopy, rapid diagnostic test, and polymerase chain reaction for accurate diagnosis of Plasmodium falciparum malaria. Malar. J. 18 (1), 1–8 (2019).
Poostchi, M., Silamut, K., Maude, R. J., Jaeger, S. & Thoma, G. Image analysis and machine learning for detecting malaria. Transl. Res. 194 , 36–55 (2018).
Article PubMed PubMed Central Google Scholar
Hanscheid, T. & Valadas, E. Malaria diagnosis. Am. J. Trop. Med. Hyg. 61 , 179. https://doi.org/10.4269/ajtmh.1999.61.179 (1999).
Article CAS PubMed Google Scholar
Alonso-Ramírez, A. A. et al. Classifying parasitized and uninfected malaria red blood cells using convolutional-recurrent neural networks. IEEE Access 10 , 97348–97359 (2022).
Krizhevsky, A., Ilya Sutskever, S. & Geoffrey, E. H. ImageNet classification with deep convolutional neural networks. Commun. ACM 60 (6), 84–90 (2017).
Redmon, J., Divvala, S., Girshick, R. & Farhadi, A. You only look once: Unified, real-time object detection. in Proceedings of the IEEE Conference on Computer Vision and Pattern Recognition , 779–788 (2016).
LeCun, Y., Bengio, Y. & Hinton, G. Deep learning. Nature 521 (7553), 436–444 (2015).
Article ADS CAS PubMed Google Scholar
Razzak, M. I., Naz, S. & Zaib, A. Deep Learning for Medical Image Processing: Overview, Challenges, and the Future 323–350 (Springer, 2018).
Praveen, S. P., Srinivasu, P. N., Shafi, J., Wozniak, M. & Ijaz, M. F. ResNet-32 and FastAI for diagnoses of ductal carcinoma from 2D tissue slides. Sci. Rep. 12 (1), 20804 (2022).
Article ADS CAS PubMed PubMed Central Google Scholar
Liang, Z. et al . CNN-based image analysis for malaria diagnosis. in IEEE International Conference on Bioinformatics and Biomedicine, IEEE , 493–496 (2016).
Krizhevsky, A., Sutskever, I. & Hinton, G. E. ImageNet classification with deep convolutional neural networks. in Proceedings of the 25th International Conference on Neural Information Processing Systems, Volume 1 (NIPS'12) , 1097–1105 (2012).
Bibin, D., Nair, M. S. & Punitha, P. Malaria parasite detection from peripheral blood smear images using deep belief networks. IEEE Access 5 , 9099–9108 (2017).
Dong, Y. et al . Evaluations of deep convolutional neural networks for automatic identification of malaria-infected cells. in EMBS International Conference on Biomedical & Health Informatics, IEEE , 101–104 (2017).
Lecun, Y., Bottou, L., Bengio, Y. & Haffner, P. Gradient-based learning applied to document recognition. Proc. IEEE 86 (11), 2278–2324 (1998).
Szegedy, C. et al . Going deeper with convolutions. in Proceedings of the 2015 (CVPR) , 1–9 (2015).
Sivaramakrishnan, R., Antani, S. & Jaeger, S. Visualizing deep learning activations for improved malaria cell classification. Med. Inf. Healthc. 1 , 40–47 (2017).
Poostchi, M., Silamut, K., Maude, R. J., Jaeger, S. & Thoma, G. Image analysis and machine learning for detecting malaria. Transl. Res. 194 (6), 36–55 (2018).
Sivaramakrishnan, R. et al. Pre-trained convolutional neural networks as feature extractors toward improved malaria parasite detection in thin blood smear images. PeerJ 6 , e4568 (2018).
Yang, F. et al. Deep learning for smartphone-based malaria parasite detection in thick blood smears. IEEE J. Biomed. Health Inform. 24 (5), 1427–1438 (2019).
Article PubMed Google Scholar
Vijayalakshmi, A. & Rajesh Kanna, B. Deep learning approach to detect malaria from microscopic images. Multimed. Tools Appl. 79 (21), 1–21 (2020).
Madhu, G. et al. Imperative dynamic routing between capsules network for malaria classification. Comput. Mater. Contin. 68 (1), 903–919 (2021).
Loddo, A., Fadda, C. & Di Ruberto, C. An empirical evaluation of convolutional networks for malaria diagnosis. J. Imaging 8 , 3. https://doi.org/10.3390/jimaging8030066 (2022).
Meng, X., Ha, Y. & Tian, J. Neighbor correlated graph convolutional network for multi-stage malaria parasite recognition. Multimed. Tools Appl. 81 , 11393–11414. https://doi.org/10.1007/s11042-022-12098-6 (2022).
Madhu, G. et al. DSCN-net: A deep Siamese capsule neural network model for automatic diagnosis of malaria parasites detection. Multimed. Tools Appl. 81 , 34105–34127. https://doi.org/10.1007/s11042-022-13008-6 (2022).
Ha, Y., Meng, X., Du, Z., Tian, J. & Yuan, Y. Semi-supervised graph learning framework for apicomplexan parasite classification. Biomed. Signal Process. Control 81 , 104502. https://doi.org/10.1016/j.bspc.2022.104502 (2022).
Sabour, S., Frosst, N. & Hinton, G. E. Dynamic routing between capsules. Adv. Neural Inf. Process. Syst. 1 , 3856–3866 (2017).
Szegedy, C. et al . Going deeper with convolutions. in Proc. CVPR 2015 , 1–9 (2015).
He, K., Zhang, X., Ren, S. & Sun, J. Delving deep into rectifiers: Surpassing human-level performance on imagenet classification. in Proceedings of the IEEE International Conference on Computer Vision , 1026–1034 (2015).
Glorot, X. & Bengio, Y. Understanding the difficulty of training deep feedforward neural networks. in Proceedings of the Thirteenth International Conference on Artificial Intelligence and Statistics , 249–256 (2010).
Das, D. K., Maiti, A. K. & Chakraborty, C. Automated system for characterization and classification of malaria-infected stages using light microscopic images of thin blood smears. J. Microsc. 257 (3), 238–252 (2015).
Díaz, G., González, F. A. & Romero, E. A semi-automatic method for quantification and classification of erythrocytes infected with malaria parasites in microscopic images. J. Biomed. Inform. 42 (2), 296–307 (2009).
Gopakumar, G. P. et al. Convolutional neural network-based malaria diagnosis from focus stack of blood smear images acquired using custom-built slide scanner. J. Biophoton. 11 (3), e201700003 (2018).
Rahman, A. et al . Improving malaria parasite detection from red blood cell using deep convolutional neural networks. (2019). arXiv:1907.10418 .
Download references
Author information
Authors and affiliations.
Department of Information Technology, VNR Vignana Jyothi Institute of Engineering and Technology, Hyderabad, Telangana, 500090, India
Golla Madhu
Operations Research Department, Faculty of Graduate Studies for Statistical Research, Cairo University, Giza, 12613, Egypt
Ali Wagdy Mohamed
Applied Science Research Center, Applied Science Private University, Amman, Jordan
LBEF Campus (Asia Pacific University of Technology & Innovation, Malaysia), Kathmandu, 44600, Nepal
Sandeep Kautish
College of Business and Economics, Kabridahar University, Po Box 250, Kabridahar, Ethiopia
Mohd Asif Shah
School of Business, Woxsen University, Kamkole, Sadasivpet, Hyderabad, 502345, Telangana, India
Division of Research and Development, Lovely Professional University, Phagwara, 144001, Punjab, India
Department of Statistics & Operations Research, Aligarh Muslim University, Aligarh, 202002, India
You can also search for this author in PubMed Google Scholar
Contributions
G.M. conceived and designed the experiments, performed the experiments, and prepared figures and/or tables. A.W.M., S.K., M.A.S. and I.A. supervised the study, analyzed the results, and provided insightful suggestions for the manuscript. All authors have read and authored or reviewed drafts of the paper and approved the final draft.
Corresponding author
Correspondence to Mohd Asif Shah .
Ethics declarations
Competing interests.
The authors declare no competing interests.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Cite this article.
Madhu, G., Mohamed, A.W., Kautish, S. et al. Intelligent diagnostic model for malaria parasite detection and classification using imperative inception-based capsule neural networks. Sci Rep 13 , 13377 (2023). https://doi.org/10.1038/s41598-023-40317-z
Download citation
Received : 13 April 2023
Accepted : 08 August 2023
Published : 17 August 2023
DOI : https://doi.org/10.1038/s41598-023-40317-z
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
This article is cited by
Language discrepancies in the performance of generative artificial intelligence models: an examination of infectious disease queries in english and arabic.
- Malik Sallam
- Kholoud Al-Mahzoum
BMC Infectious Diseases (2024)
Hybrid framework for membrane protein type prediction based on the PSSM
- Xiaoli Ruan
Scientific Reports (2024)
By submitting a comment you agree to abide by our Terms and Community Guidelines . If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily.
- Oxford Thesis Collection
- Climate Research Collection
- CC0 version of this metadata
Understanding the use of geospatial disease mapping in malaria risk stratification and intervention targeting across sub-Saharan Africa
Malaria risk maps are a critical tool to assist national malaria programmes in the prioritization and targeting of their malaria control activities to maximize efficacy and equity, especially under conditions of resource constraint. The research study sets out to answer a core series of questions pertaining to the use of malaria mapping, the actors involved, the mapped outputs produced, and approaches for their improvement and knowledge translation.
The research study conducts a na...
Email this record
Please enter the email address that the record information will be sent to.
Please add any additional information to be included within the email.
Cite this record
Chicago style, access document.
- Hawa_2022_Understanding_the_use.pdf ( Preview , Dissemination version, 6.5MB, Terms of use )
Why is the content I wish to access not available via ORA?
Content may be unavailable for the following four reasons.
- Version unsuitable We have not obtained a suitable full-text for a given research output. See the versions advice for more information.
- Recently completed Sometimes content is held in ORA but is unavailable for a fixed period of time to comply with the policies and wishes of rights holders.
- Permissions All content made available in ORA should comply with relevant rights, such as copyright. See the copyright guide for more information.
- Clearance Some thesis volumes scanned as part of the digitisation scheme funded by Dr Leonard Polonsky are currently unavailable due to sensitive material or uncleared third-party copyright content. We are attempting to contact authors whose theses are affected.
Alternative access to the full-text
Request a copy.
We require your email address in order to let you know the outcome of your request.
Provide a statement outlining the basis of your request for the information of the author.
Please note any files released to you as part of your request are subject to the terms and conditions of use for the Oxford University Research Archive unless explicitly stated otherwise by the author.
Contributors
Bibliographic details, item description, terms of use, views and downloads.
If you are the owner of this record, you can report an update to it here: Report update to this record
Report an update
We require your email address in order to let you know the outcome of your enquiry.
- Skip to main content
- Accessibility information

- Enlighten Enlighten
Enlighten Theses
- Latest Additions
- Browse by Year
- Browse by Subject
- Browse by College/School
- Browse by Author
- Browse by Funder
- Login (Library staff only)
In this section
Drug action and resistance in malaria parasites: experimental genetics models and biochemical features of fast acting novel antimalarials
Simwela, Nelson Victor (2020) Drug action and resistance in malaria parasites: experimental genetics models and biochemical features of fast acting novel antimalarials. PhD thesis, University of Glasgow.
| |
Resistance to antimalarial drugs inevitably follows their deployment in malaria endemic parts of the world. For instance, current malaria control efforts which significantly rely on artemisinin combination therapies (ACTs) are being threatened by the emergence of resistance to artemisinins and ACTs. Understanding the role of genetic determinants of artemisinin resistance is therefore important for implementation of mitigation strategies. Moreover, elucidating the mode of action for drugs that are in advanced stages of development is specifically critical as drug resistance mechanisms can be prospectively predicted and possible means of surveillance put in place.
In the present work, CRISPR-Cas9 genome editing has been used to engineer candidate artemisinin resistance mutations (Kelch13 and UBP-1) in the rodent malaria parasite Plasmodium berghei. The role of these mutations in mediating artemisinin (and chloroquine) resistance under both in vitro and in vivo conditions has been assessed which up until now, has either remained un-validated (UBP-1) or debated (Kelch13, under in vivo conditions) in human infecting Plasmodium falciparum. The results have provided an in vivo model for understanding and validating artemisinin resistance phenotypes which just like their Plasmodium falciparum equivalents do not just mediate resistance phenotypes, but also carry accompanying fitness costs.
In addition to the above findings, biochemical and drug inhibition studies have been carried out to demonstrate that small molecule inhibitors targeting ubiquitin hydrolases (to which UBP-1 is a class member) display activity in human and rodent infecting malaria parasites in vitro and in vivo. These inhibitors also show evidence of ability to potentiate artemisinin action which can be exploited to overcome the emerging resistance as combination partner drugs. Untargeted metabolomic screens have also been used to characterize the mode of action of lead antimalarial drug candidates that are emerging from the Novartis Institute of Tropical Diseases drug discovery pipeline. A common biochemical and metabolic profile of these compounds which display a very fast parasite killing rate is presented and can hopefully be used to identify compounds that can achieve a similar feat. Moreover, these profiles have pointed to possible mode of action for novel drugs whose mechanistic mode of parasite killing is still unknown or disputed.
| Item Type: | Thesis (PhD) |
|---|---|
| Qualification Level: | Doctoral |
| Keywords: | Antimalarial drugs, mode of action, resistance, genetics models, biochemistry, metabolomics. |
| Subjects: | > |
| Colleges/Schools: | > > |
| Funder's Name: | , |
| Supervisor's Name: | Waters, Professor Andy and Barrett, Professor Mike |
| Date of Award: | 2020 |
| Depositing User: | |
| Unique ID: | glathesis:2020-81876 |
| Copyright: | Copyright of this thesis is held by the author. |
| Date Deposited: | 17 Dec 2020 17:02 |
| Last Modified: | 16 Sep 2022 15:52 |
| Thesis DOI: | |
| URI: |
Actions (login required)
| View Item |
Downloads per month over past year
View more statistics

The University of Glasgow is a registered Scottish charity: Registration Number SC004401
malaria final thesis
- September 2019
- Thesis for: degree
- Advisor: SUPERVISOR BY: Dr. HAMZE ALI ABDILLAHI

- Medical lecturer.
Abstract and Figures

Discover the world's research
- 25+ million members
- 160+ million publication pages
- 2.3+ billion citations

- Elsanousi Abbas
- Suleiman Elmahi

- World Health Organization
- Ashenafi Woime
- Wilson Sama

- Mejbah Uddin
- A Cunnington
- Degifiebereka
- M Dr Ap Motsoaledi
- Recruit researchers
- Join for free
- Login Email Tip: Most researchers use their institutional email address as their ResearchGate login Password Forgot password? Keep me logged in Log in or Continue with Google Welcome back! Please log in. Email · Hint Tip: Most researchers use their institutional email address as their ResearchGate login Password Forgot password? Keep me logged in Log in or Continue with Google No account? Sign up
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List

Systematic review on traditional medicinal plants used for the treatment of malaria in Ethiopia: trends and perspectives
Getachew alebie.
1 Department of Biology, Jigjiga University, P.O. Box-1020, Jijiga, Ethiopia
Befikadu Urga
2 College of Veterinary Medicine, Jigjiga University, P.O.Box-1020, Jijiga, Ethiopia
Associated Data
All data pertaining to this study are within the manuscript and the supporting files.
Ethiopia is endowed with abundant medicinal plant resources and traditional medicinal practices. However, available research evidence on indigenous anti-malarial plants is highly fragmented in the country. The present systematic review attempted to explore, synthesize and compile ethno-medicinal research evidence on anti-malarial medicinal plants in Ethiopia.
A systematic web search analysis and review was conducted on research literature pertaining to medicinal plants used for traditional malaria treatment in Ethiopia. Data were collected from a total of 82 Ethiopian studies meeting specific inclusion criteria including published research articles and unpublished thesis reports. SPSS Version 16 was used to summarize relevant ethno-botanical/medicinal information using descriptive statistics, frequency, percentage, tables, and bar graphs.
A total of 200 different plant species (from 71 families) used for traditional malaria treatment were identified in different parts of Ethiopia. Distribution and usage pattern of anti-malarial plants showed substantial variability across different geographic settings. A higher diversity of anti-malarial plants was reported from western and southwestern parts of the country. Analysis of ethno-medicinal recipes indicated that mainly fresh leaves were used for preparation of remedies. Decoction, concoction and eating/chewing were found to be the most frequently employed herbal remedy preparation methods. Notably, anti-malarial herbal remedies were administered by oral route. Information on potential side effects of anti-malarial herbal preparations was patchy. However, some anti-malarial plants were reported to have potentially serious side effects using different local antidotes and some specific contra-indications.
The study highlighted a rich diversity of indigenous anti-malarial medicinal plants with equally divergent herbal remedy preparation and use pattern in Ethiopia. Baseline information gaps were observed in key geographic settings. Likewise, herbal remedy toxicity risks and countermeasures generally entailed more exhaustive investigation. Experimental research and advanced chemical analysis are also required to validate the therapeutic potential of anti-malarial compounds from promising plant species.
Electronic supplementary material
The online version of this article (doi:10.1186/s12936-017-1953-2) contains supplementary material, which is available to authorized users.
Malaria remains one of the world’s leading health problems, causing about 429,000 deaths in 2015, the vast majority of deaths (99%) were due to Plasmodium falciparum malaria [ 1 ]. In that year, most (92%) of the deaths were estimated to have occurred in the sub-Saharan Africa region. Children were particularly affected by the disease with 70% of malaria-caused deaths occurring among the under five-year age group [ 1 , 2 ]. In Ethiopia, the majority (around 68%) of populations live in areas deemed malarious or potentially malarious [ 3 ]. Despite recent improvements in malaria control strategies, the disease remains a major public health problem and a leading cause of outpatient consultations, admissions and death in the country [ 4 , 5 ].
In recent years, emergence of drug-resistant Plasmodium species has exacerbated the health and economic impact of malaria. In particular, P. falciparum (the most pathogenic human parasite) has developed resistance to virtually all currently available anti-malarial drugs [ 6 ]. Consequently, research for alternative anti-malarial drugs has accelerated over the last two decades [ 7 ]. Historically, medicinal plants have been the focus of many researches aimed at discovering alternative anti-malarial drugs in different parts of the world [ 8 ]. This has led to the discovery of numerous anti-malarial compounds with significant structural varieties, including quinines, triterpenes, sesquiterpenoids, quassinoids, limnoids, alkaloids, lignans, and coumarins [ 9 ].
Around 80% of Ethiopian populations (particularly rural societies) still rely on traditional medicinal plants to fight a number of diseases. This was attributed to high cost of modern drugs, paucity and inaccessibility of modern health services, and cultural acceptability of traditional medicine [ 10 , 11 ]. Communities inhabiting different localities in the country have developed their own medical plant arsenals and knowledge on their utilization, management and conservation [ 12 ]. A large variety of medicinal plants are used as traditional malaria remedy in different parts of Ethiopia [ 13 – 17 ].
Proper documentation of traditional medicine and plants used in the prophylaxis and treatment of malaria constitutes an important task not only in preserving precious indigenous knowledge and biodiversity but also in enhancing community access to and stakes in improvement of malaria control interventions. It is also crucial for stimulating future research on safety and efficacy of medicinal plants and identification of chemical entities that could be developed into new standardized phytomedicines. In contrast, ethno-botanical and ethno-pharmacological research on indigenous anti-malarial plants is still at a rudimentary stage in Ethiopia [ 18 ]. Moreover, available research evidence on indigenous anti-malarial plants is highly fragmented, which underscores serious need for systematic compilation and synthesis.
The present systematic review attempted to explore, synthesize and compile ethno-medicinal research findings on anti-malarial plants in Ethiopia.
A systematic analysis and review of research literature related to medicinal plants used for traditional malaria treatment in Ethiopia was conducted between April and October 2016.
Search strategy
A web-based systematic research literature search strategy was employed. Ethno-botanical/ethno-medicinal studies reporting on medicinal plants used for traditional malaria treatment in Ethiopia were gathered by two different search approaches, including:
- Search for unpublished MSc/PhD thesis research reports using Google search engine and local university websites;
- Search for published journal articles using international scientific databases including PubMed, Science direct, Web of Science, Google scholar, AJOL, etc.
Literature search was performed using the following key terms: Ethiopia/Ethiopian plants/Ethiopian medicinal plants/Ethiopian anti-malarial plants, Malaria/Anti-malarial/Anti-malarial plants, Traditional/Traditional knowledge/Traditional Medicine/Traditional medicinal plants, Medicinal Plants/Medicinal herbs, Indigenous/Indigenous knowledge, Plants/Herbal/Medicine/Remedies, Folk Medicine/Folk remedies/Home remedies/Herbal remedies, Ethnobotany/Ethnobotanical, Ethnopharmacology/Ethnopharmacological, Ethnomedicine/Ethnomedicinal, Ethnopharmaceutical, Medico-cultural.
Screening and criteria
Screening of search outputs was performed in two stages. First, the title and abstract of identified journal articles/theses was overviewed. Thereafter, suitable articles/theses were downloaded and critically inspected for inclusion in the review. Literature screening was based on the following inclusion and exclusion criteria.
Inclusion criteria
Published and unpublished ethno-botanical and ethno-medicinal surveys reporting on anti-malarial plant/s, conducted at any time period in Ethiopia
Exclusion criteria
The following types of research data were excluded from analysis:
- Data from review articles, historical documents or experimental studies;
- Data from published and unpublished ethno-botanical and ethno-medicinal surveys lacking information on anyone of the following: study areas/localities, informant’s involvement, scientific plant names, and not reporting information about anti-malarial medicinal plants;
- Data from non-open access journal articles or partially accessed (abstract only) articles.
Data retrieval
Relevant information pertaining to Ethiopian anti-malarial medicinal plants was retrieved using a structured Excel format by directly quoting reported values. In order to provide uniform information on preparation methods of the remedy, the following terms were established, and they signified the respective preparation processes described herein: Concoction: mixing/combining different ingredients to make a dish; Decoction: boiling the materials and extracting essences or active ingredients; Infusion: macerating/soaking the materials in a liquid or water; Homogenization: homogenizing ingredients; Pounding: grinding, pulverizing, chopping or crushing of ingredients; Cooking: preparing food (remedy) for eating by adding ingredients; Smoking: burning dry materials and inhaling the smoke; Bathing/evaporating: boiling the materials and taking the vapour or steam through intranasal and whole body.
In addition, missed information in some studies, particularly local name and habit of the plants, and misspelled scientific names were retrieved from Natural Database for Africa (NDA), Version 2.0. In case of some research papers lacked geographic locations of the study localities/districts, information was retrieved through direct web (Google) searching.
Data analysis
All data were entered into Statistical Software Packages for Social Science (SPSS, software version 16.0). A descriptive statistical methods, percentage and frequency were used to analyse ethno-botanical data on reported medicinal plants and associated indigenous knowledge. The results were presented using charts and tables.
Overview of ethno-medicinal studies on medicinal plants
Ethno-medicinal studies on plants demand standard procedures for botanical identification and reliable documentation of indigenous knowledge pertaining to plant distribution, management and traditional medicinal use. A total of 82 original ethno-medicinal studies representing ten different regions in Ethiopia were included in this review. Both published and unpublished (M.Sc. and Ph.D. theses) research reports were reviewed. Overall, the reviewed research reports exhibited comparable qualities compared to slightly modified versions of the criteria set by Willcox et al. [ 19 ]. Study quality inconsistencies were noted with regard to sampling and number of knowledgeable informants, as well as completeness of herbal remedy recipe, prescription and dosage, side effects, and antidote information reported (Table 1 ). Current findings reflect potentially important information gaps and need for standardization of ethno-medicinal studies on indigenous medicinal plants in Ethiopia.

Table 1
Characteristics of studies on medicinal plants used for the treatment of malaria in Ethiopia
| Evaluation parameters | Total number of studies (n = 82) | |
|---|---|---|
| Criterion | Frequency (%) | |
| Paper types | Published article | 64 (78.0) |
| Unpublished thesis | 18 (22.0) | |
| Botanical identification | Plant collected and verified with informant | 3 (3.6) |
| Voucher specimen in herbarium | 18 (22.0) | |
| Formal identification by botanist | 13 (15.9) | |
| All | 44 (53.6) | |
| None | 4 (4.9) | |
| Informants reliability | ≥10 informants interviewed | |
| Yes | 74 (90.2) | |
| No | 8 (9.8) | |
| ≥2 informants mention use of plant for malaria treatment | ||
| Yes | 66 (80.5) | |
| No | 16 (19.5) | |
| Informant(s) experience of treating malaria | ||
| Yes | 61 (74.4) | |
| No | 21 (25.6) | |
| Reliable (fulfill all above criteria) | ||
| Yes | 53 (64.6) | |
| No | 29 (35.4) | |
| Researcher reliability | Used same language as informants | |
| Yes | 78 (95.1) | |
| No | 4 (4.9) | |
| Recorded Ethno-medicinal information | ||
| At least PU, PM and AR | 15 (18.3) | |
| Detailed | 58 (70.7) | |
| Poor | 9 (11.0) | |
PU part used, PM preparation method, RA administration routes
Anti-malarial medicinal plants in Ethiopia
In aggregate, 82 studies identified a total 200 different plant species used in traditional malaria treatments throughout Ethiopia. Additional file 1 summarizes the distribution of the reported plants according to administrative regions and floristic areas of collection. Additional file 2 summarizes the detail of traditional herbal medicine used for the treatment of malaria in Ethiopia.
Geographic distribution of anti-malarial plants
The geographic distribution of anti-malarial plants is likely to be predicated on local trend with regard to disease risk, floral diversity and cultural diversity, including traditional medicinal practices. The western lowlands of Oromia, Amhara, Tigray, Southern Nation and Nationality People (SNNP), and almost the entire areas of Benishangul Gumuz and Gambella regions represent the major malarial hotspots in Ethiopia [ 20 ]. As shown in Fig. 1 , a higher diversity of plants used to treat malaria (94 plant species) was reported from the SNNP region [ 21 – 40 ] followed by Oromia (60) [ 41 – 64 ], Amhara (47) [ 65 – 84 ], Somali (29) [ 85 , 86 ], and Tigray (24) [ 87 – 95 ] regions. In agreement, others have indicated that medicinal plants were concentrated in southern and southwestern parts of Ethiopia, which possess high biological and cultural diversity [ 96 , 97 ]. The majority of the plants reported in Amhara (60%) and Oromia (53%) regions were shared by other regions. The Amhara and Oromia regions share boundaries with many other regions in Ethiopia and are likely to share common flora and cultural practices, including in ethno-medicine. Moreover, the limited number of plants reported from highland areas, including Addis Ababa [ 98 ] and Harari [ 99 ] regions is attributed to zero prevalence of malaria or minimal transmission. Insufficiencies of plants were also reported from the lowland arid regions, including Afar [ 53 , 100 , 101 ] and Dier Dewa [ 102 ]. Both regions are characterized by moderate malaria transmission. Despite having rich floral diversity and intense malaria transmission risk, reporting of anti-malarial plants was very low in Benishangul Gumuz [ 69 , 103 , 104 ] and nil in Gambella region (Fig. 1 ).This may reflect a lack of pertinent ethno-medicinal cultural practices, however, the prevailing gap is probably attributed to serious lapses in ethno-botanical research and documentation of medicinal knowledge and resource in the two regions.

The geographical distribution of anti-malarial plants based on malaria risk stratification map of Ethiopia (adopted from the Malaria NSP 2014–2020). Malaria risk stratification was revised in 2014 using annual parasite incidence per 1000 population (per WHO recommendation) plus altitude and expert opinions from different malaria stakeholders [ 4 ]. Malaria risk is thought to be one important factor affecting the abundance of anti-malarial plants. Hence, numbers indicated in the map represent the total amount of anti-malarial plants reported from each administrative region (e.g., 24 plants reported from Tigray region)
Diversity of anti-malarial plants
The anti-malarial plant species identified in different region of Ethiopia belonged to 71 different plant families (Additional file 2 ). Cited plant families included: Fabaceae (18), Lamiaceae (17), Euphorbiaceae (11), Asteraceae (10), Cucurbitaceae and Solanaceae (8 each), Rubiaceae and Aloaceae (6 each), Acanthaceae (5), Moraceae, Brassicaceae and Capparidaceae (4 each), Asclepiadaceae, Anacardiaceae, Apocynaceae, Apiaceae, Malvaceae, Meliaceae, Rutaceae, Ranunculaceae, Rosaceae, Menispermaceae, and Verbnaceae (3 each). The more frequently cited species were: Allium sativum (31), Carica papaya (20), Vernonia amygdalina (18), Croton macrostachyus (16), Lepidium sativum (15), Justicia schimperiana (9), Phytolacca dodecandra (8), Dodonaea angustifolia , and Melia azedarach (7 each), Clerodendrum myricoides (6), Aloe sp., Azadirachta indica , Brucea antidysenteric , Calpurnia aurea and Eucalyptus globulus (5 each), Ajuga integrifolia , Carissa spinarum , Artemisia afra , Moringa stenopetala , Ruta chalepensis , Salvadora persica , and Tamarindus indica (4 each). Frequent citation of particular plant species or families could indicate potentially higher bioactive anti-malarial content. Such evidence is pertinent for prioritizing future pharmacological research agendas.
The majority of the anti-malarial plants reported in Ethiopia were shrubs and herbs, 37 and 33.5%, respectively, while tree and climbers was least reported, 23 and 6.5%, respectively. Similar observation was reported in other countries [ 105 , 106 ]. This trend may be attributed to the abundance and easy access of these growth forms in the country. Others have suggested that shrubs may hold higher content of potential anti-malarial phytochemicals, such as alkaloids and flavonoids [ 107 ]. One possible mechanism for the link between shrubs and content of potential anti-malarial phytochemicals could be the diversity and abundance of these plants in different habitats. Secondary metabolites are thought to be required in the adaptation of plants with their environment. In light of this, abundance of shrubs in various habitats could offer a great chance to interact with diverse of biotic and abiotic factors, such as temperature, light intensity, soil nutrients, water supply, herbivore and microbial attack, which might trigger many complex biochemical processes pertaining to synthesize structurally and chemically diverse metabolites with significant anti-malarial activities, including alkaloids and flavonoids.
Recipe reports
Preparation of herbal recipes for malaria treatment.
Practitioners used either a single method (209) or combinations of two (133) and more (24) methods for preparing anti-malarial herbal remedies. Decoction, concoction, eating/chewing, infusion, and pounding represented the most common independent herbal remedy preparation. Of the herbal remedies prepared by two or more methods, 71.3% were started by pounding or crushing (Fig. 2 ). Studies from other parts of Africa have also reported that decoction was the most frequently used method of herbal remedy preparation, commonly using water as a solvent [ 105 , 108 – 111 ]. Water is a cheaply available solvent that can dissolve a high number of metabolites, and high temperature would permit a rapid extraction of active ingredients. Concoction was also noted as a common method of herbal remedy preparation in Africa [ 112 – 114 ]. This method is believed to enhance synergic effect of medicinal plants and increase the efficacy of herbal remedies. Preference for eating/chewing and pounding/crushing might be related to ease of preparation, and easily available local tools, including stones.

Frequency of herbal preparation methods
Some of the anti-malarial herbal preparations were prepared from mixtures of two or more different plant species. Notable examples reported in Ethiopia include:
- Allium sativum individually combined with one of the following plants; Girardinia diversifolia [ 41 ] , Lepidium sativum [ 50 , 88 ], Ruta chalepensis [ 87 ], Datura stramonium [ 50 ], Otostegia integrifolia [ 72 ] , Ocimum basilicum [ 45 ], Ginger officinale [ 45 , 50 ], Cicer arietinum [ 75 ], Carica papaya [ 29 , 50 ], Capsicum annuum [ 42 , 43 ], Artemisia afra [ 42 ], Croton macrostachyus [ 56 ], Brucea antidysenterica [ 65 ] or with groups of plants such as: Artemisia afra, Ruta chalepensis and Lepidium sativum [ 31 ]; Solanum dasyphyllum, Lepidium sativum , Withania Somnifera, Schinus molle , and Sida schimperi [ 65 ];
- Leucas stachydiformis with Ocimum lamiifolium [ 49 ];
- Maerua oblongifolia with Withania Somnifera [ 86 ];
- Asparagus africanus with Aloe sp. [ 86 ];
- Droguetia iners with Premna oligotricha [ 39 ];
- Rumex abysinicus with Zehneria scabra [ 69 ];
- Silene macrosolen with Echinops kebericho [ 65 ];
- Vernonia amygdalina with Ruta chalepensis [ 45 , 50 , 87 ] or Carica papaya [ 32 ];
- Justicia schimperiana with Rumex nervosus and Vernonia amygdalina [ 42 ];
- Senna italica with Indigofera sp. or Zaleya pentandra [ 100 ];
- Lepidium sativum with Echinops kebericho and Croton macrostachyus [ 31 , 49 ];
- Salvadora persica with Lycium shawii and Acalypha sp. [ 100 ];
- Aloe sp. with Asparagus africanus and Senna italica [ 86 ];
- Croton macrostachyus with Gardenia lutea or Azadirachta indica and Carica papaya [ 69 ];
- Capsicum annuum with Otostegia integrifolia, Ocimum gratissimum , Prunus persica and Schinus molle [ 69 ];
- Hagenia abyssinica with Silene macrosolen, Phytolacca dodecandra , Cucumis ficifolius and Clerodendrum myricoides [ 83 ];
- Securidaca longipedunculata with Carissa spinarum , Capparis tomentosa , Withania somnifera and Cucurbita sp. [ 73 ].
Aside from anti-malarial plants, various other additives were also used in some herbal preparations. Commonly reported additives include: animal products (egg, meat and milk), honey, sugar, tea, salt, soup, Eragrostis tef dough, coffee, lemon, injera , local alcoholic drinks ( areke, tella ). Additives were mostly used to moderate the power and/or improve the taste and enhance the efficacy and healing conditions of the remedy [ 35 , 39 , 43 , 48 , 49 , 83 , 88 ]. This could possibly be attributed to synergistic effects of the mixtures that might contain a range of pharmacologically active compounds potentially augmenting the chance of the drug interacting with numerous, varied biological targets. Their interaction might influence selectivity, availability, absorption and displacement (distribution) of the remedy, and bioactivity, including enzyme activities. Thus, such traditional practices could provide the opportunity to understand drug interaction and mechanisms of actions, and pave the way to discovering lead structures for the development of novel anti-malarial drugs.
Plant parts used and condition of preparations
The majority of anti-malarial herbal remedies were prepared from a single plant part while some were prepared from a combination of two or more plant parts. Leaf and root were the most frequently used plant parts (Fig. 3 ). Leaves were indicated to be the plant parts most commonly used by traditional medicine practitioners in many African countries [ 115 – 117 ]. Leaves are responsible for synthesizing the majority of plant secondary metabolites. This makes them an abundant source of active chemical entities, which can be extracted with relative ease. Regular harvest of leaves poses no/low threat to individual plants survival. This encourages the frequent and safe utilization of leaves in herbal preparations. Plant root structures, such as tuber and rhizome, can be rich sources of potent bio-active chemical compounds. However, frequent usage of roots for herbal preparations can be risky to the survival of a plant species. Therefore, application of proper harvesting strategies and conservation measures is necessary to ensure sustainable utilization of medicinal plant resources.

Frequency of the reported plant parts used for herbal preparations
The majority (62%) of anti-malarial herbal remedies were prepared from fresh plant materials followed by dry (20.9%) and both fresh and dry materials (5%). On the other hand, plant conditions used for this matter are not indicated in 12.1% of the study reports. The predominant use of fresh materials for herbal preparation probably reflects an attempt to capture potent, volatile substances that determine therapeutic efficacy of herbal preparations [ 118 ]. Dry materials could be preferred when the plant is poorly accessible. As reported in some of the reviewed studies, some practitioners travel a long distance to collect medicinal plants and practice long-term preservation.
Routes of administration and dosage of herbal remedies
Anti-malarial herbal remedies were primarily administered through oral route (82.7%), while rarely administered through nasal (5.5%) and whole body (2.8%). Yet, few (9%) reports failed to indicate administration routes of herbal remedies. Liquid herbal preparations made from both fresh and dry materials were taken orally. Fresh solid materials were also eaten and chewed directly upon collection or after initial pounding/crushing. Meanwhile, dry solid materials were smoked and administered through intranasal. These findings were compatible to the observations reported from other countries [ 112 – 114 ]. Malaria is a disease caused by protozoan intracellular haemo-parasites and its treatment entails delivering adequate circulating concentration of appropriate anti-protozoal chemicals. The oral route is a convenient and non-invasive method of systemic treatment. The route permits relatively rapid absorption and distribution of active chemical compounds from herbal remedies, enabling the delivery of adequate curative power [ 88 ].Potential risk of enzymatic breakdown and microbial fermentation of active chemical entities may necessitate alternative routes of herbal remedy administration.
Herbal remedy dosage was basically determined by edibility of the plant parts used. In case of remedies prepared from non-edible plants/parts, dose was prescribed based on age, physical strength and health status of patients. However, full dosage determination varied from healer to healer. Variations were noted in the measurement units used for dose estimation, and in the frequency and duration of herbal treatment prescribed. Dose of herbal preparations was usually estimated using different locally available materials/means, including plastic/glass/steel cups (could be coffee-cup, teacup, water-cup) or gourd utensils, number of drops for liquid materials; teaspoons for powders; counting the number of units for seeds, leaves and fruits; index finger estimation of root size. Generally, recommendation was made to administer the herbal remedies twice or three times per day for one, two or three consecutive days to many months or until recovery. Lack of precision and standardization is widely acknowledged to be an important drawback of traditional healthcare systems [ 119 – 122 ].
Adverse effects, antidotes and contra-indications
In settings where traditional medicine is keen, the pharmacological effect of medicinal plants is generally ascribed to their active and ‘safe’ content that will only exert quick effect when taken in large quantities. However, the majority of the reviewed reports made no mention of possible side effects to different herbal preparations. Nevertheless, herbal preparations made from some anti-malarial plants were reported to have side effects, such as vomiting, nausea, diarrhoea, headache, urination, heartburn, and nightmare [ 22 , 31 , 43 , 54 , 67 , 68 , 71 , 75 , 86 , 87 , 100 ]. This may be attributed to different underlying factors, including improper dosing, toxic plant chemicals, toxic metabolic byproducts, etc. Teff injera and porridge, Shiro wot (pulse grain sauce), coffee, milk and milk products, honey, Shoforo (infusion made from coffee peel), Tela, barley soup and juice of Sansevieria ehrenbergii were reported as antidotes for potential herbal remedy side effects [ 22 , 31 , 43 , 54 , 67 , 68 , 71 , 75 , 87 ]. Some anti-malarial plants were reported as contra-indicated to the elderly, pregnant women, children, physically weak persons, and patients with hepatitis [ 31 , 42 , 43 , 54 , 66 – 68 , 71 , 75 , 86 – 88 , 100 ] (Table 2 ). Current observations indicate existence of critical research-evidence gaps with regard to the potential toxicities and corresponding counteracting mechanisms of anti-malarial plants in Ethiopia. This gap represents an important roadblock to effective development and exploitation of indigenous medicinal plant resources.
Table 2
Side effects, antidotes and contra-indications of some plants used for traditional malaria treatment in Ethiopia
| Species | Side effects | Antidotes | Contra-indication |
|---|---|---|---|
| Diarrhoea, vomiting, headache, urination | and porridge | Pregnant women | |
| Vomiting and diarrhoea | |||
| sp. | Nausea, vomiting, diarrhoea | – | Pregnant women |
| Headache, diarrhoea, vomiting | Coffee and milk, red porridge, a lot of | Pregnant women | |
| Vomiting, nausea, diarrhoea | – | – | |
| Vomiting, nausea, diarrhoea | – | – | |
| Vomiting and diarrhoea | Honey | – | |
| Headache | – | ||
| Vomiting | – | ||
| Nausea and vomiting | – | ||
| Headache, heartburn, nausea/vomiting and nightmare | Juice of | – | |
| Vomiting and diarrhoea | Milk, red porridge, coffee, | Children, pregnant women, patient with hepatitis | |
| Vomiting and diarrhoea | Milk, red porridge | Children, pregnant women | |
| – | – | Pregnant women | |
| – | – | Patient with hepatitis, babies/old people, pregnant women | |
| Vomiting and diarrhoea | Boiled coffee, milk or barley soup | Pregnant women, physically weak person |
Trends in anti-malarial plant research and development
In different African countries, many of the anti-malarial plants identified in this paper have demonstrated promising therapeutic potential on pre-clinical and clinical investigations. Notable examples were Artemisia annua [ 123 , 124 ], Ajuga remota [ 125 ], Azadirachta indica [ 126 – 128 ], Argemone mexicana [ 129 , 130 ], Vernonia amygdalina [ 131 – 135 ], Asparagus africanus [ 136 ], Uvaria leptocladon [ 137 ], and Gossypium spp. [ 138 ]. In parallel, multiple promising candidate anti-malarial compounds have been identified from these plant resources [ 139 – 145 ]. Consequently, international market demand (Switzerland, France, China, etc.) for African medicinal plants has exhibited sustained growth. Export of promising indigenous medicinal plant resources offers substantial contribution to the economy and growth of African countries. For instance, export of traditional medicines contributed an estimated R2.9 billion to South Africa’s economy [ 146 ]. Likewise, Egypt’s 2008 exports of selected medicinal plants amounted to 77,850,312 kg with a reported value of US $174,227,384 [ 147 ].
Despite the remarkable historic success of traditional medicinal practices and abundance of indigenous medicinal plant resources (Additional file 1 ), anti-malarial ethno-pharmacological research in Ethiopia remains at primitive stage, with scope limited to evaluating crude extracts from various anti-malarial plants against Plasmodium berghei . A prominent gap is evident with regard to research geared towards identifying plant bioactive entities, and establishing the efficacy and safety of medical plants through in vitro assays using human Plasmodium parasites, in vivo assay involving higher animal models and randomized clinical trials. Absence of favourable medicinal plant research and development impedes optimum exploitation of potential economic benefits. Thus, despite holding one of the richest (diversity and quantity) resources in the continent, large-scale production and export of medicinal plants has remained limited in Ethiopia. Prevailing scenarios underscore a pressing need for enhancing pre-clinical and clinical research aimed at developing safe, effective and affordable alternative anti-malarial agents from indigenous plant resources. This requires collaborative engagement involving government bodies, researchers, traditional healers, and prospective business investors.
The study highlighted that a rich diversity of indigenous medicinal plants were commonly used for traditional treatment of malaria in Ethiopia. Ethno-medicinal research on distribution and usage pattern of anti-malarial plants shows substantial variability across a spectrum of geographic and social strata in the country. Baseline information gaps are evident in key geographic settings, such as the Beshangul Gumuz and Gambella regions. Divergent preparation and use patterns of anti-malarial herbal remedies, as well as associated toxicity risks and countermeasures, generally demand deeper, exhaustive investigations. Experimental research and advanced chemical analysis are required to identify and validate the therapeutic potential of anti-malarial chemical compounds from promising plant species, with due consideration to efficacy and safety issues. Sustainable development and exploitation of indigenous medicinal plant resources entails coordinated multidisciplinary research programmes that give due credit to traditional practitioners and engage with commercial investors.
Additional files
Authors’ contributions.
GA, BU and AW separately attained materials from different sources and jointly prepared the initial draft paper. All authors systematically reviewed the final version of the manuscript. All authors read and approved the final manuscript.
Acknowledgements
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Availability of data and materials
Consent for publication, ethics approval and consent to participate, publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Getachew Alebie, Email: moc.liamg@eibelahceg .
Befikadu Urga, Email: moc.liamg@agruekif .
Amha Worku, Email: moc.liamg@2lilonaramaz .
- Open access
- Published: 16 November 2022
Malaria among under-five children in Ethiopia: a systematic review and meta-analysis
- Gebeyaw Biset 1 , 2 ,
- Abay Woday Tadess 2 , 3 ,
- Kirubel Dagnaw Tegegne 4 ,
- Lehulu Tilahun 5 &
- Natnael Atnafu 6
Malaria Journal volume 21 , Article number: 338 ( 2022 ) Cite this article
5177 Accesses
7 Citations
2 Altmetric
Metrics details
Globally, malaria is among the leading cause of under-five mortality and morbidity. Despite various malaria elimination strategies being implemented in the last decades, malaria remains a major public health concern, particularly in tropical and sub-tropical regions. Furthermore, there have been limited and inconclusive studies in Ethiopia to generate information for action towards malaria in under-five children. Additionally, there is a considerable disparity between the results of the existing studies. Therefore, the pooled estimate from this study will provide a more conclusive result to take evidence-based interventional measures against under-five malaria.
The protocol of this review is registered at PROSPERO with registration number CRD42020157886. All appropriate databases and grey literature were searched to find relevant articles. Studies reporting the prevalence or risk factors of malaria among under-five children were included. The quality of each study was assessed using the Newcastle–Ottawa Quality Assessment Scale (NOS). Data was extracted using Microsoft Excel 2016 and analysis was done using STATA 16.0 statistical software. The pooled prevalence and its associated factors of malaria were determined using a random effect model. Heterogeneity between studies was assessed using the Cochrane Q-test statistics and I 2 test. Furthermore, publication bias was checked by the visual inspection of the funnel plot and using Egger’s and Begg’s statistical tests.
Twelve studies with 34,842 under-five children were included. The pooled prevalence of under-five malaria was 22.03% (95% CI 12.25%, 31.80%). Lack of insecticide-treated mosquito net utilization (AOR: 5.67, 95% CI 3.6, 7.74), poor knowledge of child caretakers towards malaria transmission (AOR: 2.79, 95% CI 1.70, 3.89), and living near mosquito breeding sites (AOR: 5.05, 95% CI 2.92, 7.19) were risk factors of under-five malaria.
More than one in five children aged under five years were infected with malaria. This suggests the rate of under-five malaria is far off the 2030 national malaria elimination programme of Ethiopia. The Government should strengthen malaria control strategies such as disseminating insecticide-treated mosquito nets (ITNs), advocating the utilization of ITNs, and raising community awareness regarding malaria transmission.
Malaria is a major public health concern in tropical and sub-tropical regions of the world, affecting hundreds of millions of people. Nearly 3.2 billion people are at risk of malaria worldwide with a substantial risk among pregnant women and children under five years old. In the year 2020, nearly 241 million people were infected by Plasmodium species and half a million died due to malaria. Evidence suggested that more than 95% of malaria infections and deaths are concentrated in African countries. Similarly, more than 90% of malaria-related infections and deaths occur in sub-Saharan regions [ 1 , 2 ].
Malaria is a major public health problem in Ethiopia where approximately 68% of the land mass has favourable conditions for malaria transmission and 60% of the population is at risk of the disease [ 3 , 4 ]. Despite various preventive measures undertaken in the last decades, malaria remains among the top ten causes of under-five morbidity and mortality in Ethiopia [ 5 , 6 , 7 , 8 ]. Malaria transmission is highly seasonal and varies significantly with respect to geographical altitude, rainfall and population movement. In addition, due to unstable malaria transmission patterns, Ethiopia is prone to focused and large-scale cyclic malaria epidemics [ 9 ].
Studies show that children aged under five years are among the most vulnerable to malaria infections. More than 61% of all malaria deaths worldwide and 80% of sub-Saharan malaria deaths occurred among children under 5 years [ 10 , 11 ]. Similarly, the highest malaria-related morbidity and mortality in Ethiopia is reported among children under 5 years [ 12 , 13 , 14 , 15 ]. The 2019 Ethiopian demographic health survey (EDHS) showed that 59 under-fives died in 1,000 live births due to malaria [ 16 ].
Despite inconsistency among existing studies, several factors were associated with high prevalence of malaria among children under 5 years. Insecticide-treated mosquito net (ITN) utilization, presence of forest cover, altitude of residence, household density, living near mosquito breeding sites such as stagnant water, seasonal variation, and housing conditions were major predictors of malaria infection among children aged under 5 years. In addition, the low protective immunity of under-fives make them highly susceptible to malaria infection [ 17 , 18 , 19 ].
Several malaria elimination strategies have been implemented at national and international levels to control the burden of malaria. Consequently, over 6 million malaria deaths were averted between 2000 and 2015 in African countries. Despite this significant decline, malaria remains a major public health concern in tropical and sub-tropical regions of the world [ 20 , 21 , 22 ]. Furthermore, there are limited and inconclusive studies in Ethiopia to generate information for action against malaria. There is a considerable discrepancy among the results of existing studies. Therefore, the pooled estimate from the current study will provide a more conclusive result to take evidence-based interventional measures against malaria in under-fives in Ethiopia [ 23 ].
Study design
This study was designed based on the updated guideline of the Preferred Reporting Items for Systematic Review and Meta-analyses (PRISMA 2020) statements to report the findings [ 24 ]. The protocol has been registered at PROSPERO with registration number CRD42020157886. Moreover, the authors have used the guideline of PROSPERO for registering the protocol.
Eligibility criteria
The inclusion criteria for this review and meta-analysis were: (1) studies among under-five children in Ethiopia; (2) observational studies (cross-sectional, case-control, cohort studies, longitudinal studies); (3) studies that report the prevalence or factors of under-five malaria; and, (4) English language articles published in peer-reviewed journals not limited by year of study. However, studies that did not define the age of a child, studies that were not fully accessed or failed to contact the primary author (s), case reports, expert opinions, and qualitative studies were excluded.
Searching strategy
This review and meta-analysis was prepared and presented in accordance to the updated guideline of the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA 2020) [ 24 ]. The international databases PubMed/MEDLINE, HINARI, African Journal of Online (AJOL), and Google Scholar were accessed to find relevant articles. A Medical Subject Headings (MeSH), keyword terms and phrases were used both in separation and in combination using the Boolean operators “OR” and “AND” to search for eligible articles. The authors have also used snowballing of the references of identified articles for accessing potentially relevant studies (Additional file 1 ).
The keywords and phrases “under-five children”, “0–59 months old children”, pediatrics, ‘‘preschool children’’, malaria, “ Plasmodium falciparum ”, “ Plasmodium vivax ”, “mixed malaria”, “ Plasmodium species”, prevalence, incidence, magnitude, burden, proportion, determinants, “risk factors”, predictors, causes, ‘‘associated factors”, and Ethiopia were used in separation or in combination to retrieve relevant articles on malaria in under-fives in Ethiopia.
Study selection
Exhaustively, 1067 studies were retrieved using online databases, reference lists of retrieved articles, and manual searches. All the retrieved articles were exported to Endnote X8 reference managers and 495 articles were removed due to duplication. The title and abstract of the remaining 572 articles were reviewed and 524 articles were removed by title and abstract. Some 48 full articles were assessed for eligibility, which resulted in further exclusion of 36 articles due to lack of outcome reports. Finally, 16 studies were included. Of these 16, 12 studies were used to determine the pooled prevalence of malaria, 11 studies to determine the association between mosquito net utilization and under-five malaria, 5 studies to determine the association between living near mosquito breeding sites and under-five malaria, and 5 studies to determine the association between knowledge of malaria transmission and the incidence of under-five malaria (Fig. 1 ).
Flow chart illustrating the process of literature search and selection of studies included in the present systematic review and meta-analysis
Outcome measurement
The primary outcome of this systematic review and meta-analysis is the pooled prevalence of malaria among under-five children. The pooled prevalence of malaria was calculated by dividing the number of under-fives with malaria by the total number of children included in the study multiplied by 100. The second outcome was determinants of malaria among under-five children, which was estimated by the pooled odds ratio with a 95% confidence interval using a random effect meta-analysis.
Data extraction and management
Data were extracted using Microsoft Excel spreadsheet imported into STATA version 16.0 statistical software for further analysis. Three authors (AW, KD, GB) extracted the data independently. Discrepancies between authors during data extraction were managed through consensus and a fourth author was consulted to resolve disagreements. For each included article, the first author’s last name, year of publication, study setting, study design, study period, sample size, response rate, study population, outcome definition, comparison groups, and effect estimate was recorded.
Quality assessment
The quality of each study was assessed using NOS adapted for meta-analysis. Studies were assessed for representativeness of sample size, non-respondents, ascertainment of exposure, comparability, assessment of the outcome, and statistical tests. Stars were assigned to evaluate study quality, with 9–10 stars indicating “very good” quality, 7–8 stars “good” quality, 5–6 stars “satisfactory” quality, and 0–4 stars “unsatisfactory” quality. Four authors (GB, AW, KD, NA) performed the quality appraisal independently and the average assessment scale was used for the final decision [ 25 ].
Publication bias and heterogeneity
Heterogeneity was evaluated using Cochran’s Q statistic with a 5% level of significance and the I 2 statistical test. The I 2 test statistics of 25, 50 and 75% were declared as low, moderate, and high heterogeneity respectively [ 26 ]. The possible risk of publication bias was examined by the inspection of the funnel plot and statistically using Begg’s correlation and Egger’s regression test. Sub-group analysis was conducted by region, sample size and study design to minimize random variation among studies. Besides, sensitivity analysis was performed to examine the influence of a single study on the overall estimate.
Data analysis
The prevalence of malaria was calculated by dividing the number of under-five children with malaria by the total number of children included in the study multiplied by 100. The standard error of the prevalence was calculated using the formula: \(se\;\left(p\right)=\frac{\sqrt{\text{p}(1-\text{p})}}{n}\) . Adjusted odds ratio (AOR) with its upper and lower bounds were extracted for significant variables. The standard error of the OR was calculated using the formula: \(\text{SE}\; \log \;(\text{OR})=\left[\frac{UB\;cl-LB\;cl}{2Za/2}\right]\) . Then the extracted data were exported into STATA 16.0 statistical software for further analysis. Taking the variability between individual studies and the observed heterogeneity among the included studies, a random effect model analysis was employed. Additionally, sub-group analysis, publications bias and sensitivity analysis were performed.
Study characteristics
Of the 16 included studies, five were in Amhara region, three in Oromia region, four in South Nation Nationality and Peoples of Ethiopia (SNNP) region, two in Binishangulgumz, one in Afar region, and one was a national study. The highest malaria prevalence was reported in Afar region (64.04%) [ 27 ], whereas the lowest prevalence was reported in Binishangulgumz (3.93%) [ 28 ]. Regarding the study design, 12 studies were cross-sectional, three retrospectives, and one Bayesian study (Table 1 ).
Prevalence of malaria in under-five children
In this meta-analysis, 12 primary studies with 34,842 study participants were included to estimate the pooled prevalence of malaria among under-five children using a random effect model [ 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 , 35 , 36 , 37 , 38 ]. The pooled prevalence of malaria among under-five children was 22.03% (95% CI 12.25%, 31.8%, I 2 : 99.81, P < 0.001) (Fig. 2 ).
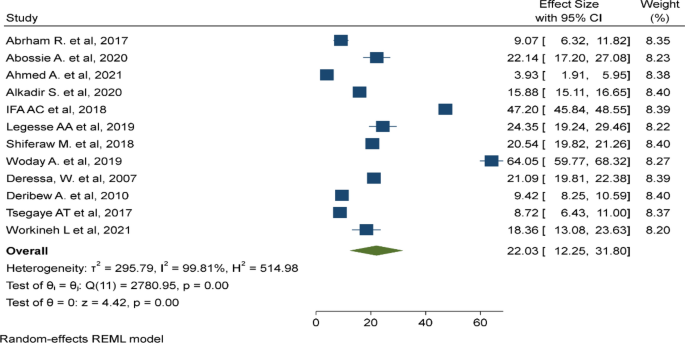
Pooled prevalence malaria among under-five children in Ethiopia, August 2022
Sub-group analysis
There was a significant heterogeneity among the included studies (I 2 : 99.81, p < 0.001). As a result, sub-group analyses was done by region (Fig. 3 ), sample size (Fig. 4 ), and study design (Fig. 5 ). The highest pooled prevalence of under-five malaria was reported in SNNP (25.81%, 95% CI 10.22–41.39%) next to a single study in Afar region (64.5%, 95% CI 59.77–68.32%). The pooled prevalence of malaria was higher among studies with a sample size < 500 (23.61%, 95% CI 6.56–40.67%). Similarly, the pooled prevalence of malaria was higher in studies with a retrospective design (27.87%, 95% CI 8.75–46.98%).
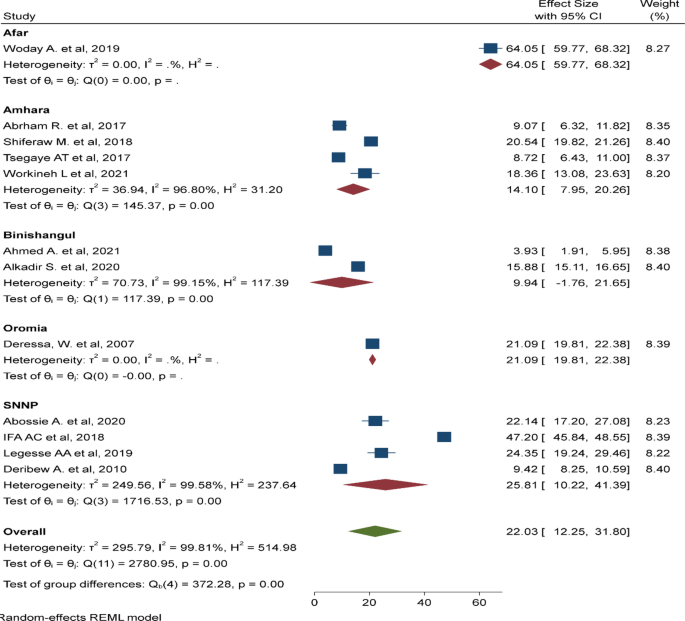
Subgroup analysis based on the region where the studies are conducted, Ethiopia, August 2022
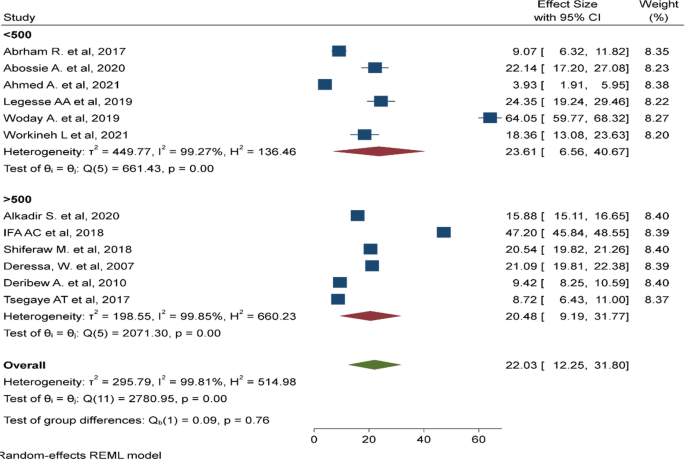
Subgroup analysis based on sample size, Ethiopia, August 2022
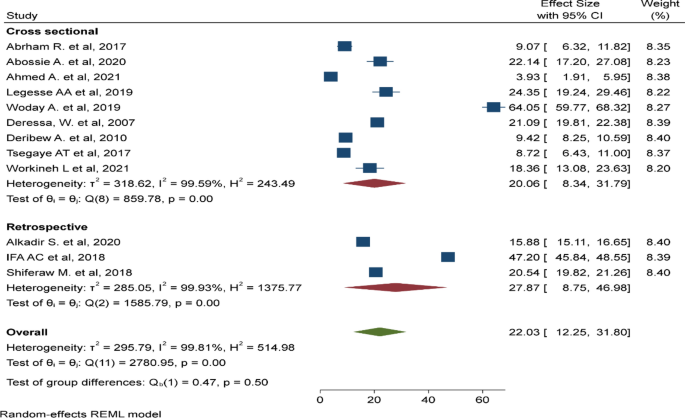
Subgroup analysis based on study design, Ethiopia, August 2022
Publication bias and sensitivity analysis
The funnel plot showed a significant publication bias with substantial asymmetry (Fig. 6 ). The funnel plot is a subjective technique; as a result, objective statistical tests Egger’s test and Begg’s test [ 39 ] were done to confirm the presence of publication bias. Consequently, the Egger’s (p = 0.43) and Begg’s statistical tests (p = 0.63) showed no statistical evidence of publication bias. Sensitivity analysis was performed with the random effect model to see the effect of a single study on the overall estimate. However, the analysis showed no evidence for the influence of a single study on the overall estimate (Fig. 7 ).
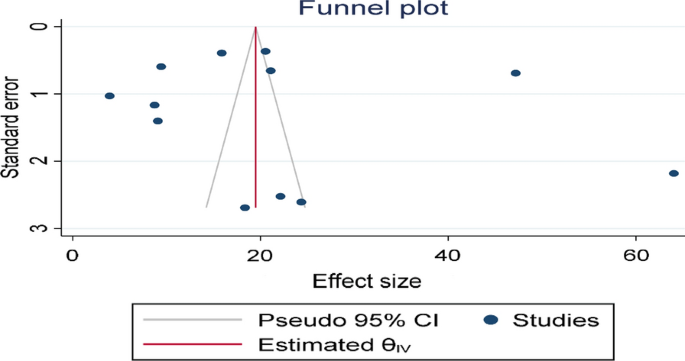
Funnel plot to determine the presence of publication bias among the included studies
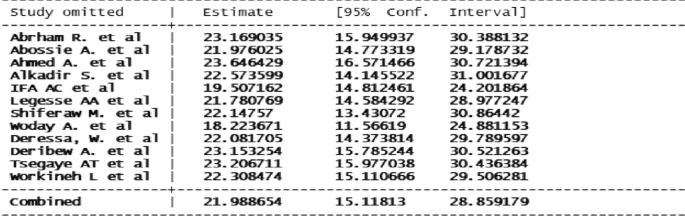
Sensitivity analysis for the study of malaria among under five children in Ethiopia, August 2022
Risk factors of malaria in under-five children
Several primary studies were included to determine the pooled factors associated with malaria among children age under 5 years. Eleven primary studies were included to assess the association between ITN utilization, five studies for assessing the association between living near mosquito breeding sites, and five studies to determine the relationship between knowledge of malaria transmission and the prevalence of malaria among under-five children.
Insecticide-treated mosquito net utilization
In this study, 11 primary studies were included to assess the association of ITN utilization and the prevalence of malaria among children aged under 5 years [ 27 , 28 , 30 , 35 , 37 , 38 , 40 , 41 , 42 , 43 , 44 ]. Children age under 5 years who did not utilize an ITN were nearly 6 times (AOR: 5.67, 95% CI 3.6–7.74) more likely to have malaria than children who did utilize an ITN (Fig. 8 ).
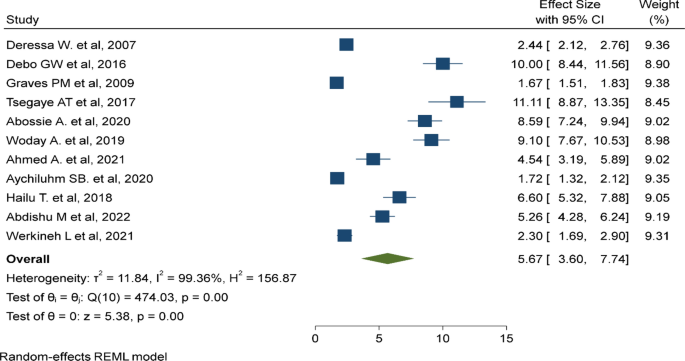
Lack of insecticide treated bed net is a risk factor of malaria among under five children
Living near mosquito breeding sites
In this meta-analysis, five primary studies were included to assess the association between living near mosquito breeding sites and the rate of malaria in under-five children [ 28 , 30 , 37 , 38 , 44 ]. which was five times (AOR: 5.05, 95% CI 2.92–7.19) more likely to be infected by malaria parasites compared to children living near a non-breeding site (Fig. 9 ).
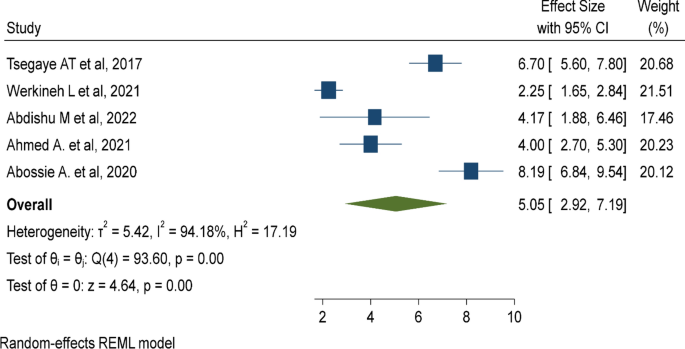
Mosquito breeding site is a risk factor of malaria among under five children
Knowledge of malaria transmission
Five primary studies were included to determine the effect of knowledge of child caretakers about malaria transmission on the incidence of under-five malaria [ 27 , 28 , 29 , 42 , 44 ], which showed that under-five children with poor knowledge of caretakers toward malaria transmission were nearly three times (AOR: 2.79, 95% CI 1.7–3.89) at high risk of malaria compared to their counterparts (Fig. 10 ).
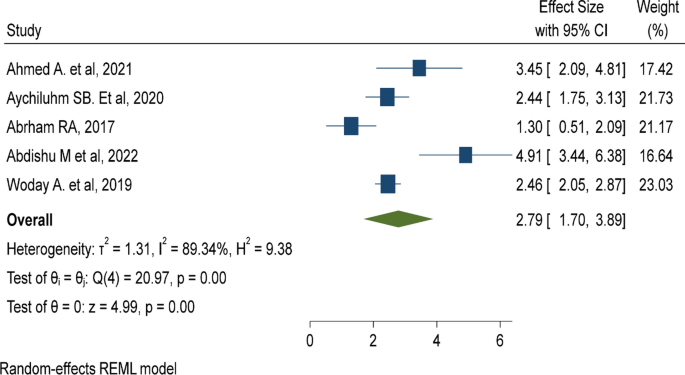
Poor knowledge of malaria transmission method is a risk factor of malaria among under five children
This study explores and synthesizes the available evidence on malaria in children under 5 years old and its associated factors in Ethiopia. By gathering, synthesizing, and pooling available studies, the finding provides strong evidence on under-five malaria in Ethiopia. The finding revealed that more than one in five children are infected with malaria Plasmodium species. The finding warned the 2030 national malaria elimination programme in Ethiopia [ 45 ] needs to emphasize malaria control strategies in the country.
In this study, 22.03% (95% CI 12.25%, 31.80%) under-five children were infected with malaria. The finding is comparable to the pooled estimate in sub-Saharan countries (18.8%) [ 46 ], (21%) [ 47 ], and (26%) [ 48 ], the national malaria survey in Gambella (21%) [ 49 ], a study in Uganda (19.04%) [ 50 ], the national malaria survey in Ghana (21%) [ 51 ], and the national malaria survey in Nigeria (27%) [ 52 ]. These findings pointed out that malaria is still a concern for children under 5 years old. The reason could be their low protective immunity makes them highly susceptible to malaria infection, and could be associated with poor malaria control strategies or lack of monitoring and evaluation of malaria control programmes.
The finding shows a nearly 16-fold higher estimate than the national malaria indicator survey in Ethiopia (1.4%) [ 49 ]. The Government of Ethiopia need to strengthen malaria control strategies. The finding is threefold higher than the demographic and health survey in Tanzania (7%) [ 53 ] and the malaria indicator survey in Kenya (6%) [ 54 ]. The reason could be demographic health surveys and malaria indicator surveys are conducted at national level, including low malaria-endemic areas, which thereby lower the report of under-five malaria. However, studies included in this meta-analysis were conducted in high malaria areas of Ethiopia which might increase the prevalence of under-five malaria.
Conversely, the finding was lower than the report in sub-Saharan countries (36%) [ 55 ], a study in Malawi (37%) [ 56 ], the malaria indicator survey in Liberia (45%) [ 57 ], the malaria indicator survey in south Sudan (32%) [ 58 ], and a meta-analysis in the Democratic Republic of the Congo (37.4%) [ 59 ]. The possible explanation for the discrepancy could be countries might have different malaria monitoring programmes and different levels of achievements in malaria control strategies. The reason could also be due to the fact that Ethiopia had low malaria prevalence compared to most other malaria-endemic countries in Africa [ 45 ].
Lack of access to or non-utilization of ITNs is a risk factor for higher prevalence of malaria among under-five children, which is similar to the study in Ethiopia [ 42 ], a study in Malawi [ 60 ], a study in sub-Saharan countries [ 47 ], and a study in Uganda [ 61 ]. The reason is the fact that children who utilize ITNs are not exposed to Plasmodium species and are less likely to get infected by the parasite. The Government should disseminate ITNs and advocate utilization to prevent high malaria transmission among under-five children.
Poor knowledge of child caretakers about malaria transmission increases the likelihood of malaria infection. The finding is similar to studies in sub-Saharan countries [ 62 ], in Rwanda [ 63 ], Ghana [ 64 ], Uganda [ 50 ], Malawi [ 56 ], and Nigeria [ 65 ]. The reason could be child caretakers who are not aware of malaria transmission are less likely to protect their child from Plasmodium species. Community awareness regarding malaria transmission and its prevention should be strengthened.
Residing near mosquito breeding sites such as stagnant water increases malaria transmission. The finding is similar to the pooled estimate in sub-Saharan countries [ 46 ], studies in Ethiopia [ 66 ], Malawi [ 67 ], South Africa [ 68 ], and Nigeria [ 65 ]. The reason is mosquitoes can multiply and survive in stagnant and unprotected water sources. As a result, under-five children living near these areas are at higher risk of malaria infection. Cleaning and removing mosquito breeding sites is advisable to reduce malaria in under-fives in Ethiopia.
Strength and limitations of the study
A limitation of the study is pooling prevalence and odds ratio despite high heterogeneity. There are outliers in the included studies such as the study from Afar region this might result in an exaggerated pooled estimate. Another limitation of the study is its narrow scope, which included studies involving only children aged under 5 years. In addition, only a few factors were considered by excluding factors that were reported in a few studies this might cause bias on the factors of under-five malaria. Similarly, excluding articles that were published other than in English language might cause publication bias.
The finding revealed that more than one in five children aged under 5 years were infected with malaria. The risk factors identified are mostly preventable, including ITN under-utilization, living near mosquito breeding sites, and poor knowledge of malaria transmission and its prevention methods by caretakers. The Government should strengthen malaria control strategies such as eradicating mosquito breeding sites, ensure access to ITNs for all under-five children living in malaria-endemic areas, and raise awareness regarding malaria transmission and its preventative methods.
Availability of data and materials
All materials and data related to this article are included in the main document of the manuscript.
Abbreviations
Ethiopian Demographic and Health Survey
Insecticide-treated nets
Low and Middle-Income Countries
Medical Subject Headings
Newcastle–Ottawa Quality Assessment Scale
Population, Exposure, Comparison, and Outcome
Preferred Reporting Items for Systematic Review and Meta-Analysis
South Nation Nationality and Peoples
World Health Organization
WHO. World malaria report. Geneva: World Health Organization; 2017.
Google Scholar
WHO. World-malaria-report-2016. Geneva: World Health Organization; 2016.
Taffese HS, Hemming-Schroeder E, Koepfli C, Tesfaye G, Lee M-C, Kazura J, et al. Malaria epidemiology and interventions in Ethiopia from 2001 to 2016. Infect Dis Poverty. 2018;7:103.
Article PubMed PubMed Central Google Scholar
Ethiopian Public Health Institute. Ethiopian malaria indicator survey 2015 (EMIS-2015). Ethiopia, Addis Ababa; 2016.
United Nations. The millenium development goals 2015 report. New York; 2015.
Hajia Y, Fogartyb. AW, Deressa W. Prevalence and associated factors of malaria among febrile children in Ethiopia: a cross-sectional health facility-based study. Acta Trop. 2016;155:63–70.
Article Google Scholar
Tadesse F, Fogarty AW, Deressa W. Prevalence and associated risk factors of malaria among adults in East Shewa Zone of Oromia Regional State, Ethiopia: a cross-sectional study. BMC Public Health. 2018;18:25.
Federal Ministry of Finance and Economic Development. Assessing progress towards the millenium development goals: Ethiopia MDGs report 2012. Addis Ababa, Ethiopia; 2012.
Federal Ministry of Health. Health and health related indicators. Addis Ababa, Ethiopia; 2013.
Kassam R, Sekiwunga R, MacLeod D, Tembe J, Liow E. Patterns of treatment-seeking behaviors among caregivers of febrile young children: a Ugandan multiple case study. BMC Public Health. 2016;16:160.
WHO. World malaria report 2019. Geneva: World Health Organization; 2019.
Ashton RA, Kefyalew T, Tesfaye G, Pullan RL, Yadeta D, Reithinger R, et al. School-based surveys of malaria in Oromia Regional State, Ethiopia: a rapid survey method for malaria in low transmission settings. Malar J. 2011;10:25.
Ayalew S. The prevalence of malaria and the associated risk factors in Jiga area, Northwest Ethiopia. Thesis, Addis Ababa University; 2014.
Debo GW, Kassa DH. Prevalence of malaria and associated factors in Benna Tsemay district of pastoralist community, southern Ethiopia. Trop Dis Travel Med Vaccines. 2016;2:16.
Molla E, Ayele B. Prevalence of malaria and associated factors in Dilla Town and the surrounding rural areas, Gedeo Zone, southern Ethiopia. J Bacteriol Parasitol. 2015;6:5.
Ethiopian Public Health Institute (EPHI) and ICF. Ethiopia mini demographic and health survey 2019: key indicators. Rockville, Maryland, USA, Addis Ababa, Ethiopia; 2019.
Roberts D, Matthews G. Risk factors of malaria in children under the age of five years old in Uganda. Malar J. 2016;15:246.
Tassew A, Hopkins R, Deressa W. Factors influencing the ownership and utilization of long-lasting insecticidal nets for malaria prevention in Ethiopia. Malar J. 2017;16:262.
Central Statistical Agency. Ethiopia demographic and health survey 2016. Addis Ababa, Ethiopia; 2017.
Federal Ministry of Health. Health sector development program IV (2010/11–2014/15) final draft. Addis Ababa, Ethiopia; 2010.
Federal Ministry of Health. Health sector transformation plan (HSTP 2016–2020). Addis Ababa, Ethiopia; 2015.
United Nations. Transforming our world: the 2030 agenda for sustainable development. New York; 2015. https://stg-wedocs.unep.org/bitstream/handle/20.500.11822/11125/unepswiosm1inf7sdg.pdf?sequence=1 .
Jima D, Wondabeku M, Alemu A, Teferra A, Awel N, Deressa W, et al. Analysis of malaria surveillance data in Ethiopia: what can be learned from the Integrated disease surveillance and response system? Malar J. 2012;11:330.
MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 2021;372:n71. https://doi.org/10.1136/bmj.n71 .
Stang A. Critical evaluation of the Newcastle–Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25:603–5.
Article PubMed Google Scholar
Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539–58.
Woday A, Mohammed A, Gebre A, Urmale K. Prevalence and associated factors of malaria among febrile children in Afar region, Ethiopia: a health facility based study. Ethiop J Health Sci. 2019;29:5.
Ahmed A, Mulatu K, Elfu B. Prevalence of malaria and associated factors among under-five children in Sherkole refugee camp, Benishangul-Gumuz region, Ethiopia. A cross-sectional study. PLoS ONE. 2021;16:e0246895.
Article CAS PubMed PubMed Central Google Scholar
Abrham R. Preventing malaria among under five children in Damot Gale Woreda, Wolayta Zone, Ethiopia: the role of parents knowledge and treatment seeking. Prim Health Care. 2017;7:284.
Abossie A, Yohanes T, Nedu A, Tafesse W, Damitie M. Prevalence of malaria and associated risk factors among febrile children under five years: a cross-sectional study in Arba Minch Zuria district, south Ethiopia. Infect Drug Resist. 2020;13:363.
Alkadir S, Gelana T, Gebresilassie A. A five year trend analysis of malaria prevalence in Guba district, Benishangul-Gumuz regional state, western Ethiopia: a retrospective study. Trop Dis Travel Med Vaccines. 2020;6:18.
Chemeda Ifa A. Trend in malaria prevalence among children under five years of age in the Hadiya Zone, southern Ethiopia: a five-year retrospective study. Fam Med Prim Care Rev. 2018;20:337–40.
Legesse AA. Prevalence of malaria and associated risk factors among febrile children under 5 years in Gamo-Gofa, Ethiopia: an institutional based cross sectional study. 30th EPHA annual conference; 2019.
Shiferaw M, Alemu M, Tedla K, Tadesse D, Bayissa S, Bugssa G. The prevalence of malaria in Tselemti Wereda, North Ethiopia: a retrospective study. Ethiop J Health Sci. 2018;28:5.
Deressa W, Ali A, Berhane Y. Household and socioeconomic factors associated with childhood febrile illnesses and treatment seeking behaviour in an area of epidemic malaria in rural Ethiopia. Trans R Soc Trop Med Hyg. 2007;101:939–47.
Deribew A, Alemseged F, Tessema F, Sena L, Birhanu Z, Zeynudin A, et al. Malaria and under-nutrition: a community based study among under-five children at risk of malaria, south-west Ethiopia. PLoS ONE. 2010;5:e10775.
Tsegaye AT, Ayele A, Birhanu S. Prevalence and associated factors of malaria in children under the age of five years in Wogera district, northwest Ethiopia: a cross-sectional study. PLoS ONE. 2021;16:e0257944.
Workineh L, Lakew M, Dires S, Kiros T, Damtie S, Hailemichael W, et al. Prevalence of malaria and associated factors among children attending health institutions at South Gondar Zone, Northwest Ethiopia: a cross-sectional study. Glob Pediatr Health. 2021;8:2333794X211059107.
PubMed PubMed Central Google Scholar
Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–34.
Debo GW, Kassa DH. Prevalence of malaria and associated factors in Benna Tsemay district of pastoralist community, Southern Ethiopia. Trop Dis Travel Med Vaccines. 2016;2:9.
Graves PM, Richards FO, Ngondi J, Emerson PM, Shargie EB, Endeshaw T, et al. Individual, household and environmental risk factors for malaria infection in Amhara, Oromia and SNNP regions of Ethiopia. Trans R Soc Trop Med Hyg. 2009;103:1211–20.
Aychiluhm SB, Gelaye KA, Angaw DA, Dagne GA, Tadesse AW, Abera A, et al. Determinants of malaria among under-five children in Ethiopia: Bayesian multilevel analysis. BMC Public Health. 2020;20:1468.
Hailu T, Alemu M, Mulu W, Abera B. Incidence of Plasmodium infections and determinant factors among febrile children in a district of Northwest Ethiopia; a cross-sectional study. Trop Dis Travel Med Vaccines. 2018;4:6.
Abdishu M, Gobena T, Damena M, Abdi H, Birhanu A. Determinants of malaria morbidity among school-aged children living in East Hararghe Zone, Oromia, Ethiopia: a community-based case–control study. Pediatr Health Med Ther. 2022;13:183.
Federal Democratic Republic of Ethiopia Ministry of Health. National malaria strategic plan 2021–2025. Addis Ababa, Ethiopia; 2020.
Yang D, He Y, Wu B, Deng Y, Li M, Yang Q, et al. Drinking water and sanitation conditions are associated with the risk of malaria among children under five years old in sub-Saharan Africa: a logistic regression model analysis of national survey data. J Adv Res. 2019;21:1–13.
Tusting LS, Gething PW, Gibson HS, Greenwood B, Knudsen J, Lindsay SW, et al. Housing and child health in sub-Saharan Africa: a cross-sectional analysis. PLoS Med. 2020;17:e1003055.
Taylor C, Namaste SM, Lowell J, Useem J, Yé Y. Estimating the fraction of severe malaria among malaria-positive children: analysis of household surveys in 19 malaria-endemic countries in Africa. Am J Trop Med Hyg. 2021;104:1375.
Federal Democratic Republic of Ethiopia Ministry of Health. Ethiopia national malaria indicator survey 2015. Addis Ababa, Ethiopia; 2016.
Wanzira H, Katamba H, Okullo AE, Agaba B, Kasule M, Rubahika D. Factors associated with malaria parasitaemia among children under 5 years in Uganda: a secondary data analysis of the 2014 malaria indicator survey dataset. Malar J. 2017;16:191.
Ghana Statistical Service (GSS). Ghana Health Service (GHS), and ICF. Ghana malaria indicator survey 2016. Accra, Ghana, and Rockville, USA. 2017.
National Malaria Elimination Programme (NMEP), National Population Commission (NPopC). National Bureau of Statistics (NBS), and ICF International. Nigeria malaria indicator survey 2015. Abuja, Nigeria; 2016.
Ministry of Health. Community Development, Gender, Elderly and Children [Tanzania Mainland], Ministry of Health [Zanzibar], National Bureau of Statistics, Office of the Chief Government Statistics, and ICF. Tanzania malaria indicator survey (TMIS) 2017: malaria atlas. Dar se Saalam, Tanzania, and Rockville, USA; 2017.
Division of National Malaria Programme [Kenya] and ICF. Kenya malaria indicator survey 2020. Nairobi, Kenya and Rockville, Maryland, USA; 2021.
Berendsen ML, Van Gijzel SW, Smits J, De Mast Q, Aaby P, Benn CS, et al. BCG vaccination is associated with reduced malaria prevalence in children under the age of 5 years in sub-Saharan Africa. BMJ Glob Health. 2019;4:e001862.
Gaston RT, Ramroop S. Prevalence of and factors associated with malaria in children under five years of age in Malawi, using malaria indicator survey data. Heliyon. 2020;6:e03946.
National Malaria Control Programme [Liberia], Ministry of Health, Liberia Institute of Statistics and Geo-Information Services, and ICF. Liberia malaria indicator survey 2016. Liberia, Monrovia and Rockville, USA; 2017.
Ministry of Health, Republic of South Sudan. Malaria Indicator Survey (MIS) 2017. Juba, South Sudan; 2017.
Levitz L, Janko M, Mwandagalirwa K, Thwai KL, Likwela JL, Tshefu AK, et al. Effect of individual and community-level bed net usage on malaria prevalence among under-fives in the Democratic Republic of Congo. Malar J. 2018;17:39.
Chitunhu S, Musenge E. Direct and indirect determinants of childhood malaria morbidity in Malawi: a survey cross-sectional analysis based on malaria indicator survey data for 2012. Malar J. 2015;14:265.
Kooko R, Wafula ST, Orishaba P. Socio-economic determinants of malaria prevalence among under five children in Uganda: evidence from 2018–19 Uganda malaria indicator survey. J Vector Borne Dis. 2022. Online ahead of print.
Obasohan PE, Walters SJ, Jacques R, Khatab K. A scoping review of selected studies on predictor variables associated with the malaria status among children under five years in sub-Saharan Africa. Int J Environ Res Public Health. 2021;18:2119.
Ayele DG, Zewotir TT, Mwambi HG. The risk factor indicators of malaria in Ethiopia. Malar J. 2012;11:195.
Ejigu BA, Wencheko E. Spatial prevalence and determinants of malaria among under-five Children in Ghana. MedRxiv. 2021. https://doi.org/10.1101/2021.03.12.21253436 .
Adefemi K, Awolaran O, Wuraola C. Social and environmental determinants of malaria in under five children in Nigeria: a review. Int J Community Med Public Health. 2015;2:345–50.
McMahon A, Mihretie A, Ahmed AA, Lake M, Awoke W, Wimberly MC. Remote sensing of environmental risk factors for malaria in different geographic contexts. Int J Health Geograph. 2021;20:28.
Hajison PL, Feresu SA, Mwakikunga BW. Malaria in children under-five: a comparison of risk factors in lakeshore and highland areas, Zomba district, Malawi. PLoS ONE. 2018;13:e0207207.
Adeola AM, Botai O, Olwoch JM, Rautenbach CdW, Adisa O, Taiwo O, et al. Environmental factors and population at risk of malaria in Nkomazi municipality, South Africa. Trop Med Int Health. 2016;21:675–86.
Article CAS PubMed Google Scholar
Download references
Acknowledgements
We would like to thank Wollo University’s librarian and information and communication technology (ICT) center staff for availing us of uninterrupted internet connection. We also acknowledge PROSPERO for registration of this protocol.
No funding was used.
Author information
Authors and affiliations.
Department of Pediatrics and Child Health Nursing, School of Nursing and Midwifery, College of Medicine and Health Sciences, Wollo University, Dessie, Ethiopia
Gebeyaw Biset
Dream Science and Technology College, Dessie, Ethiopia
Gebeyaw Biset & Abay Woday Tadess
Department of Public Health, College of Medicine and Health Sciences, Samara University, Samara, Ethiopia
Abay Woday Tadess
Department of Adult Health Nursing, School of Nursing and Midwifery, College of Medicine and Health Sciences, Wollo University, Dessie, Ethiopia
Kirubel Dagnaw Tegegne
Department of Emergency and Critical Care Nursing, School of Nursing and Midwifery, College of Medicine and Health Sciences, Wollo University, Dessie, Ethiopia
Lehulu Tilahun
School of Midwifery, College of Health Science and Medicine, Wolaita Sodo University, Wolaita Sodo, Ethiopia
Natnael Atnafu
You can also search for this author in PubMed Google Scholar
Contributions
GB, AWT, KDT, NA, and LT have conceived the title and written the protocol. GB, AWT, and KDT have performed the search strategy. GB, NA, and LT performed the quality assessment, data extraction, and the analysis. All authors have been involved in the final write-up of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Correspondence to Gebeyaw Biset .
Ethics declarations
Ethics approval and consent to participate.
Not applicable.
Consent for publication
Competing interests.
The authors declare no competing interests.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1..
PRISMA 2020 Checklist for reporting the finding.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Biset, G., Tadess, A.W., Tegegne, K.D. et al. Malaria among under-five children in Ethiopia: a systematic review and meta-analysis. Malar J 21 , 338 (2022). https://doi.org/10.1186/s12936-022-04370-9
Download citation
Received : 19 August 2022
Accepted : 07 November 2022
Published : 16 November 2022
DOI : https://doi.org/10.1186/s12936-022-04370-9
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Under-five children
- Systematic review
- Meta-analysis
Malaria Journal
ISSN: 1475-2875
- Submission enquiries: [email protected]
IEEE Account
- Change Username/Password
- Update Address
Purchase Details
- Payment Options
- Order History
- View Purchased Documents
Profile Information
- Communications Preferences
- Profession and Education
- Technical Interests
- US & Canada: +1 800 678 4333
- Worldwide: +1 732 981 0060
- Contact & Support
- About IEEE Xplore
- Accessibility
- Terms of Use
- Nondiscrimination Policy
- Privacy & Opting Out of Cookies
A not-for-profit organization, IEEE is the world's largest technical professional organization dedicated to advancing technology for the benefit of humanity. © Copyright 2024 IEEE - All rights reserved. Use of this web site signifies your agreement to the terms and conditions.

COMMENTS
PhD, Chemical Engineering. Thesis. Controlled-release of mosquito repellents from microporous polymer strands. Prof WW Focke. Ms Masego Dilebo. MSc, Biochemistry. Dissertation. Mechanistic investigation of novel artemisinin-based combination that targets Plasmodium falciparum parasites. Prof L Birkholtz, Dr D Coertzen, RK Haynes.
ii Dissertation Abstract Background: Recently, malaria has become a major global health priority.As a result there has been renewed interest in malaria control, elimination, and eradication. Zambia is one of the Elimination 8 countries and one of the President's Malaria Initiative focus
As member of the Board of Examiners of the MSc Thesis Open Defense Examination, we certify that we have read, evaluated the thesis prepared by Tesfagabr G/Eyesus and examined ... Malaria Vector Mosquitoes and their Geographical Distribution 14 2.8. The Life Cycle of Plasmodium 16 2.9. Factors that Affect the Epidemiology of Malaria 18
The Relationship Between Malaria Status in Under-five Children and Some Household Demographic, Socioeconomic and ... MSc in Project Management, University of Roehampton BSc Biological Sciences, University of Sierra Leone . A thesis submitted to the graduate faculty of Georgia State University in partial fulfillment of the requirements for the ...
TOOLS AND TECHNIQUES FOR THE STUDY AND EVALUATION OF MALARIA CONTROL MEASURES IN WEST AFRICA Malarial disease caused by Plasmodium genus parasites remains as one of the most pressing public health matters of our time. Malaria still kills roughly 500,000 people a year, predominately children under 5 years of age, mostly in sub-Saharan Africa [1].
Malaria is an acute fever sickness caused by the Plasmodium parasite and spread by infected Anopheles female mosquitoes. It causes catastrophic illness if left untreated for an extended period ...
Malaria risk maps are a critical tool to assist national malaria programmes in the prioritization and targeting of their malaria control activities to maximize efficacy and equity, especially under conditions of resource constraint. ... [Master's thesis]. University of Oxford. ... MSc by Research Level of award: Masters ...
Malaria is an acute febrile illness caused by the Plasmodium parasites, transmitted from person to person by the bites of infected female Anopheles mosquitoes. (World Health Organization [WHO], 2016). Five parasite species are responsible for malaria infection in humans, with two of these (P. falciparum and P. vivax) posing the biggest threat.
malaria originated from the Medieval Italian " mala aria", meaning " bad air." This is because by then, the disease was associated with the air in the swamps and marshland (2). 1.2 Global incidence, prevalence, and epidemiology of malaria Malaria occurs mostly in the tropical and the subtropical regions of the world. The prevalent
iv | P a g e malaria vectors over 15 months in 12 villages. Overall, the MET caught proportionately fewer An. gambiae s.l. than the HLC (mean estimated number of 0.78 versus 1.82 indoors, and 1.05 versus 2.04 outdoors). However provided a consistent representation of vector species composition, seasonal
rea showed fluctuating trends. The prevalence of malaria was 14% in year 2017, 12.5% in 2018, 15% in 2019, 13% in 2020 and 13% in year2021 (Table 1).The highest annual prevalence of malaria (15%) was recordedduring the year 2019; which was much higher than that of other years.The second larger malaria cas.
Malaria is a life-threatening infectious disease with an estimated 229 million cases in the year 2019 worldwide. Plasmodium falciparum 1-deoxy-d-xylulose-5-phosphate reductoisomerase (PfDXR) is one of the key enzymes in the biosynthetic pathway of isoprenoid, (required for parasite growth and survival) and considered as an attractive target for designing anti-malarial drugs.
November 2019. ersity, HaramayaHARAMAYA UNIVERSITYPOSTGRADUATE PROGRAM DIRECTORATEI hereby certify that I have read and evaluated this Thesis entitled "Mathematical Modeling of Malaria Transmission: the Case of West. hewa Zone, Nono Wereda" prepared under our guidance, by Dagne Diro. We. as fulfilling the thesis re.
The thesis seeks to assess the economic impacts of malaria in Burkina Faso via a systematic literature review. Synthesising existing research findings will provide insights into the economic burden of malaria and its implications for healthcare, labor productivity, and socio-economic development in the country.
Resistance to antimalarial drugs inevitably follows their deployment in malaria endemic parts of the world. For instance, current malaria control efforts which significantly rely on artemisinin combination therapies (ACTs) are being threatened by the emergence of resistance to artemisinins and ACTs. Understanding the role of genetic determinants of artemisinin resistance is therefore important ...
Results. Eighty-one (19.0%) microscopically confirmed malaria cases were recorded, P.vivax was the most frequently detected species (n = 58; 71.6%).Interestingly, 73.2% (n = 309) of the participant did not utilize LLINs due to the fear of toxicity (37.4%, n = 158), misconception (21.6%, n = 91), and shortage (14.2%, n = 60).The data showed age, gender, marital status, family size, usage of ...
Abstract and Figures. SUPERVISOR BY: Dr. HAMZE ALI ABDILLAHI. .5 your occupations. 13 IS malaria a common disease in your community. 19 shows the 52% answer to yes ,33.8% n0 and 13.8%l don't know ...
Malaria remains one of the world's leading health problems, causing about 429,000 deaths in 2015, the vast majority of deaths (99%) were due to Plasmodium falciparum malaria . In that year, most (92%) of the deaths were estimated to have occurred in the sub-Saharan Africa region. ... Search for unpublished MSc/PhD thesis research reports ...
Malaria research is of a great contribution to the socio-economic improvement of Africa since the groups most vulnerable to this disease are of great economic importance to the development of Africa. 1.1.2 Malaria transmission. Malaria is caused by protozoan parasite belonging to genus Plasmodium and transmitted by female Anopheles mosquito [4].
a prevention combination of insecticide-treated nets (ITNs) and either intermittent preventive treatment in pregnancy (IPTp) with sulfadoxine-pyrimethamine for women who are HIV-negative,3,4 or daily co-trimoxazole prophylaxis for HIV-positive women.5 In areas in Asia and Latin America with low transmission, control of malaria in pregnancy ...
A total of 200 different plant species (from 71 families) used for traditional malaria treatment were identified in different parts of Ethiopia. Distribution and usage pattern of anti-malarial plants showed substantial variability across different geographic settings. ... Search for unpublished MSc/PhD thesis research reports using Google ...
Globally, malaria is among the leading cause of under-five mortality and morbidity. Despite various malaria elimination strategies being implemented in the last decades, malaria remains a major public health concern, particularly in tropical and sub-tropical regions. Furthermore, there have been limited and inconclusive studies in Ethiopia to generate information for action towards malaria in ...
Malaria is the deadliest disease in the earth and big hectic work for the health department. The traditional way of diagnosing malaria is by schematic examining blood smears of human beings for parasite-infected red blood cells under the microscope by lab or qualified technicians. This process is inefficient and the diagnosis depends on the experience and well knowledgeable person needed for ...