
- Science Notes Posts
- Contact Science Notes
- Todd Helmenstine Biography
- Anne Helmenstine Biography
- Free Printable Periodic Tables (PDF and PNG)
- Periodic Table Wallpapers
- Interactive Periodic Table
- Periodic Table Posters
- Science Experiments for Kids
- How to Grow Crystals
- Chemistry Projects
- Fire and Flames Projects
- Holiday Science
- Chemistry Problems With Answers
- Physics Problems
- Unit Conversion Example Problems
- Chemistry Worksheets
- Biology Worksheets
- Periodic Table Worksheets
- Physical Science Worksheets
- Science Lab Worksheets
- My Amazon Books

Control Group Definition and Examples

The control group is the set of subjects that does not receive the treatment in a study. In other words, it is the group where the independent variable is held constant. This is important because the control group is a baseline for measuring the effects of a treatment in an experiment or study. A controlled experiment is one which includes one or more control groups.
- The experimental group experiences a treatment or change in the independent variable. In contrast, the independent variable is constant in the control group.
- A control group is important because it allows meaningful comparison. The researcher compares the experimental group to it to assess whether or not there is a relationship between the independent and dependent variable and the magnitude of the effect.
- There are different types of control groups. A controlled experiment has one more control group.
Control Group vs Experimental Group
The only difference between the control group and experimental group is that subjects in the experimental group receive the treatment being studied, while participants in the control group do not. Otherwise, all other variables between the two groups are the same.
Control Group vs Control Variable
A control group is not the same thing as a control variable. A control variable or controlled variable is any factor that is held constant during an experiment. Examples of common control variables include temperature, duration, and sample size. The control variables are the same for both the control and experimental groups.
Types of Control Groups
There are different types of control groups:
- Placebo group : A placebo group receives a placebo , which is a fake treatment that resembles the treatment in every respect except for the active ingredient. Both the placebo and treatment may contain inactive ingredients that produce side effects. Without a placebo group, these effects might be attributed to the treatment.
- Positive control group : A positive control group has conditions that guarantee a positive test result. The positive control group demonstrates an experiment is capable of producing a positive result. Positive controls help researchers identify problems with an experiment.
- Negative control group : A negative control group consists of subjects that are not exposed to a treatment. For example, in an experiment looking at the effect of fertilizer on plant growth, the negative control group receives no fertilizer.
- Natural control group : A natural control group usually is a set of subjects who naturally differ from the experimental group. For example, if you compare the effects of a treatment on women who have had children, the natural control group includes women who have not had children. Non-smokers are a natural control group in comparison to smokers.
- Randomized control group : The subjects in a randomized control group are randomly selected from a larger pool of subjects. Often, subjects are randomly assigned to either the control or experimental group. Randomization reduces bias in an experiment. There are different methods of randomly assigning test subjects.
Control Group Examples
Here are some examples of different control groups in action:
Negative Control and Placebo Group
For example, consider a study of a new cancer drug. The experimental group receives the drug. The placebo group receives a placebo, which contains the same ingredients as the drug formulation, minus the active ingredient. The negative control group receives no treatment. The reason for including the negative group is because the placebo group experiences some level of placebo effect, which is a response to experiencing some form of false treatment.
Positive and Negative Controls
For example, consider an experiment looking at whether a new drug kills bacteria. The experimental group exposes bacterial cultures to the drug. If the group survives, the drug is ineffective. If the group dies, the drug is effective.
The positive control group has a culture of bacteria that carry a drug resistance gene. If the bacteria survive drug exposure (as intended), then it shows the growth medium and conditions allow bacterial growth. If the positive control group dies, it indicates a problem with the experimental conditions. A negative control group of bacteria lacking drug resistance should die. If the negative control group survives, something is wrong with the experimental conditions.
- Bailey, R. A. (2008). Design of Comparative Experiments . Cambridge University Press. ISBN 978-0-521-68357-9.
- Chaplin, S. (2006). “The placebo response: an important part of treatment”. Prescriber . 17 (5): 16–22. doi: 10.1002/psb.344
- Hinkelmann, Klaus; Kempthorne, Oscar (2008). Design and Analysis of Experiments, Volume I: Introduction to Experimental Design (2nd ed.). Wiley. ISBN 978-0-471-72756-9.
- Pithon, M.M. (2013). “Importance of the control group in scientific research.” Dental Press J Orthod . 18 (6):13-14. doi: 10.1590/s2176-94512013000600003
- Stigler, Stephen M. (1992). “A Historical View of Statistical Concepts in Psychology and Educational Research”. American Journal of Education . 101 (1): 60–70. doi: 10.1086/444032
Related Posts
Have a language expert improve your writing
Run a free plagiarism check in 10 minutes, automatically generate references for free.
- Knowledge Base
- Methodology
- Control Groups and Treatment Groups | Uses & Examples
Control Groups & Treatment Groups | Uses & Examples
Published on 6 May 2022 by Lauren Thomas . Revised on 13 April 2023.
In a scientific study, a control group is used to establish a cause-and-effect relationship by isolating the effect of an independent variable .
Researchers change the independent variable in the treatment group and keep it constant in the control group. Then they compare the results of these groups.
Using a control group means that any change in the dependent variable can be attributed to the independent variable.
Table of contents
Control groups in experiments, control groups in non-experimental research, importance of control groups, frequently asked questions about control groups.
Control groups are essential to experimental design . When researchers are interested in the impact of a new treatment, they randomly divide their study participants into at least two groups:
- The treatment group (also called the experimental group ) receives the treatment whose effect the researcher is interested in.
- The control group receives either no treatment, a standard treatment whose effect is already known, or a placebo (a fake treatment).
The treatment is any independent variable manipulated by the experimenters, and its exact form depends on the type of research being performed. In a medical trial, it might be a new drug or therapy. In public policy studies, it could be a new social policy that some receive and not others.
In a well-designed experiment, all variables apart from the treatment should be kept constant between the two groups. This means researchers can correctly measure the entire effect of the treatment without interference from confounding variables .
- You pay the students in the treatment group for achieving high grades.
- Students in the control group do not receive any money.
Studies can also include more than one treatment or control group. Researchers might want to examine the impact of multiple treatments at once, or compare a new treatment to several alternatives currently available.
- The treatment group gets the new pill.
- Control group 1 gets an identical-looking sugar pill (a placebo).
- Control group 2 gets a pill already approved to treat high blood pressure.
Since the only variable that differs between the three groups is the type of pill, any differences in average blood pressure between the three groups can be credited to the type of pill they received.
- The difference between the treatment group and control group 1 demonstrates the effectiveness of the pill as compared to no treatment.
- The difference between the treatment group and control group 2 shows whether the new pill improves on treatments already available on the market.
Prevent plagiarism, run a free check.
Although control groups are more common in experimental research, they can be used in other types of research too. Researchers generally rely on non-experimental control groups in two cases: quasi-experimental or matching design.
Control groups in quasi-experimental design
While true experiments rely on random assignment to the treatment or control groups, quasi-experimental design uses some criterion other than randomisation to assign people.
Often, these assignments are not controlled by researchers, but are pre-existing groups that have received different treatments. For example, researchers could study the effects of a new teaching method that was applied in some classes in a school but not others, or study the impact of a new policy that is implemented in one region but not in the neighbouring region.
In these cases, the classes that did not use the new teaching method, or the region that did not implement the new policy, is the control group.
Control groups in matching design
In correlational research , matching represents a potential alternate option when you cannot use either true or quasi-experimental designs.
In matching designs, the researcher matches individuals who received the ‘treatment’, or independent variable under study, to others who did not – the control group.
Each member of the treatment group thus has a counterpart in the control group identical in every way possible outside of the treatment. This ensures that the treatment is the only source of potential differences in outcomes between the two groups.
Control groups help ensure the internal validity of your research. You might see a difference over time in your dependent variable in your treatment group. However, without a control group, it is difficult to know whether the change has arisen from the treatment. It is possible that the change is due to some other variables.
If you use a control group that is identical in every other way to the treatment group, you know that the treatment – the only difference between the two groups – must be what has caused the change.
For example, people often recover from illnesses or injuries over time regardless of whether they’ve received effective treatment or not. Thus, without a control group, it’s difficult to determine whether improvements in medical conditions come from a treatment or just the natural progression of time.
Risks from invalid control groups
If your control group differs from the treatment group in ways that you haven’t accounted for, your results may reflect the interference of confounding variables instead of your independent variable.
Minimising this risk
A few methods can aid you in minimising the risk from invalid control groups.
- Ensure that all potential confounding variables are accounted for , preferably through an experimental design if possible, since it is difficult to control for all the possible confounders outside of an experimental environment.
- Use double-blinding . This will prevent the members of each group from modifying their behavior based on whether they were placed in the treatment or control group, which could then lead to biased outcomes.
- Randomly assign your subjects into control and treatment groups. This method will allow you to not only minimise the differences between the two groups on confounding variables that you can directly observe, but also those you cannot.
An experimental group, also known as a treatment group, receives the treatment whose effect researchers wish to study, whereas a control group does not. They should be identical in all other ways.
A true experiment (aka a controlled experiment) always includes at least one control group that doesn’t receive the experimental treatment.
However, some experiments use a within-subjects design to test treatments without a control group. In these designs, you usually compare one group’s outcomes before and after a treatment (instead of comparing outcomes between different groups).
For strong internal validity , it’s usually best to include a control group if possible. Without a control group, it’s harder to be certain that the outcome was caused by the experimental treatment and not by other variables.
In a controlled experiment , all extraneous variables are held constant so that they can’t influence the results. Controlled experiments require:
- A control group that receives a standard treatment, a fake treatment, or no treatment
- Random assignment of participants to ensure the groups are equivalent
Depending on your study topic, there are various other methods of controlling variables .
A confounding variable , also called a confounder or confounding factor, is a third variable in a study examining a potential cause-and-effect relationship.
A confounding variable is related to both the supposed cause and the supposed effect of the study. It can be difficult to separate the true effect of the independent variable from the effect of the confounding variable.
In your research design , it’s important to identify potential confounding variables and plan how you will reduce their impact.
Cite this Scribbr article
If you want to cite this source, you can copy and paste the citation or click the ‘Cite this Scribbr article’ button to automatically add the citation to our free Reference Generator.
Thomas, L. (2023, April 13). Control Groups & Treatment Groups | Uses & Examples. Scribbr. Retrieved 9 September 2024, from https://www.scribbr.co.uk/research-methods/control-groups/
Is this article helpful?

Lauren Thomas
Other students also liked, controlled experiments | methods & examples of control, a quick guide to experimental design | 5 steps & examples, correlation vs causation | differences, designs & examples.
Experimental Method In Psychology
Saul McLeod, PhD
Editor-in-Chief for Simply Psychology
BSc (Hons) Psychology, MRes, PhD, University of Manchester
Saul McLeod, PhD., is a qualified psychology teacher with over 18 years of experience in further and higher education. He has been published in peer-reviewed journals, including the Journal of Clinical Psychology.
Learn about our Editorial Process
Olivia Guy-Evans, MSc
Associate Editor for Simply Psychology
BSc (Hons) Psychology, MSc Psychology of Education
Olivia Guy-Evans is a writer and associate editor for Simply Psychology. She has previously worked in healthcare and educational sectors.
On This Page:
The experimental method involves the manipulation of variables to establish cause-and-effect relationships. The key features are controlled methods and the random allocation of participants into controlled and experimental groups .
What is an Experiment?
An experiment is an investigation in which a hypothesis is scientifically tested. An independent variable (the cause) is manipulated in an experiment, and the dependent variable (the effect) is measured; any extraneous variables are controlled.
An advantage is that experiments should be objective. The researcher’s views and opinions should not affect a study’s results. This is good as it makes the data more valid and less biased.
There are three types of experiments you need to know:
1. Lab Experiment
A laboratory experiment in psychology is a research method in which the experimenter manipulates one or more independent variables and measures the effects on the dependent variable under controlled conditions.
A laboratory experiment is conducted under highly controlled conditions (not necessarily a laboratory) where accurate measurements are possible.
The researcher uses a standardized procedure to determine where the experiment will take place, at what time, with which participants, and in what circumstances.
Participants are randomly allocated to each independent variable group.
Examples are Milgram’s experiment on obedience and Loftus and Palmer’s car crash study .
- Strength : It is easier to replicate (i.e., copy) a laboratory experiment. This is because a standardized procedure is used.
- Strength : They allow for precise control of extraneous and independent variables. This allows a cause-and-effect relationship to be established.
- Limitation : The artificiality of the setting may produce unnatural behavior that does not reflect real life, i.e., low ecological validity. This means it would not be possible to generalize the findings to a real-life setting.
- Limitation : Demand characteristics or experimenter effects may bias the results and become confounding variables .
2. Field Experiment
A field experiment is a research method in psychology that takes place in a natural, real-world setting. It is similar to a laboratory experiment in that the experimenter manipulates one or more independent variables and measures the effects on the dependent variable.
However, in a field experiment, the participants are unaware they are being studied, and the experimenter has less control over the extraneous variables .
Field experiments are often used to study social phenomena, such as altruism, obedience, and persuasion. They are also used to test the effectiveness of interventions in real-world settings, such as educational programs and public health campaigns.
An example is Holfing’s hospital study on obedience .
- Strength : behavior in a field experiment is more likely to reflect real life because of its natural setting, i.e., higher ecological validity than a lab experiment.
- Strength : Demand characteristics are less likely to affect the results, as participants may not know they are being studied. This occurs when the study is covert.
- Limitation : There is less control over extraneous variables that might bias the results. This makes it difficult for another researcher to replicate the study in exactly the same way.
3. Natural Experiment
A natural experiment in psychology is a research method in which the experimenter observes the effects of a naturally occurring event or situation on the dependent variable without manipulating any variables.
Natural experiments are conducted in the day (i.e., real life) environment of the participants, but here, the experimenter has no control over the independent variable as it occurs naturally in real life.
Natural experiments are often used to study psychological phenomena that would be difficult or unethical to study in a laboratory setting, such as the effects of natural disasters, policy changes, or social movements.
For example, Hodges and Tizard’s attachment research (1989) compared the long-term development of children who have been adopted, fostered, or returned to their mothers with a control group of children who had spent all their lives in their biological families.
Here is a fictional example of a natural experiment in psychology:
Researchers might compare academic achievement rates among students born before and after a major policy change that increased funding for education.
In this case, the independent variable is the timing of the policy change, and the dependent variable is academic achievement. The researchers would not be able to manipulate the independent variable, but they could observe its effects on the dependent variable.
- Strength : behavior in a natural experiment is more likely to reflect real life because of its natural setting, i.e., very high ecological validity.
- Strength : Demand characteristics are less likely to affect the results, as participants may not know they are being studied.
- Strength : It can be used in situations in which it would be ethically unacceptable to manipulate the independent variable, e.g., researching stress .
- Limitation : They may be more expensive and time-consuming than lab experiments.
- Limitation : There is no control over extraneous variables that might bias the results. This makes it difficult for another researcher to replicate the study in exactly the same way.
Key Terminology
Ecological validity.
The degree to which an investigation represents real-life experiences.
Experimenter effects
These are the ways that the experimenter can accidentally influence the participant through their appearance or behavior.
Demand characteristics
The clues in an experiment lead the participants to think they know what the researcher is looking for (e.g., the experimenter’s body language).
Independent variable (IV)
The variable the experimenter manipulates (i.e., changes) is assumed to have a direct effect on the dependent variable.
Dependent variable (DV)
Variable the experimenter measures. This is the outcome (i.e., the result) of a study.
Extraneous variables (EV)
All variables which are not independent variables but could affect the results (DV) of the experiment. EVs should be controlled where possible.
Confounding variables
Variable(s) that have affected the results (DV), apart from the IV. A confounding variable could be an extraneous variable that has not been controlled.
Random Allocation
Randomly allocating participants to independent variable conditions means that all participants should have an equal chance of participating in each condition.
The principle of random allocation is to avoid bias in how the experiment is carried out and limit the effects of participant variables.
Order effects
Changes in participants’ performance due to their repeating the same or similar test more than once. Examples of order effects include:
(i) practice effect: an improvement in performance on a task due to repetition, for example, because of familiarity with the task;
(ii) fatigue effect: a decrease in performance of a task due to repetition, for example, because of boredom or tiredness.
- Skip to secondary menu
- Skip to main content
- Skip to primary sidebar
Statistics By Jim
Making statistics intuitive
Control Group in an Experiment
By Jim Frost 3 Comments
A control group in an experiment does not receive the treatment. Instead, it serves as a comparison group for the treatments. Researchers compare the results of a treatment group to the control group to determine the effect size, also known as the treatment effect.

Imagine that a treatment group receives a vaccine and it has an infection rate of 10%. By itself, you don’t know if that’s an improvement. However, if you also have an unvaccinated control group with an infection rate of 20%, you know the vaccine improved the outcome by 10 percentage points.
By serving as a basis for comparison, the control group reveals the treatment’s effect.
Related post : Effect Sizes in Statistics
Using Control Groups in Experiments
Most experiments include a control group and at least one treatment group. In an ideal experiment, the subjects in all groups start with the same overall characteristics except that those in the treatment groups receive a treatment. When the groups are otherwise equivalent before treatment begins, you can attribute differences after the experiment to the treatments.
Randomized controlled trials (RCTs) assign subjects to the treatment and control groups randomly. This process helps ensure the groups are comparable when treatment begins. Consequently, treatment effects are the most likely cause for differences between groups at the end of the study. Statisticians consider RCTs to be the gold standard. To learn more about this process, read my post, Random Assignment in Experiments .
Observational studies either can’t use randomized groups or don’t use them because they’re too costly or problematic. In these studies, the characteristics of the control group might be different from the treatment groups at the start of the study, making it difficult to estimate the treatment effect accurately at the end. Case-Control studies are a specific type of observational study that uses a control group.
For these types of studies, analytical methods and design choices, such as regression analysis and matching, can help statistically mitigate confounding variables. Matching involves selecting participants with similar characteristics. For each participant in the treatment group, the researchers find a subject with comparable traits to include in the control group. To learn more about this type of study and matching, read my post, Observational Studies Explained .
Control groups are key way to increase the internal validity of an experiment. To learn more, read my post about internal and external validity .
Randomized versus non-randomized control groups are just several of the different types you can have. We’ll look at more kinds later!
Related posts : When to Use Regression Analysis
Example of a Control Group
Suppose we want to determine whether regular vitamin consumption affects the risk of dying. Our experiment has the following two experimental groups:
- Control group : Does not consume vitamin supplements
- Treatment group : Regularly consumes vitamin supplements.
In this experiment, we randomly assign subjects to the two groups. Because we use random assignment, the two groups start with similar characteristics, including healthy habits, physical attributes, medical conditions, and other factors affecting the outcome. The intentional introduction of vitamin supplements in the treatment group is the only systematic difference between the groups.
After the experiment is complete, we compare the death risk between the treatment and control groups. Because the groups started roughly equal, we can reasonably attribute differences in death risk at the end of the study to vitamin consumption. By having the control group as the basis of comparison, the effect of vitamin consumption becomes clear!
Types of Control Groups
Researchers can use different types of control groups in their experiments. Earlier, you learned about the random versus non-random kinds, but there are other variations. You can use various types depending on your research goals, constraints, and ethical issues, among other things.
Negative Control Group
The group introduces a condition that the researchers expect won’t have an effect. This group typically receives no treatment. These experiments compare the effectiveness of the experimental treatment to no treatment. For example, in a vaccine study, a negative control group does not get the vaccine.
Positive Control Group
Positive control groups typically receive a standard treatment that science has already proven effective. These groups serve as a benchmark for the performance of a conventional treatment. In this vein, experiments with positive control groups compare the effectiveness of a new treatment to a standard one.
For example, an old blood pressure medicine can be the treatment in a positive control group, while the treatment group receives the new, experimental blood pressure medicine. The researchers want to determine whether the new treatment is better than the previous treatment.
In these studies, subjects can still take the standard medication for their condition, a potentially critical ethics issue.
Placebo Control Group
Placebo control groups introduce a treatment lookalike that will not affect the outcome. Standard examples of placebos are sugar pills and saline solution injections instead of genuine medicine. The key is that the placebo looks like the actual treatment. Researchers use this approach when the recipients’ belief that they’re receiving the treatment might influence their outcomes. By using placebos, the experiment controls for these psychological benefits. The researchers want to determine whether the treatment performs better than the placebo effect.
Learn more about the Placebo Effect .
Blinded Control Groups
If the subject’s awareness of their group assignment might affect their outcomes, the researchers can use a blinded experimental design that does not tell participants their group membership. Typically, blinded control groups will receive placebos, as described above. In a double-blinded control group, both subjects and researchers don’t know group assignments.
Waitlist Control Group
When there is a waitlist to receive a new treatment, those on the waitlist can serve as a control group until they receive treatment. This type of design avoids ethical concerns about withholding a better treatment until the study finishes. This design can be a variation of a positive control group because the subjects might be using conventional medicines while on the waitlist.
Historical Control Group
When historical data for a comparison group exists, it can serve as a control group for an experiment. The group doesn’t exist in the study, but the researchers compare the treatment group to the existing data. For example, the researchers might have infection rate data for unvaccinated individuals to compare to the infection rate among the vaccinated participants in their study. This approach allows everyone in the experiment to receive the new treatment. However, differences in place, time, and other circumstances can reduce the value of these comparisons. In other words, other factors might account for the apparent effects.
Share this:

Reader Interactions
December 19, 2021 at 9:17 am
Thank you very much Jim for your quick and comprehensive feedback. Extremely helpful!! Regards, Arthur
December 17, 2021 at 4:46 pm
Thank you very much Jim, very interesting article.
Can I select a control group at the end of intervention/experiment? Currently I am managing a project in rural Cambodia in five villages, however I did not select any comparison/control site at the beginning. Since I know there are other villages which have not been exposed to any type of intervention, can i select them as a control site during my end-line data collection or it will not be a legitimate control? Thank you very much, Arthur
December 18, 2021 at 1:51 am
You might be able to use that approach, but it’s not ideal. The ideal is to have control groups defined at the beginning of the study. You can use the untreated villages as a type of historical control groups that I talk about in this article. Or, if they’re awaiting to receive the intervention, it might be akin to a waitlist control group.
If you go that route, you’ll need to consider whether there was some systematic reason why these villages have not received any intervention. For example, are the villages in question more remote? And, if there is a systematic reason, would that affect your outcome variable? More generally, are they systematically different? How well do the untreated villages represent your target population?
If you had selected control villages at the beginning, you’d have been better able to ensure there weren’t any systematic differences between the villages receiving interventions and those that didn’t.
If the villages that didn’t receive any interventions are systematically different, you’ll need to incorporate that into your interpretation of the results. Are they different in ways that affect the outcomes you’re measuring? Can those differences account for the difference in outcomes between the treated and untreated villages? Hopefully, you’d be able to measure those differences between untreated/treated villages.
So, yes, you can use that approach. It’s not perfect and there will potentially be more things for you to consider and factor into your conclusions. Despite these drawbacks, it’s possible that using a pseudo control group like that is better than not doing that because at least you can make comparisons to something. Otherwise, you won’t know whether the outcomes in the intervention villages represent an improvement! Just be aware of the extra considerations!
Best of luck with your research!
Comments and Questions Cancel reply

- History & Society
- Science & Tech
- Biographies
- Animals & Nature
- Geography & Travel
- Arts & Culture
- Games & Quizzes
- On This Day
- One Good Fact
- New Articles
- Lifestyles & Social Issues
- Philosophy & Religion
- Politics, Law & Government
- World History
- Health & Medicine
- Browse Biographies
- Birds, Reptiles & Other Vertebrates
- Bugs, Mollusks & Other Invertebrates
- Environment
- Fossils & Geologic Time
- Entertainment & Pop Culture
- Sports & Recreation
- Visual Arts
- Demystified
- Image Galleries
- Infographics
- Top Questions
- Britannica Kids
- Saving Earth
- Space Next 50
- Student Center
- When did science begin?
- Where was science invented?

control group
Our editors will review what you’ve submitted and determine whether to revise the article.
- Verywell Mind - What Is a Control Group?
- National Center for Biotechnology Information - PubMed Central - Control Group Design: Enhancing Rigor in Research of Mind-Body Therapies for Depression
control group , the standard to which comparisons are made in an experiment. Many experiments are designed to include a control group and one or more experimental groups; in fact, some scholars reserve the term experiment for study designs that include a control group. Ideally, the control group and the experimental groups are identical in every way except that the experimental groups are subjected to treatments or interventions believed to have an effect on the outcome of interest while the control group is not. Inclusion of a control group greatly strengthens researchers’ ability to draw conclusions from a study. Indeed, only in the presence of a control group can a researcher determine whether a treatment under investigation truly has a significant effect on an experimental group, and the possibility of making an erroneous conclusion is reduced. See also scientific method .
A typical use of a control group is in an experiment in which the effect of a treatment is unknown and comparisons between the control group and the experimental group are used to measure the effect of the treatment. For instance, in a pharmaceutical study to determine the effectiveness of a new drug on the treatment of migraines , the experimental group will be administered the new drug and the control group will be administered a placebo (a drug that is inert, or assumed to have no effect). Each group is then given the same questionnaire and asked to rate the effectiveness of the drug in relieving symptoms . If the new drug is effective, the experimental group is expected to have a significantly better response to it than the control group. Another possible design is to include several experimental groups, each of which is given a different dosage of the new drug, plus one control group. In this design, the analyst will compare results from each of the experimental groups to the control group. This type of experiment allows the researcher to determine not only if the drug is effective but also the effectiveness of different dosages. In the absence of a control group, the researcher’s ability to draw conclusions about the new drug is greatly weakened, due to the placebo effect and other threats to validity. Comparisons between the experimental groups with different dosages can be made without including a control group, but there is no way to know if any of the dosages of the new drug are more or less effective than the placebo.
It is important that every aspect of the experimental environment be as alike as possible for all subjects in the experiment. If conditions are different for the experimental and control groups, it is impossible to know whether differences between groups are actually due to the difference in treatments or to the difference in environment. For example, in the new migraine drug study, it would be a poor study design to administer the questionnaire to the experimental group in a hospital setting while asking the control group to complete it at home. Such a study could lead to a misleading conclusion, because differences in responses between the experimental and control groups could have been due to the effect of the drug or could have been due to the conditions under which the data were collected. For instance, perhaps the experimental group received better instructions or was more motivated by being in the hospital setting to give accurate responses than the control group.
In non-laboratory and nonclinical experiments, such as field experiments in ecology or economics , even well-designed experiments are subject to numerous and complex variables that cannot always be managed across the control group and experimental groups. Randomization, in which individuals or groups of individuals are randomly assigned to the treatment and control groups, is an important tool to eliminate selection bias and can aid in disentangling the effects of the experimental treatment from other confounding factors. Appropriate sample sizes are also important.
A control group study can be managed in two different ways. In a single-blind study, the researcher will know whether a particular subject is in the control group, but the subject will not know. In a double-blind study , neither the subject nor the researcher will know which treatment the subject is receiving. In many cases, a double-blind study is preferable to a single-blind study, since the researcher cannot inadvertently affect the results or their interpretation by treating a control subject differently from an experimental subject.
- Bipolar Disorder
- Therapy Center
- When To See a Therapist
- Types of Therapy
- Best Online Therapy
- Best Couples Therapy
- Managing Stress
- Sleep and Dreaming
- Understanding Emotions
- Self-Improvement
- Healthy Relationships
- Student Resources
- Personality Types
- Sweepstakes
- Guided Meditations
- Verywell Mind Insights
- 2024 Verywell Mind 25
- Mental Health in the Classroom
- Editorial Process
- Meet Our Review Board
- Crisis Support
What Is a Control Group?
Control Groups vs. Experimental Groups in Psychology Research
Doug Corrance/The Image Bank/Getty Images
Control Group vs. Experimental Group
Types of control groups.
In simple terms, the control group comprises participants who do not receive the experimental treatment. When conducting an experiment, these people are randomly assigned to this group. They also closely resemble the participants who are in the experimental group or the individuals who receive the treatment.
Experimenters utilize variables to make comparisons between an experimental group and a control group. A variable is something that researchers can manipulate, measure, and control in an experiment. The independent variable is the aspect of the experiment that the researchers manipulate (or the treatment). The dependent variable is what the researchers measure to see if the independent variable had an effect.
While they do not receive the treatment, the control group does play a vital role in the research process. Experimenters compare the experimental group to the control group to determine if the treatment had an effect.
By serving as a comparison group, researchers can isolate the independent variable and look at the impact it had.
The simplest way to determine the difference between a control group and an experimental group is to determine which group receives the treatment and which does not. To ensure that the results can then be compared accurately, the two groups should be otherwise identical.
Not exposed to the treatment (the independent variable)
Used to provide a baseline to compare results against
May receive a placebo treatment
Exposed to the treatment
Used to measure the effects of the independent variable
Identical to the control group aside from their exposure to the treatment
Why a Control Group Is Important
While the control group does not receive treatment, it does play a critical role in the experimental process. This group serves as a benchmark, allowing researchers to compare the experimental group to the control group to see what sort of impact changes to the independent variable produced.
Because participants have been randomly assigned to either the control group or the experimental group, it can be assumed that the groups are comparable.
Any differences between the two groups are, therefore, the result of the manipulations of the independent variable. The experimenters carry out the exact same procedures with both groups with the exception of the manipulation of the independent variable in the experimental group.
There are a number of different types of control groups that might be utilized in psychology research. Some of these include:
- Positive control groups : In this case, researchers already know that a treatment is effective but want to learn more about the impact of variations of the treatment. In this case, the control group receives the treatment that is known to work, while the experimental group receives the variation so that researchers can learn more about how it performs and compares to the control.
- Negative control group : In this type of control group, the participants are not given a treatment. The experimental group can then be compared to the group that did not experience any change or results.
- Placebo control group : This type of control group receives a placebo treatment that they believe will have an effect. This control group allows researchers to examine the impact of the placebo effect and how the experimental treatment compared to the placebo treatment.
- Randomized control group : This type of control group involves using random selection to help ensure that the participants in the control group accurately reflect the demographics of the larger population.
- Natural control group : This type of control group is naturally selected, often by situational factors. For example, researchers might compare people who have experienced trauma due to war to people who have not experienced war. The people who have not experienced war-related trauma would be the control group.
Examples of Control Groups
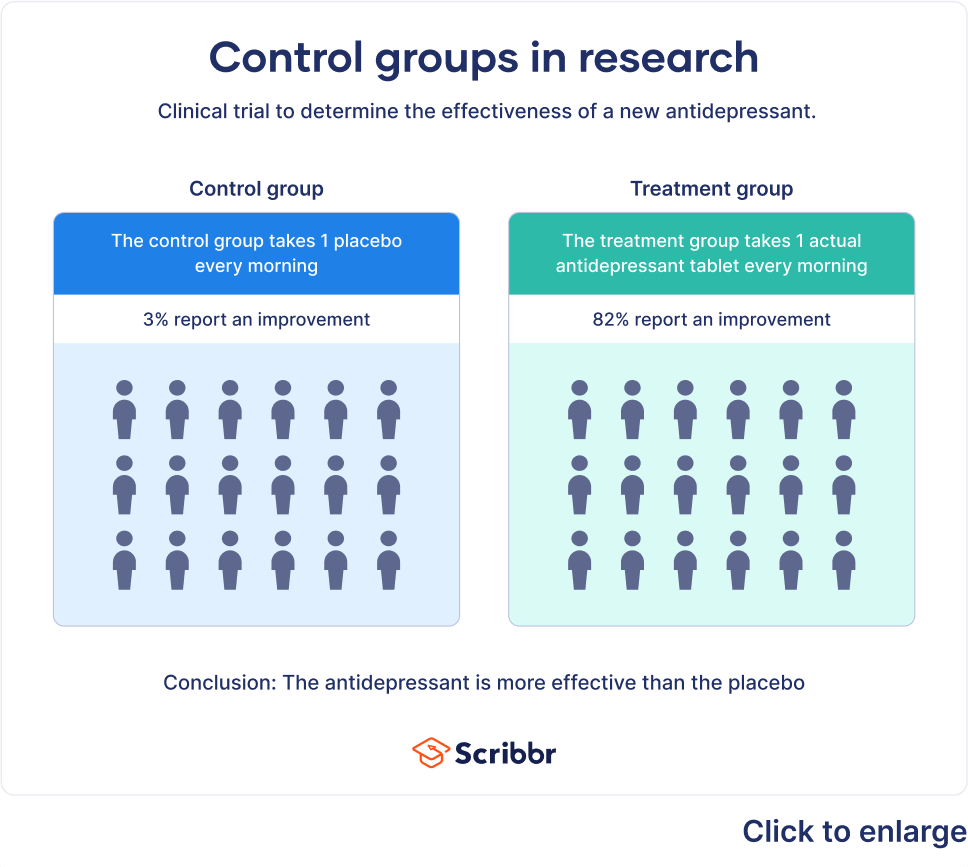
Control groups can be used in a variety of situations. For example, imagine a study in which researchers example how distractions during an exam influence test results. The control group would take an exam in a setting with no distractions, while the experimental groups would be exposed to different distractions. The results of the exam would then be compared to see the effects that distractions had on test scores.
Experiments that look at the effects of medications on certain conditions are also examples of how a control group can be used in research. For example, researchers looking at the effectiveness of a new antidepressant might use a control group that receives a placebo and an experimental group that receives the new medication. At the end of the study, researchers would compare measures of depression for both groups to determine what impact the new medication had.
After the experiment is complete, researchers can then look at the test results and start making comparisons between the control group and the experimental group.
Uses for Control Groups
Researchers utilize control groups to conduct research in a range of different fields. Some common uses include:
- Psychology : Researchers utilize control groups to learn more about mental health, behaviors, and treatments.
- Medicine : Control groups can be used to learn more about certain health conditions, assess how well medications work to treat these conditions, and assess potential side effects that may result.
- Education : Educational researchers utilize control groups to learn more about how different curriculums, programs, or instructional methods impact student outcomes.
- Marketing : Researchers utilize control groups to learn more about how consumers respond to advertising and marketing efforts.
Malay S, Chung KC. The choice of controls for providing validity and evidence in clinical research . Plast Reconstr Surg. 2012 Oct;130(4):959-965. doi:10.1097/PRS.0b013e318262f4c8
National Cancer Institute. Control group.
Pithon MM. Importance of the control group in scientific research . Dental Press J Orthod. 2013;18(6):13-14. doi:10.1590/s2176-94512013000600003
Karlsson P, Bergmark A. Compared with what? An analysis of control-group types in Cochrane and Campbell reviews of psychosocial treatment efficacy with substance use disorders . Addiction . 2015;110(3):420-8. doi:10.1111/add.12799
Myers A, Hansen C. Experimental Psychology . Belmont, CA: Cengage Learning; 2012.
By Kendra Cherry, MSEd Kendra Cherry, MS, is a psychosocial rehabilitation specialist, psychology educator, and author of the "Everything Psychology Book."
User Preferences
Content preview.
Arcu felis bibendum ut tristique et egestas quis:
- Ut enim ad minim veniam, quis nostrud exercitation ullamco laboris
- Duis aute irure dolor in reprehenderit in voluptate
- Excepteur sint occaecat cupidatat non proident
Keyboard Shortcuts
1.4.4 - control and placebo groups.
A control group is an experimental condition that does not receive the actual treatment and may serve as a baseline. A control group may receive a placebo or they may receive no treatment at all. A placebo is something that appears to the participants to be an active treatment, but does not actually contain the active treatment. For example, a placebo pill is a sugar pill that participants may take not knowing that it does not contain any active medicine. This can lead to a psychological phenomena called the placebo effect which occurs when participants who are given a placebo treatment experience a change even though they are not receiving any active treatment. Researchers use placebos in the control group to determine if any differences between groups are due to the active medicine or the participants' perceptions (the placebo effect).
Example: Vitamin B Energy Study Section
Researchers want to know if adults who consume a drink that is high in vitamin B-12 have increased energy. They obtain a representative sample of adults. All participants are given a drink that they are told to consume every morning. They are not told what is in the drink. Half are given a drink that is high in vitamin B-12 while the other half are given a drink that tastes the same but contains no vitamin B-12.
The participants who received the drink with no vitamin B-12 are the placebo group . The purpose of the placebo group in this study is to make the two groups equivalent except for the presence of the vitamin B-12. By comparing these two groups, the researchers will be able to determine what impact the vitamin B-12 had on the response variable. We could also say that this served as a control group because this group did not receive any active ingredients.
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- v.20(10); 2019 Oct 4

Why control an experiment?
John s torday.
1 Department of Pediatrics, Harbor‐UCLA Medical Center, Torrance, CA, USA
František Baluška
2 IZMB, University of Bonn, Bonn, Germany
Empirical research is based on observation and experimentation. Yet, experimental controls are essential for overcoming our sensory limits and generating reliable, unbiased and objective results.

We made a deliberate decision to become scientists and not philosophers, because science offers the opportunity to test ideas using the scientific method. And once we began our formal training as scientists, the greatest challenge beyond formulating a testable or refutable hypothesis was designing appropriate controls for an experiment. In theory, this seems trivial, but in practice, it is often difficult. But where and when did this concept of controlling an experiment start? It is largely attributed to Roger Bacon, who emphasized the use of artificial experiments to provide additional evidence for observations in his Novum Organum Scientiarum in 1620. Other philosophers took up the concept of empirical research: in 1877, Charles Peirce redefined the scientific method in The Fixation of Belief as the most efficient and reliable way to prove a hypothesis. In the 1930s, Karl Popper emphasized the necessity of refuting hypotheses in The Logic of Scientific Discoveries . While these influential works do not explicitly discuss controls as an integral part of experiments, their importance for generating solid and reliable results is nonetheless implicit.
… once we began our formal training as scientists, the greatest challenge beyond formulating a testable or refutable hypothesis was designing appropriate controls for an experiment.
But the scientific method based on experimentation and observation has come under criticism of late in light of the ever more complex problems faced in physics and biology. Chris Anderson, the editor of Wired Magazine, proposed that we should turn to statistical analysis, machine learning, and pattern recognition instead of creating and testing hypotheses, based on the Informatics credo that if you cannot answer the question, you need more data. However, this attitude subsumes that we already have enough data and that we just cannot make sense of it. This assumption is in direct conflict with David Bohm's thesis that there are two “Orders”, the Explicate and Implicate 1 . The Explicate Order is the way in which our subjective sensory systems perceive the world 2 . In contrast, Bohm's Implicate Order would represent the objective reality beyond our perception. This view—that we have only a subjective understanding of reality—dates back to Galileo Galilei who, in 1623, criticized the Aristotelian concept of absolute and objective qualities of our sensory perceptions 3 and to Plato's cave allegory that reality is only what our senses allow us to see.
The only way for systematically overcoming the limits of our sensory apparatus and to get a glimpse of the Implicate Order is through the scientific method, through hypothesis‐testing, controlled experimentation. Beyond the methodology, controlling an experiment is critically important to ensure that the observed results are not just random events; they help scientists to distinguish between the “signal” and the background “noise” that are inherent in natural and living systems. For example, the detection method for the recent discovery of gravitational waves used four‐dimensional reference points to factor out the background noise of the Cosmos. Controls also help to account for errors and variability in the experimental setup and measuring tools: The negative control of an enzyme assay, for instance, tests for any unrelated background signals from the assay or measurement. In short, controls are essential for the unbiased, objective observation and measurement of the dependent variable in response to the experimental setup.
The only way for systematically overcoming the limits of our sensory apparatus […] is through the Scientific Method, through hypothesis‐testing, controlled experimentation.
Nominally, both positive and negative controls are material and procedural; that is, they control for variability of the experimental materials and the procedure itself. But beyond the practical issues to avoid procedural and material artifacts, there is an underlying philosophical question. The need for experimental controls is a subliminal recognition of the relative and subjective nature of the Explicate Order. It requires controls as “reference points” in order to transcend it, and to approximate the Implicate Order.
This is similar to Peter Rowlands’ 4 dictum that everything in the Universe adds up to zero, the universal attractor in mathematics. Prior to the introduction of zero, mathematics lacked an absolute reference point similar to a negative or positive control in an experiment. The same is true of biology, where the cell is the reference point owing to its negative entropy: It appears as an attractor for the energy of its environment. Hence, there is a need for careful controls in biology: The homeostatic balance that is inherent to life varies during the course of an experiment and therefore must be precisely controlled to distinguish noise from signal and approximate the Implicate Order of life.
P < 0.05 tacitly acknowledges the explicate order
Another example of the “subjectivity” of our perception is the level of accuracy we accept for differences between groups. For example, when we use statistical methods to determine if an observed difference between control and experimental groups is a random occurrence or a specific effect, we conventionally consider a p value of less than or equal to 5% as statistically significant; that is, there is a less than 0.05 probability that the effect is random. The efficacy of this arbitrary convention has been debated for decades; suffice to say that despite questioning the validity of that convention, a P value of < 0.05 reflects our acceptance of the subjectivity of our perception of reality.
… controls are essential for the unbiased, objective observation and measurement of the dependent variable in response to the experimental setup.
Thus, if we do away with hypothesis‐testing science in favor of informatics based on data and statistics—referring to Anderson's suggestion—it reflects our acceptance of the noise in the system. However, mere data analysis without any underlying hypothesis is tantamount to “garbage in‐garbage out”, in contrast to well‐controlled imaginative experiments to separate the wheat from the chaff. Albert Einstein was quoted as saying that imagination was more important than knowledge.
The ultimate purpose of the scientific method is to understand ourselves and our place in Nature. Conventionally, we subscribe to the Anthropic Principle, that we are “in” this Universe, whereas the Endosymbiosis Theory, advocated by Lynn Margulis, stipulates that we are “of” this Universe as a result of the assimilation of the physical environment. According to this theory, the organism endogenizes external factors to make them physiologically “useful”, such as iron as the core of the hemoglobin molecule, or ancient bacteria as mitochondria.
… there is a fundamental difference between knowing via believing and knowing based on empirical research.
By applying the developmental mechanism of cell–cell communication to phylogeny, we have revealed the interrelationships between cells and explained evolution from its origin as the unicellular state to multicellularity via cell–cell communication. The ultimate outcome of this research is that consciousness is the product of cellular processes and cell–cell communication in order to react to the environment and better anticipate future events 5 , 6 . Consciousness is an essential prerequisite for transcending the Explicate Order toward the Implicate Order via cellular sensory and cognitive systems that feed an ever‐expanding organismal knowledge about both the environment and itself.
It is here where the empirical approach to understanding nature comes in with its emphasis that knowledge comes only from sensual experience rather than innate ideas or traditions. In the context of the cell or higher systems, knowledge about the environment can only be gained by sensing and analyzing the environment. Empiricism is similar to an equation in which the variables and terms form a product, or a chemical reaction, or a biological process where the substrates, aka sensory data, form products, that is, knowledge. However, it requires another step—imagination, according to Albert Einstein—to transcend the Explicate Order in order to gain insight into the Implicate Order. Take for instance, Dmitri Ivanovich Mendeleev's Periodic Table of Elements: his brilliant insight was not just to use Atomic Number to organize it, but also to consider the chemical reactivities of the Elements by sorting them into columns. By introducing chemical reactivity to the Periodic Table, Mendeleev provided something like the “fourth wall” in Drama, which gives the audience an omniscient, god‐like perspective on what is happening on stage.
The capacity to transcend the subjective Explicate Order to approximate the objective Implicate Order is not unlike Eastern philosophies like Buddhism or Taoism, which were practiced long before the scientific method. An Indian philosopher once pointed out that the Hindus have known for 30,000 years that the Earth revolves around the sun, while the Europeans only realized this a few hundred years ago based on the work of Copernicus, Brahe, and Galileo. However, there is a fundamental difference between knowing via believing and knowing based on empirical research. A similar example is Aristotle's refusal to test whether a large stone would fall faster than a small one, as he knew the answer already 7 . Galileo eventually performed the experiment from the Leaning Tower in Pisa to demonstrate that the fall time of two objects is independent of their mass—which disproved Aristotle's theory of gravity that stipulated that objects fall at a speed proportional to their mass. Again, it demonstrates the power of empiricism and experimentation as formulated by Francis Bacon, John Locke, and others, over intuition and rationalizing.
Even if our scientific instruments provide us with objective data, we still need to apply our consciousness to evaluate and interpret such data.
Following the evolution from the unicellular state to multicellular organisms—and reverse‐engineering it to a minimal‐cell state—reveals that biologic diversity is an artifact of the Explicate Order. Indeed, the unicell seems to be the primary level of selection in the Implicate Order, as it remains proximate to the First Principles of Physiology, namely negative entropy (negentropy), chemiosmosis, and homeostasis. The first two principles are necessary for growth and proliferation, whereas the last reflects Newton's Third Law of Motion that every action has an equal and opposite reaction so as to maintain homeostasis.
All organisms interact with their surroundings and assimilate their experience as epigenetic marks. Such marks extend to the DNA of germ cells and thus change the phenotypic expression of the offspring. The offspring, in turn, interacts with the environment in response to such epigenetic modifications, giving rise to the concept of the phenotype as an agent that actively and purposefully interacts with its environment in order to adapt and survive. This concept of phenotype based on agency linked to the Explicate Order fundamentally differs from its conventional description as a mere set of biologic characteristics. Organisms’ capacities to anticipate future stress situations from past memories are obvious in simple animals such as nematodes, as well as in plants and bacteria 8 , suggesting that the subjective Explicate Order controls both organismal behavior and trans‐generational evolution.
That perspective offers insight to the nature of consciousness: not as a “mind” that is separate from a “body”, but as an endogenization of physical matter, which complies with the Laws of Nature. In other words, consciousness is the physiologic manifestation of endogenized physical surroundings, compartmentalized, and made essential for all organisms by forming the basis for their physiology. Endocytosis and endocytic/synaptic vesicles contribute to endogenization of cellular surroundings, allowing eukaryotic organisms to gain knowledge about the environment. This is true not only for neurons in brains, but also for all eukaryotic cells 5 .
Such a view of consciousness offers insight to our awareness of our physical surroundings as the basis for self‐referential self‐organization. But this is predicated on our capacity to “experiment” with our environment. The burgeoning idea that we are entering the Anthropocene, a man‐made world founded on subjective senses instead of Natural Laws, is a dangerous step away from our innate evolutionary arc. Relying on just our senses and emotions, without experimentation and controls to understand the Implicate Order behind reality, is not just an abandonment of the principles of the Enlightenment, but also endangers the planet and its diversity of life.
Further reading
Anderson C (2008) The End of Theory: the data deluge makes the scientific method obsolete. Wired (December 23, 2008)
Bacon F (1620, 2011) Novum Organum Scientiarum. Nabu Press
Baluška F, Gagliano M, Witzany G (2018) Memory and Learning in Plants. Springer Nature
Charlesworth AG, Seroussi U, Claycomb JM (2019) Next‐Gen learning: the C. elegans approach. Cell 177: 1674–1676
Eliezer Y, Deshe N, Hoch L, Iwanir S, Pritz CO, Zaslaver A (2019) A memory circuit for coping with impending adversity. Curr Biol 29: 1573–1583
Gagliano M, Renton M, Depczynski M, Mancuso S (2014) Experience teaches plants to learn faster and forget slower in environments where it matters. Oecologia 175: 63–72
Gagliano M, Vyazovskiy VV, Borbély AA, Grimonprez M, Depczynski M (2016) Learning by association in plants. Sci Rep 6: 38427
Katz M, Shaham S (2019) Learning and memory: mind over matter in C. elegans . Curr Biol 29: R365‐R367
Kováč L (2007) Information and knowledge in biology – time for reappraisal. Plant Signal Behav 2: 65–73
Kováč L (2008) Bioenergetics – a key to brain and mind. Commun Integr Biol 1: 114–122
Koshland DE Jr (1980) Bacterial chemotaxis in relation to neurobiology. Annu Rev Neurosci 3: 43–75
Lyon P (2015) The cognitive cell: bacterial behavior reconsidered. Front Microbiol 6: 264
Margulis L (2001) The conscious cell. Ann NY Acad Sci 929: 55–70
Maximillian N (2018) The Metaphysics of Science and Aim‐Oriented Empiricism. Springer: New York
Mazzocchi F (2015) Could Big Data be the end of theory in science? EMBO Rep 16: 1250–1255
Moore RS, Kaletsky R, Murphy CT (2019) Piwi/PRG‐1 argonaute and TGF‐β mediate transgenerational learned pathogenic avoidance. Cell 177: 1827–1841
Peirce CS (1877) The Fixation of Belief. Popular Science Monthly 12: 1–15
Pigliucci M (2009) The end of theory in science? EMBO Rep 10: 534
Popper K (1959) The Logic of Scientific Discovery. Routledge: London
Posner R, Toker IA, Antonova O, Star E, Anava S, Azmon E, Hendricks M, Bracha S, Gingold H, Rechavi O (2019) Neuronal small RNAs control behavior transgenerationally. Cell 177: 1814–1826
Russell B (1912) The Problems of Philosophy. Henry Holt and Company: New York
Scerri E (2006) The Periodic Table: It's Story and Significance. Oxford University Press, Oxford
Shapiro JA (2007) Bacteria are small but not stupid: cognition, natural genetic engineering and socio‐bacteriology. Stud Hist Philos Biol Biomed Sci 38: 807–818
Torday JS, Miller WB Jr (2016) Biologic relativity: who is the observer and what is observed? Prog Biophys Mol Biol 121: 29–34
Torday JS, Rehan VK (2017) Evolution, the Logic of Biology. Wiley: Hoboken
Torday JS, Miller WB Jr (2016) Phenotype as agent for epigenetic inheritance. Biology (Basel) 5: 30
Wasserstein RL, Lazar NA (2016) The ASA's statement on p‐values: context, process and purpose. Am Statist 70: 129–133
Yamada T, Yang Y, Valnegri P, Juric I, Abnousi A, Markwalter KH, Guthrie AN, Godec A, Oldenborg A, Hu M, Holy TE, Bonni A (2019) Sensory experience remodels genome architecture in neural circuit to drive motor learning. Nature 569: 708–713
Ladislav Kováč discussed the advantages and drawbacks of the inductive method for science and the logic of scientific discoveries 9 . Obviously, technological advances have enabled scientists to expand the borders of knowledge, and informatics allows us to objectively analyze ever larger data‐sets. It was the telescope that enabled Tycho Brahe, Johannes Kepler, and Galileo Galilei to make accurate observations and infer the motion of the planets. The microscope provided Robert Koch and Louis Pasteur insights into the microbial world and determines the nature of infectious diseases. Particle colliders now give us a glimpse into the birth of the Universe, while DNA sequencing and bioinformatics have enormously advanced biology's goal to understand the molecular basis of life.
However, Kováč also reminds us that Bayesian inferences and reasoning have serious drawbacks, as documented in the instructive example of Bertrand Russell's “inductivist turkey”, which collected large amounts of reproducible data each morning about feeding time. Based on these observations, the turkey correctly predicted the feeding time for the next morning—until Christmas Eve when the turkey's throat was cut 9 . In order to avoid the fate of the “inductivist turkey”, mankind should also rely on Popperian deductive science, namely formulating theories, concepts, and hypotheses, which are either confirmed or refuted via stringent experimentation and proper controls. Even if our scientific instruments provide us with objective data, we still need to apply our consciousness to evaluate and interpret such data. Moreover, before we start using our scientific instruments, we need to pose scientific questions. Therefore, as suggested by Albert Szent‐Györgyi, we need both Dionysian and Apollonian types of scientists 10 . Unfortunately, as was the case in Szent‐Györgyi's times, the Dionysians are still struggling to get proper support.
There have been pleas for reconciling philosophy and science, which parted ways owing to the rise of empiricism. This essay recognizes the centrality experiments and their controls for the advancement of scientific thought, and the attendant advance in philosophy needed to cope with many extant and emerging issues in science and society. We need a common “will” to do so. The rationale is provided herein, if only.

Acknowledgements
John Torday has been a recipient of NIH Grant HL055268. František Baluška is thankful to numerous colleagues for very stimulating discussions on topics analyzed in this article.
EMBO Reports (2019) 20 : e49110 [ PMC free article ] [ PubMed ] [ Google Scholar ]
Contributor Information
John S Torday, Email: ude.alcu@yadrotj .
František Baluška, Email: ed.nnob-inu@aksulab .
What are Controlled Experiments?
Determining Cause and Effect
skynesher / Getty Images
- Research, Samples, and Statistics
- Key Concepts
- Major Sociologists
- News & Issues
- Recommended Reading
- Archaeology
A controlled experiment is a highly focused way of collecting data and is especially useful for determining patterns of cause and effect. This type of experiment is used in a wide variety of fields, including medical, psychological, and sociological research. Below, we’ll define what controlled experiments are and provide some examples.
Key Takeaways: Controlled Experiments
- A controlled experiment is a research study in which participants are randomly assigned to experimental and control groups.
- A controlled experiment allows researchers to determine cause and effect between variables.
- One drawback of controlled experiments is that they lack external validity (which means their results may not generalize to real-world settings).
Experimental and Control Groups
To conduct a controlled experiment , two groups are needed: an experimental group and a control group . The experimental group is a group of individuals that are exposed to the factor being examined. The control group, on the other hand, is not exposed to the factor. It is imperative that all other external influences are held constant . That is, every other factor or influence in the situation needs to remain exactly the same between the experimental group and the control group. The only thing that is different between the two groups is the factor being researched.
For example, if you were studying the effects of taking naps on test performance, you could assign participants to two groups: participants in one group would be asked to take a nap before their test, and those in the other group would be asked to stay awake. You would want to ensure that everything else about the groups (the demeanor of the study staff, the environment of the testing room, etc.) would be equivalent for each group. Researchers can also develop more complex study designs with more than two groups. For example, they might compare test performance among participants who had a 2-hour nap, participants who had a 20-minute nap, and participants who didn’t nap.
Assigning Participants to Groups
In controlled experiments, researchers use random assignment (i.e. participants are randomly assigned to be in the experimental group or the control group) in order to minimize potential confounding variables in the study. For example, imagine a study of a new drug in which all of the female participants were assigned to the experimental group and all of the male participants were assigned to the control group. In this case, the researchers couldn’t be sure if the study results were due to the drug being effective or due to gender—in this case, gender would be a confounding variable.
Random assignment is done in order to ensure that participants are not assigned to experimental groups in a way that could bias the study results. A study that compares two groups but does not randomly assign participants to the groups is referred to as quasi-experimental, rather than a true experiment.
Blind and Double-Blind Studies
In a blind experiment, participants don’t know whether they are in the experimental or control group. For example, in a study of a new experimental drug, participants in the control group may be given a pill (known as a placebo ) that has no active ingredients but looks just like the experimental drug. In a double-blind study , neither the participants nor the experimenter knows which group the participant is in (instead, someone else on the research staff is responsible for keeping track of group assignments). Double-blind studies prevent the researcher from inadvertently introducing sources of bias into the data collected.
Example of a Controlled Experiment
If you were interested in studying whether or not violent television programming causes aggressive behavior in children, you could conduct a controlled experiment to investigate. In such a study, the dependent variable would be the children’s behavior, while the independent variable would be exposure to violent programming. To conduct the experiment, you would expose an experimental group of children to a movie containing a lot of violence, such as martial arts or gun fighting. The control group, on the other hand, would watch a movie that contained no violence.
To test the aggressiveness of the children, you would take two measurements : one pre-test measurement made before the movies are shown, and one post-test measurement made after the movies are watched. Pre-test and post-test measurements should be taken of both the control group and the experimental group. You would then use statistical techniques to determine whether the experimental group showed a significantly greater increase in aggression, compared to participants in the control group.
Studies of this sort have been done many times and they usually find that children who watch a violent movie are more aggressive afterward than those who watch a movie containing no violence.
Strengths and Weaknesses
Controlled experiments have both strengths and weaknesses. Among the strengths is the fact that results can establish causation. That is, they can determine cause and effect between variables. In the above example, one could conclude that being exposed to representations of violence causes an increase in aggressive behavior. This kind of experiment can also zero-in on a single independent variable, since all other factors in the experiment are held constant.
On the downside, controlled experiments can be artificial. That is, they are done, for the most part, in a manufactured laboratory setting and therefore tend to eliminate many real-life effects. As a result, analysis of a controlled experiment must include judgments about how much the artificial setting has affected the results. Results from the example given might be different if, say, the children studied had a conversation about the violence they watched with a respected adult authority figure, like a parent or teacher, before their behavior was measured. Because of this, controlled experiments can sometimes have lower external validity (that is, their results might not generalize to real-world settings).
Updated by Nicki Lisa Cole, Ph.D.
- An Overview of Qualitative Research Methods
- Using Ethnomethodology to Understand Social Order
- Pros and Cons of Secondary Data Analysis
- Immersion Definition: Cultural, Language, and Virtual
- Sociology Explains Why Some People Cheat on Their Spouses
- What Is Participant Observation Research?
- The Differences Between Indexes and Scales
- Definition and Overview of Grounded Theory
- Deductive Versus Inductive Reasoning
- The Study of Cultural Artifacts via Content Analysis
- Units of Analysis as Related to Sociology
- Data Sources For Sociological Research
- Full-Text Sociology Journals Online
- How Race and Gender Biases Impact Students in Higher Ed
- The Racial Wealth Gap
- A Review of Software Tools for Quantitative Data Analysis

- Activities, Experiments, Online Games, Visual Aids
- Activities, Experiments, and Investigations
- Experimental Design and the Scientific Method
Experimental Design - Independent, Dependent, and Controlled Variables
To view these resources with no ads, please login or subscribe to help support our content development. school subscriptions can access more than 175 downloadable unit bundles in our store for free (a value of $1,500). district subscriptions provide huge group discounts for their schools. email for a quote: [email protected] ..
Scientific experiments are meant to show cause and effect of a phenomena (relationships in nature). The “ variables ” are any factor, trait, or condition that can be changed in the experiment and that can have an effect on the outcome of the experiment.
An experiment can have three kinds of variables: i ndependent, dependent, and controlled .
- The independent variable is one single factor that is changed by the scientist followed by observation to watch for changes. It is important that there is just one independent variable, so that results are not confusing.
- The dependent variable is the factor that changes as a result of the change to the independent variable.
- The controlled variables (or constant variables) are factors that the scientist wants to remain constant if the experiment is to show accurate results. To be able to measure results, each of the variables must be able to be measured.
For example, let’s design an experiment with two plants sitting in the sun side by side. The controlled variables (or constants) are that at the beginning of the experiment, the plants are the same size, get the same amount of sunlight, experience the same ambient temperature and are in the same amount and consistency of soil (the weight of the soil and container should be measured before the plants are added). The independent variable is that one plant is getting watered (1 cup of water) every day and one plant is getting watered (1 cup of water) once a week. The dependent variables are the changes in the two plants that the scientist observes over time.
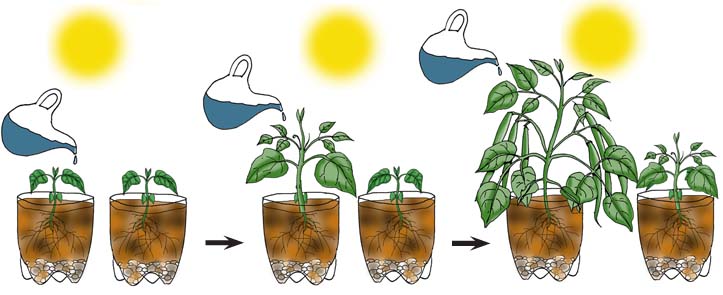
Can you describe the dependent variable that may result from this experiment? After four weeks, the dependent variable may be that one plant is taller, heavier and more developed than the other. These results can be recorded and graphed by measuring and comparing both plants’ height, weight (removing the weight of the soil and container recorded beforehand) and a comparison of observable foliage.
Using What You Learned: Design another experiment using the two plants, but change the independent variable. Can you describe the dependent variable that may result from this new experiment?
Think of another simple experiment and name the independent, dependent, and controlled variables. Use the graphic organizer included in the PDF below to organize your experiment's variables.
Please Login or Subscribe to access downloadable content.
Citing Research References
When you research information you must cite the reference. Citing for websites is different from citing from books, magazines and periodicals. The style of citing shown here is from the MLA Style Citations (Modern Language Association).
When citing a WEBSITE the general format is as follows. Author Last Name, First Name(s). "Title: Subtitle of Part of Web Page, if appropriate." Title: Subtitle: Section of Page if appropriate. Sponsoring/Publishing Agency, If Given. Additional significant descriptive information. Date of Electronic Publication or other Date, such as Last Updated. Day Month Year of access < URL >.
Here is an example of citing this page:
Amsel, Sheri. "Experimental Design - Independent, Dependent, and Controlled Variables" Exploring Nature Educational Resource ©2005-2024. March 25, 2024 < http://www.exploringnature.org/db/view/Experimental-Design-Independent-Dependent-and-Controlled-Variables >
Exploringnature.org has more than 2,000 illustrated animals. Read about them, color them, label them, learn to draw them.

Frequently asked questions
What is the difference between a control group and an experimental group.
An experimental group, also known as a treatment group, receives the treatment whose effect researchers wish to study, whereas a control group does not. They should be identical in all other ways.
Frequently asked questions: Methodology
Attrition refers to participants leaving a study. It always happens to some extent—for example, in randomized controlled trials for medical research.
Differential attrition occurs when attrition or dropout rates differ systematically between the intervention and the control group . As a result, the characteristics of the participants who drop out differ from the characteristics of those who stay in the study. Because of this, study results may be biased .
Action research is conducted in order to solve a particular issue immediately, while case studies are often conducted over a longer period of time and focus more on observing and analyzing a particular ongoing phenomenon.
Action research is focused on solving a problem or informing individual and community-based knowledge in a way that impacts teaching, learning, and other related processes. It is less focused on contributing theoretical input, instead producing actionable input.
Action research is particularly popular with educators as a form of systematic inquiry because it prioritizes reflection and bridges the gap between theory and practice. Educators are able to simultaneously investigate an issue as they solve it, and the method is very iterative and flexible.
A cycle of inquiry is another name for action research . It is usually visualized in a spiral shape following a series of steps, such as “planning → acting → observing → reflecting.”
To make quantitative observations , you need to use instruments that are capable of measuring the quantity you want to observe. For example, you might use a ruler to measure the length of an object or a thermometer to measure its temperature.
Criterion validity and construct validity are both types of measurement validity . In other words, they both show you how accurately a method measures something.
While construct validity is the degree to which a test or other measurement method measures what it claims to measure, criterion validity is the degree to which a test can predictively (in the future) or concurrently (in the present) measure something.
Construct validity is often considered the overarching type of measurement validity . You need to have face validity , content validity , and criterion validity in order to achieve construct validity.
Convergent validity and discriminant validity are both subtypes of construct validity . Together, they help you evaluate whether a test measures the concept it was designed to measure.
- Convergent validity indicates whether a test that is designed to measure a particular construct correlates with other tests that assess the same or similar construct.
- Discriminant validity indicates whether two tests that should not be highly related to each other are indeed not related. This type of validity is also called divergent validity .
You need to assess both in order to demonstrate construct validity. Neither one alone is sufficient for establishing construct validity.
- Discriminant validity indicates whether two tests that should not be highly related to each other are indeed not related
Content validity shows you how accurately a test or other measurement method taps into the various aspects of the specific construct you are researching.
In other words, it helps you answer the question: “does the test measure all aspects of the construct I want to measure?” If it does, then the test has high content validity.
The higher the content validity, the more accurate the measurement of the construct.
If the test fails to include parts of the construct, or irrelevant parts are included, the validity of the instrument is threatened, which brings your results into question.
Face validity and content validity are similar in that they both evaluate how suitable the content of a test is. The difference is that face validity is subjective, and assesses content at surface level.
When a test has strong face validity, anyone would agree that the test’s questions appear to measure what they are intended to measure.
For example, looking at a 4th grade math test consisting of problems in which students have to add and multiply, most people would agree that it has strong face validity (i.e., it looks like a math test).
On the other hand, content validity evaluates how well a test represents all the aspects of a topic. Assessing content validity is more systematic and relies on expert evaluation. of each question, analyzing whether each one covers the aspects that the test was designed to cover.
A 4th grade math test would have high content validity if it covered all the skills taught in that grade. Experts(in this case, math teachers), would have to evaluate the content validity by comparing the test to the learning objectives.
Snowball sampling is a non-probability sampling method . Unlike probability sampling (which involves some form of random selection ), the initial individuals selected to be studied are the ones who recruit new participants.
Because not every member of the target population has an equal chance of being recruited into the sample, selection in snowball sampling is non-random.
Snowball sampling is a non-probability sampling method , where there is not an equal chance for every member of the population to be included in the sample .
This means that you cannot use inferential statistics and make generalizations —often the goal of quantitative research . As such, a snowball sample is not representative of the target population and is usually a better fit for qualitative research .
Snowball sampling relies on the use of referrals. Here, the researcher recruits one or more initial participants, who then recruit the next ones.
Participants share similar characteristics and/or know each other. Because of this, not every member of the population has an equal chance of being included in the sample, giving rise to sampling bias .
Snowball sampling is best used in the following cases:
- If there is no sampling frame available (e.g., people with a rare disease)
- If the population of interest is hard to access or locate (e.g., people experiencing homelessness)
- If the research focuses on a sensitive topic (e.g., extramarital affairs)
The reproducibility and replicability of a study can be ensured by writing a transparent, detailed method section and using clear, unambiguous language.
Reproducibility and replicability are related terms.
- Reproducing research entails reanalyzing the existing data in the same manner.
- Replicating (or repeating ) the research entails reconducting the entire analysis, including the collection of new data .
- A successful reproduction shows that the data analyses were conducted in a fair and honest manner.
- A successful replication shows that the reliability of the results is high.
Stratified sampling and quota sampling both involve dividing the population into subgroups and selecting units from each subgroup. The purpose in both cases is to select a representative sample and/or to allow comparisons between subgroups.
The main difference is that in stratified sampling, you draw a random sample from each subgroup ( probability sampling ). In quota sampling you select a predetermined number or proportion of units, in a non-random manner ( non-probability sampling ).
Purposive and convenience sampling are both sampling methods that are typically used in qualitative data collection.
A convenience sample is drawn from a source that is conveniently accessible to the researcher. Convenience sampling does not distinguish characteristics among the participants. On the other hand, purposive sampling focuses on selecting participants possessing characteristics associated with the research study.
The findings of studies based on either convenience or purposive sampling can only be generalized to the (sub)population from which the sample is drawn, and not to the entire population.
Random sampling or probability sampling is based on random selection. This means that each unit has an equal chance (i.e., equal probability) of being included in the sample.
On the other hand, convenience sampling involves stopping people at random, which means that not everyone has an equal chance of being selected depending on the place, time, or day you are collecting your data.
Convenience sampling and quota sampling are both non-probability sampling methods. They both use non-random criteria like availability, geographical proximity, or expert knowledge to recruit study participants.
However, in convenience sampling, you continue to sample units or cases until you reach the required sample size.
In quota sampling, you first need to divide your population of interest into subgroups (strata) and estimate their proportions (quota) in the population. Then you can start your data collection, using convenience sampling to recruit participants, until the proportions in each subgroup coincide with the estimated proportions in the population.
A sampling frame is a list of every member in the entire population . It is important that the sampling frame is as complete as possible, so that your sample accurately reflects your population.
Stratified and cluster sampling may look similar, but bear in mind that groups created in cluster sampling are heterogeneous , so the individual characteristics in the cluster vary. In contrast, groups created in stratified sampling are homogeneous , as units share characteristics.
Relatedly, in cluster sampling you randomly select entire groups and include all units of each group in your sample. However, in stratified sampling, you select some units of all groups and include them in your sample. In this way, both methods can ensure that your sample is representative of the target population .
A systematic review is secondary research because it uses existing research. You don’t collect new data yourself.
The key difference between observational studies and experimental designs is that a well-done observational study does not influence the responses of participants, while experiments do have some sort of treatment condition applied to at least some participants by random assignment .
An observational study is a great choice for you if your research question is based purely on observations. If there are ethical, logistical, or practical concerns that prevent you from conducting a traditional experiment , an observational study may be a good choice. In an observational study, there is no interference or manipulation of the research subjects, as well as no control or treatment groups .
It’s often best to ask a variety of people to review your measurements. You can ask experts, such as other researchers, or laypeople, such as potential participants, to judge the face validity of tests.
While experts have a deep understanding of research methods , the people you’re studying can provide you with valuable insights you may have missed otherwise.
Face validity is important because it’s a simple first step to measuring the overall validity of a test or technique. It’s a relatively intuitive, quick, and easy way to start checking whether a new measure seems useful at first glance.
Good face validity means that anyone who reviews your measure says that it seems to be measuring what it’s supposed to. With poor face validity, someone reviewing your measure may be left confused about what you’re measuring and why you’re using this method.
Face validity is about whether a test appears to measure what it’s supposed to measure. This type of validity is concerned with whether a measure seems relevant and appropriate for what it’s assessing only on the surface.
Statistical analyses are often applied to test validity with data from your measures. You test convergent validity and discriminant validity with correlations to see if results from your test are positively or negatively related to those of other established tests.
You can also use regression analyses to assess whether your measure is actually predictive of outcomes that you expect it to predict theoretically. A regression analysis that supports your expectations strengthens your claim of construct validity .
When designing or evaluating a measure, construct validity helps you ensure you’re actually measuring the construct you’re interested in. If you don’t have construct validity, you may inadvertently measure unrelated or distinct constructs and lose precision in your research.
Construct validity is often considered the overarching type of measurement validity , because it covers all of the other types. You need to have face validity , content validity , and criterion validity to achieve construct validity.
Construct validity is about how well a test measures the concept it was designed to evaluate. It’s one of four types of measurement validity , which includes construct validity, face validity , and criterion validity.
There are two subtypes of construct validity.
- Convergent validity : The extent to which your measure corresponds to measures of related constructs
- Discriminant validity : The extent to which your measure is unrelated or negatively related to measures of distinct constructs
Naturalistic observation is a valuable tool because of its flexibility, external validity , and suitability for topics that can’t be studied in a lab setting.
The downsides of naturalistic observation include its lack of scientific control , ethical considerations , and potential for bias from observers and subjects.
Naturalistic observation is a qualitative research method where you record the behaviors of your research subjects in real world settings. You avoid interfering or influencing anything in a naturalistic observation.
You can think of naturalistic observation as “people watching” with a purpose.
A dependent variable is what changes as a result of the independent variable manipulation in experiments . It’s what you’re interested in measuring, and it “depends” on your independent variable.
In statistics, dependent variables are also called:
- Response variables (they respond to a change in another variable)
- Outcome variables (they represent the outcome you want to measure)
- Left-hand-side variables (they appear on the left-hand side of a regression equation)
An independent variable is the variable you manipulate, control, or vary in an experimental study to explore its effects. It’s called “independent” because it’s not influenced by any other variables in the study.
Independent variables are also called:
- Explanatory variables (they explain an event or outcome)
- Predictor variables (they can be used to predict the value of a dependent variable)
- Right-hand-side variables (they appear on the right-hand side of a regression equation).
As a rule of thumb, questions related to thoughts, beliefs, and feelings work well in focus groups. Take your time formulating strong questions, paying special attention to phrasing. Be careful to avoid leading questions , which can bias your responses.
Overall, your focus group questions should be:
- Open-ended and flexible
- Impossible to answer with “yes” or “no” (questions that start with “why” or “how” are often best)
- Unambiguous, getting straight to the point while still stimulating discussion
- Unbiased and neutral
A structured interview is a data collection method that relies on asking questions in a set order to collect data on a topic. They are often quantitative in nature. Structured interviews are best used when:
- You already have a very clear understanding of your topic. Perhaps significant research has already been conducted, or you have done some prior research yourself, but you already possess a baseline for designing strong structured questions.
- You are constrained in terms of time or resources and need to analyze your data quickly and efficiently.
- Your research question depends on strong parity between participants, with environmental conditions held constant.
More flexible interview options include semi-structured interviews , unstructured interviews , and focus groups .
Social desirability bias is the tendency for interview participants to give responses that will be viewed favorably by the interviewer or other participants. It occurs in all types of interviews and surveys , but is most common in semi-structured interviews , unstructured interviews , and focus groups .
Social desirability bias can be mitigated by ensuring participants feel at ease and comfortable sharing their views. Make sure to pay attention to your own body language and any physical or verbal cues, such as nodding or widening your eyes.
This type of bias can also occur in observations if the participants know they’re being observed. They might alter their behavior accordingly.
The interviewer effect is a type of bias that emerges when a characteristic of an interviewer (race, age, gender identity, etc.) influences the responses given by the interviewee.
There is a risk of an interviewer effect in all types of interviews , but it can be mitigated by writing really high-quality interview questions.
A semi-structured interview is a blend of structured and unstructured types of interviews. Semi-structured interviews are best used when:
- You have prior interview experience. Spontaneous questions are deceptively challenging, and it’s easy to accidentally ask a leading question or make a participant uncomfortable.
- Your research question is exploratory in nature. Participant answers can guide future research questions and help you develop a more robust knowledge base for future research.
An unstructured interview is the most flexible type of interview, but it is not always the best fit for your research topic.
Unstructured interviews are best used when:
- You are an experienced interviewer and have a very strong background in your research topic, since it is challenging to ask spontaneous, colloquial questions.
- Your research question is exploratory in nature. While you may have developed hypotheses, you are open to discovering new or shifting viewpoints through the interview process.
- You are seeking descriptive data, and are ready to ask questions that will deepen and contextualize your initial thoughts and hypotheses.
- Your research depends on forming connections with your participants and making them feel comfortable revealing deeper emotions, lived experiences, or thoughts.
The four most common types of interviews are:
- Structured interviews : The questions are predetermined in both topic and order.
- Semi-structured interviews : A few questions are predetermined, but other questions aren’t planned.
- Unstructured interviews : None of the questions are predetermined.
- Focus group interviews : The questions are presented to a group instead of one individual.
Deductive reasoning is commonly used in scientific research, and it’s especially associated with quantitative research .
In research, you might have come across something called the hypothetico-deductive method . It’s the scientific method of testing hypotheses to check whether your predictions are substantiated by real-world data.
Deductive reasoning is a logical approach where you progress from general ideas to specific conclusions. It’s often contrasted with inductive reasoning , where you start with specific observations and form general conclusions.
Deductive reasoning is also called deductive logic.
There are many different types of inductive reasoning that people use formally or informally.
Here are a few common types:
- Inductive generalization : You use observations about a sample to come to a conclusion about the population it came from.
- Statistical generalization: You use specific numbers about samples to make statements about populations.
- Causal reasoning: You make cause-and-effect links between different things.
- Sign reasoning: You make a conclusion about a correlational relationship between different things.
- Analogical reasoning: You make a conclusion about something based on its similarities to something else.
Inductive reasoning is a bottom-up approach, while deductive reasoning is top-down.
Inductive reasoning takes you from the specific to the general, while in deductive reasoning, you make inferences by going from general premises to specific conclusions.
In inductive research , you start by making observations or gathering data. Then, you take a broad scan of your data and search for patterns. Finally, you make general conclusions that you might incorporate into theories.
Inductive reasoning is a method of drawing conclusions by going from the specific to the general. It’s usually contrasted with deductive reasoning, where you proceed from general information to specific conclusions.
Inductive reasoning is also called inductive logic or bottom-up reasoning.
A hypothesis states your predictions about what your research will find. It is a tentative answer to your research question that has not yet been tested. For some research projects, you might have to write several hypotheses that address different aspects of your research question.
A hypothesis is not just a guess — it should be based on existing theories and knowledge. It also has to be testable, which means you can support or refute it through scientific research methods (such as experiments, observations and statistical analysis of data).
Triangulation can help:
- Reduce research bias that comes from using a single method, theory, or investigator
- Enhance validity by approaching the same topic with different tools
- Establish credibility by giving you a complete picture of the research problem
But triangulation can also pose problems:
- It’s time-consuming and labor-intensive, often involving an interdisciplinary team.
- Your results may be inconsistent or even contradictory.
There are four main types of triangulation :
- Data triangulation : Using data from different times, spaces, and people
- Investigator triangulation : Involving multiple researchers in collecting or analyzing data
- Theory triangulation : Using varying theoretical perspectives in your research
- Methodological triangulation : Using different methodologies to approach the same topic
Many academic fields use peer review , largely to determine whether a manuscript is suitable for publication. Peer review enhances the credibility of the published manuscript.
However, peer review is also common in non-academic settings. The United Nations, the European Union, and many individual nations use peer review to evaluate grant applications. It is also widely used in medical and health-related fields as a teaching or quality-of-care measure.
Peer assessment is often used in the classroom as a pedagogical tool. Both receiving feedback and providing it are thought to enhance the learning process, helping students think critically and collaboratively.
Peer review can stop obviously problematic, falsified, or otherwise untrustworthy research from being published. It also represents an excellent opportunity to get feedback from renowned experts in your field. It acts as a first defense, helping you ensure your argument is clear and that there are no gaps, vague terms, or unanswered questions for readers who weren’t involved in the research process.
Peer-reviewed articles are considered a highly credible source due to this stringent process they go through before publication.
In general, the peer review process follows the following steps:
- First, the author submits the manuscript to the editor.
- Reject the manuscript and send it back to author, or
- Send it onward to the selected peer reviewer(s)
- Next, the peer review process occurs. The reviewer provides feedback, addressing any major or minor issues with the manuscript, and gives their advice regarding what edits should be made.
- Lastly, the edited manuscript is sent back to the author. They input the edits, and resubmit it to the editor for publication.
Exploratory research is often used when the issue you’re studying is new or when the data collection process is challenging for some reason.
You can use exploratory research if you have a general idea or a specific question that you want to study but there is no preexisting knowledge or paradigm with which to study it.
Exploratory research is a methodology approach that explores research questions that have not previously been studied in depth. It is often used when the issue you’re studying is new, or the data collection process is challenging in some way.
Explanatory research is used to investigate how or why a phenomenon occurs. Therefore, this type of research is often one of the first stages in the research process , serving as a jumping-off point for future research.
Exploratory research aims to explore the main aspects of an under-researched problem, while explanatory research aims to explain the causes and consequences of a well-defined problem.
Explanatory research is a research method used to investigate how or why something occurs when only a small amount of information is available pertaining to that topic. It can help you increase your understanding of a given topic.
Clean data are valid, accurate, complete, consistent, unique, and uniform. Dirty data include inconsistencies and errors.
Dirty data can come from any part of the research process, including poor research design , inappropriate measurement materials, or flawed data entry.
Data cleaning takes place between data collection and data analyses. But you can use some methods even before collecting data.
For clean data, you should start by designing measures that collect valid data. Data validation at the time of data entry or collection helps you minimize the amount of data cleaning you’ll need to do.
After data collection, you can use data standardization and data transformation to clean your data. You’ll also deal with any missing values, outliers, and duplicate values.
Every dataset requires different techniques to clean dirty data , but you need to address these issues in a systematic way. You focus on finding and resolving data points that don’t agree or fit with the rest of your dataset.
These data might be missing values, outliers, duplicate values, incorrectly formatted, or irrelevant. You’ll start with screening and diagnosing your data. Then, you’ll often standardize and accept or remove data to make your dataset consistent and valid.
Data cleaning is necessary for valid and appropriate analyses. Dirty data contain inconsistencies or errors , but cleaning your data helps you minimize or resolve these.
Without data cleaning, you could end up with a Type I or II error in your conclusion. These types of erroneous conclusions can be practically significant with important consequences, because they lead to misplaced investments or missed opportunities.
Data cleaning involves spotting and resolving potential data inconsistencies or errors to improve your data quality. An error is any value (e.g., recorded weight) that doesn’t reflect the true value (e.g., actual weight) of something that’s being measured.
In this process, you review, analyze, detect, modify, or remove “dirty” data to make your dataset “clean.” Data cleaning is also called data cleansing or data scrubbing.
Research misconduct means making up or falsifying data, manipulating data analyses, or misrepresenting results in research reports. It’s a form of academic fraud.
These actions are committed intentionally and can have serious consequences; research misconduct is not a simple mistake or a point of disagreement but a serious ethical failure.
Anonymity means you don’t know who the participants are, while confidentiality means you know who they are but remove identifying information from your research report. Both are important ethical considerations .
You can only guarantee anonymity by not collecting any personally identifying information—for example, names, phone numbers, email addresses, IP addresses, physical characteristics, photos, or videos.
You can keep data confidential by using aggregate information in your research report, so that you only refer to groups of participants rather than individuals.
Research ethics matter for scientific integrity, human rights and dignity, and collaboration between science and society. These principles make sure that participation in studies is voluntary, informed, and safe.
Ethical considerations in research are a set of principles that guide your research designs and practices. These principles include voluntary participation, informed consent, anonymity, confidentiality, potential for harm, and results communication.
Scientists and researchers must always adhere to a certain code of conduct when collecting data from others .
These considerations protect the rights of research participants, enhance research validity , and maintain scientific integrity.
In multistage sampling , you can use probability or non-probability sampling methods .
For a probability sample, you have to conduct probability sampling at every stage.
You can mix it up by using simple random sampling , systematic sampling , or stratified sampling to select units at different stages, depending on what is applicable and relevant to your study.
Multistage sampling can simplify data collection when you have large, geographically spread samples, and you can obtain a probability sample without a complete sampling frame.
But multistage sampling may not lead to a representative sample, and larger samples are needed for multistage samples to achieve the statistical properties of simple random samples .
These are four of the most common mixed methods designs :
- Convergent parallel: Quantitative and qualitative data are collected at the same time and analyzed separately. After both analyses are complete, compare your results to draw overall conclusions.
- Embedded: Quantitative and qualitative data are collected at the same time, but within a larger quantitative or qualitative design. One type of data is secondary to the other.
- Explanatory sequential: Quantitative data is collected and analyzed first, followed by qualitative data. You can use this design if you think your qualitative data will explain and contextualize your quantitative findings.
- Exploratory sequential: Qualitative data is collected and analyzed first, followed by quantitative data. You can use this design if you think the quantitative data will confirm or validate your qualitative findings.
Triangulation in research means using multiple datasets, methods, theories and/or investigators to address a research question. It’s a research strategy that can help you enhance the validity and credibility of your findings.
Triangulation is mainly used in qualitative research , but it’s also commonly applied in quantitative research . Mixed methods research always uses triangulation.
In multistage sampling , or multistage cluster sampling, you draw a sample from a population using smaller and smaller groups at each stage.
This method is often used to collect data from a large, geographically spread group of people in national surveys, for example. You take advantage of hierarchical groupings (e.g., from state to city to neighborhood) to create a sample that’s less expensive and time-consuming to collect data from.
No, the steepness or slope of the line isn’t related to the correlation coefficient value. The correlation coefficient only tells you how closely your data fit on a line, so two datasets with the same correlation coefficient can have very different slopes.
To find the slope of the line, you’ll need to perform a regression analysis .
Correlation coefficients always range between -1 and 1.
The sign of the coefficient tells you the direction of the relationship: a positive value means the variables change together in the same direction, while a negative value means they change together in opposite directions.
The absolute value of a number is equal to the number without its sign. The absolute value of a correlation coefficient tells you the magnitude of the correlation: the greater the absolute value, the stronger the correlation.
These are the assumptions your data must meet if you want to use Pearson’s r :
- Both variables are on an interval or ratio level of measurement
- Data from both variables follow normal distributions
- Your data have no outliers
- Your data is from a random or representative sample
- You expect a linear relationship between the two variables
Quantitative research designs can be divided into two main categories:
- Correlational and descriptive designs are used to investigate characteristics, averages, trends, and associations between variables.
- Experimental and quasi-experimental designs are used to test causal relationships .
Qualitative research designs tend to be more flexible. Common types of qualitative design include case study , ethnography , and grounded theory designs.
A well-planned research design helps ensure that your methods match your research aims, that you collect high-quality data, and that you use the right kind of analysis to answer your questions, utilizing credible sources . This allows you to draw valid , trustworthy conclusions.
The priorities of a research design can vary depending on the field, but you usually have to specify:
- Your research questions and/or hypotheses
- Your overall approach (e.g., qualitative or quantitative )
- The type of design you’re using (e.g., a survey , experiment , or case study )
- Your sampling methods or criteria for selecting subjects
- Your data collection methods (e.g., questionnaires , observations)
- Your data collection procedures (e.g., operationalization , timing and data management)
- Your data analysis methods (e.g., statistical tests or thematic analysis )
A research design is a strategy for answering your research question . It defines your overall approach and determines how you will collect and analyze data.
Questionnaires can be self-administered or researcher-administered.
Self-administered questionnaires can be delivered online or in paper-and-pen formats, in person or through mail. All questions are standardized so that all respondents receive the same questions with identical wording.
Researcher-administered questionnaires are interviews that take place by phone, in-person, or online between researchers and respondents. You can gain deeper insights by clarifying questions for respondents or asking follow-up questions.
You can organize the questions logically, with a clear progression from simple to complex, or randomly between respondents. A logical flow helps respondents process the questionnaire easier and quicker, but it may lead to bias. Randomization can minimize the bias from order effects.
Closed-ended, or restricted-choice, questions offer respondents a fixed set of choices to select from. These questions are easier to answer quickly.
Open-ended or long-form questions allow respondents to answer in their own words. Because there are no restrictions on their choices, respondents can answer in ways that researchers may not have otherwise considered.
A questionnaire is a data collection tool or instrument, while a survey is an overarching research method that involves collecting and analyzing data from people using questionnaires.
The third variable and directionality problems are two main reasons why correlation isn’t causation .
The third variable problem means that a confounding variable affects both variables to make them seem causally related when they are not.
The directionality problem is when two variables correlate and might actually have a causal relationship, but it’s impossible to conclude which variable causes changes in the other.
Correlation describes an association between variables : when one variable changes, so does the other. A correlation is a statistical indicator of the relationship between variables.
Causation means that changes in one variable brings about changes in the other (i.e., there is a cause-and-effect relationship between variables). The two variables are correlated with each other, and there’s also a causal link between them.
While causation and correlation can exist simultaneously, correlation does not imply causation. In other words, correlation is simply a relationship where A relates to B—but A doesn’t necessarily cause B to happen (or vice versa). Mistaking correlation for causation is a common error and can lead to false cause fallacy .
Controlled experiments establish causality, whereas correlational studies only show associations between variables.
- In an experimental design , you manipulate an independent variable and measure its effect on a dependent variable. Other variables are controlled so they can’t impact the results.
- In a correlational design , you measure variables without manipulating any of them. You can test whether your variables change together, but you can’t be sure that one variable caused a change in another.
In general, correlational research is high in external validity while experimental research is high in internal validity .
A correlation is usually tested for two variables at a time, but you can test correlations between three or more variables.
A correlation coefficient is a single number that describes the strength and direction of the relationship between your variables.
Different types of correlation coefficients might be appropriate for your data based on their levels of measurement and distributions . The Pearson product-moment correlation coefficient (Pearson’s r ) is commonly used to assess a linear relationship between two quantitative variables.
A correlational research design investigates relationships between two variables (or more) without the researcher controlling or manipulating any of them. It’s a non-experimental type of quantitative research .
A correlation reflects the strength and/or direction of the association between two or more variables.
- A positive correlation means that both variables change in the same direction.
- A negative correlation means that the variables change in opposite directions.
- A zero correlation means there’s no relationship between the variables.
Random error is almost always present in scientific studies, even in highly controlled settings. While you can’t eradicate it completely, you can reduce random error by taking repeated measurements, using a large sample, and controlling extraneous variables .
You can avoid systematic error through careful design of your sampling , data collection , and analysis procedures. For example, use triangulation to measure your variables using multiple methods; regularly calibrate instruments or procedures; use random sampling and random assignment ; and apply masking (blinding) where possible.
Systematic error is generally a bigger problem in research.
With random error, multiple measurements will tend to cluster around the true value. When you’re collecting data from a large sample , the errors in different directions will cancel each other out.
Systematic errors are much more problematic because they can skew your data away from the true value. This can lead you to false conclusions ( Type I and II errors ) about the relationship between the variables you’re studying.
Random and systematic error are two types of measurement error.
Random error is a chance difference between the observed and true values of something (e.g., a researcher misreading a weighing scale records an incorrect measurement).
Systematic error is a consistent or proportional difference between the observed and true values of something (e.g., a miscalibrated scale consistently records weights as higher than they actually are).
On graphs, the explanatory variable is conventionally placed on the x-axis, while the response variable is placed on the y-axis.
- If you have quantitative variables , use a scatterplot or a line graph.
- If your response variable is categorical, use a scatterplot or a line graph.
- If your explanatory variable is categorical, use a bar graph.
The term “ explanatory variable ” is sometimes preferred over “ independent variable ” because, in real world contexts, independent variables are often influenced by other variables. This means they aren’t totally independent.
Multiple independent variables may also be correlated with each other, so “explanatory variables” is a more appropriate term.
The difference between explanatory and response variables is simple:
- An explanatory variable is the expected cause, and it explains the results.
- A response variable is the expected effect, and it responds to other variables.
In a controlled experiment , all extraneous variables are held constant so that they can’t influence the results. Controlled experiments require:
- A control group that receives a standard treatment, a fake treatment, or no treatment.
- Random assignment of participants to ensure the groups are equivalent.
Depending on your study topic, there are various other methods of controlling variables .
There are 4 main types of extraneous variables :
- Demand characteristics : environmental cues that encourage participants to conform to researchers’ expectations.
- Experimenter effects : unintentional actions by researchers that influence study outcomes.
- Situational variables : environmental variables that alter participants’ behaviors.
- Participant variables : any characteristic or aspect of a participant’s background that could affect study results.
An extraneous variable is any variable that you’re not investigating that can potentially affect the dependent variable of your research study.
A confounding variable is a type of extraneous variable that not only affects the dependent variable, but is also related to the independent variable.
In a factorial design, multiple independent variables are tested.
If you test two variables, each level of one independent variable is combined with each level of the other independent variable to create different conditions.
Within-subjects designs have many potential threats to internal validity , but they are also very statistically powerful .
Advantages:
- Only requires small samples
- Statistically powerful
- Removes the effects of individual differences on the outcomes
Disadvantages:
- Internal validity threats reduce the likelihood of establishing a direct relationship between variables
- Time-related effects, such as growth, can influence the outcomes
- Carryover effects mean that the specific order of different treatments affect the outcomes
While a between-subjects design has fewer threats to internal validity , it also requires more participants for high statistical power than a within-subjects design .
- Prevents carryover effects of learning and fatigue.
- Shorter study duration.
- Needs larger samples for high power.
- Uses more resources to recruit participants, administer sessions, cover costs, etc.
- Individual differences may be an alternative explanation for results.
Yes. Between-subjects and within-subjects designs can be combined in a single study when you have two or more independent variables (a factorial design). In a mixed factorial design, one variable is altered between subjects and another is altered within subjects.
In a between-subjects design , every participant experiences only one condition, and researchers assess group differences between participants in various conditions.
In a within-subjects design , each participant experiences all conditions, and researchers test the same participants repeatedly for differences between conditions.
The word “between” means that you’re comparing different conditions between groups, while the word “within” means you’re comparing different conditions within the same group.
Random assignment is used in experiments with a between-groups or independent measures design. In this research design, there’s usually a control group and one or more experimental groups. Random assignment helps ensure that the groups are comparable.
In general, you should always use random assignment in this type of experimental design when it is ethically possible and makes sense for your study topic.
To implement random assignment , assign a unique number to every member of your study’s sample .
Then, you can use a random number generator or a lottery method to randomly assign each number to a control or experimental group. You can also do so manually, by flipping a coin or rolling a dice to randomly assign participants to groups.
Random selection, or random sampling , is a way of selecting members of a population for your study’s sample.
In contrast, random assignment is a way of sorting the sample into control and experimental groups.
Random sampling enhances the external validity or generalizability of your results, while random assignment improves the internal validity of your study.
In experimental research, random assignment is a way of placing participants from your sample into different groups using randomization. With this method, every member of the sample has a known or equal chance of being placed in a control group or an experimental group.
“Controlling for a variable” means measuring extraneous variables and accounting for them statistically to remove their effects on other variables.
Researchers often model control variable data along with independent and dependent variable data in regression analyses and ANCOVAs . That way, you can isolate the control variable’s effects from the relationship between the variables of interest.
Control variables help you establish a correlational or causal relationship between variables by enhancing internal validity .
If you don’t control relevant extraneous variables , they may influence the outcomes of your study, and you may not be able to demonstrate that your results are really an effect of your independent variable .
A control variable is any variable that’s held constant in a research study. It’s not a variable of interest in the study, but it’s controlled because it could influence the outcomes.
Including mediators and moderators in your research helps you go beyond studying a simple relationship between two variables for a fuller picture of the real world. They are important to consider when studying complex correlational or causal relationships.
Mediators are part of the causal pathway of an effect, and they tell you how or why an effect takes place. Moderators usually help you judge the external validity of your study by identifying the limitations of when the relationship between variables holds.
If something is a mediating variable :
- It’s caused by the independent variable .
- It influences the dependent variable
- When it’s taken into account, the statistical correlation between the independent and dependent variables is higher than when it isn’t considered.
A confounder is a third variable that affects variables of interest and makes them seem related when they are not. In contrast, a mediator is the mechanism of a relationship between two variables: it explains the process by which they are related.
A mediator variable explains the process through which two variables are related, while a moderator variable affects the strength and direction of that relationship.
There are three key steps in systematic sampling :
- Define and list your population , ensuring that it is not ordered in a cyclical or periodic order.
- Decide on your sample size and calculate your interval, k , by dividing your population by your target sample size.
- Choose every k th member of the population as your sample.
Systematic sampling is a probability sampling method where researchers select members of the population at a regular interval – for example, by selecting every 15th person on a list of the population. If the population is in a random order, this can imitate the benefits of simple random sampling .
Yes, you can create a stratified sample using multiple characteristics, but you must ensure that every participant in your study belongs to one and only one subgroup. In this case, you multiply the numbers of subgroups for each characteristic to get the total number of groups.
For example, if you were stratifying by location with three subgroups (urban, rural, or suburban) and marital status with five subgroups (single, divorced, widowed, married, or partnered), you would have 3 x 5 = 15 subgroups.
You should use stratified sampling when your sample can be divided into mutually exclusive and exhaustive subgroups that you believe will take on different mean values for the variable that you’re studying.
Using stratified sampling will allow you to obtain more precise (with lower variance ) statistical estimates of whatever you are trying to measure.
For example, say you want to investigate how income differs based on educational attainment, but you know that this relationship can vary based on race. Using stratified sampling, you can ensure you obtain a large enough sample from each racial group, allowing you to draw more precise conclusions.
In stratified sampling , researchers divide subjects into subgroups called strata based on characteristics that they share (e.g., race, gender, educational attainment).
Once divided, each subgroup is randomly sampled using another probability sampling method.
Cluster sampling is more time- and cost-efficient than other probability sampling methods , particularly when it comes to large samples spread across a wide geographical area.
However, it provides less statistical certainty than other methods, such as simple random sampling , because it is difficult to ensure that your clusters properly represent the population as a whole.
There are three types of cluster sampling : single-stage, double-stage and multi-stage clustering. In all three types, you first divide the population into clusters, then randomly select clusters for use in your sample.
- In single-stage sampling , you collect data from every unit within the selected clusters.
- In double-stage sampling , you select a random sample of units from within the clusters.
- In multi-stage sampling , you repeat the procedure of randomly sampling elements from within the clusters until you have reached a manageable sample.
Cluster sampling is a probability sampling method in which you divide a population into clusters, such as districts or schools, and then randomly select some of these clusters as your sample.
The clusters should ideally each be mini-representations of the population as a whole.
If properly implemented, simple random sampling is usually the best sampling method for ensuring both internal and external validity . However, it can sometimes be impractical and expensive to implement, depending on the size of the population to be studied,
If you have a list of every member of the population and the ability to reach whichever members are selected, you can use simple random sampling.
The American Community Survey is an example of simple random sampling . In order to collect detailed data on the population of the US, the Census Bureau officials randomly select 3.5 million households per year and use a variety of methods to convince them to fill out the survey.
Simple random sampling is a type of probability sampling in which the researcher randomly selects a subset of participants from a population . Each member of the population has an equal chance of being selected. Data is then collected from as large a percentage as possible of this random subset.
Quasi-experimental design is most useful in situations where it would be unethical or impractical to run a true experiment .
Quasi-experiments have lower internal validity than true experiments, but they often have higher external validity as they can use real-world interventions instead of artificial laboratory settings.
A quasi-experiment is a type of research design that attempts to establish a cause-and-effect relationship. The main difference with a true experiment is that the groups are not randomly assigned.
Blinding is important to reduce research bias (e.g., observer bias , demand characteristics ) and ensure a study’s internal validity .
If participants know whether they are in a control or treatment group , they may adjust their behavior in ways that affect the outcome that researchers are trying to measure. If the people administering the treatment are aware of group assignment, they may treat participants differently and thus directly or indirectly influence the final results.
- In a single-blind study , only the participants are blinded.
- In a double-blind study , both participants and experimenters are blinded.
- In a triple-blind study , the assignment is hidden not only from participants and experimenters, but also from the researchers analyzing the data.
Blinding means hiding who is assigned to the treatment group and who is assigned to the control group in an experiment .
A true experiment (a.k.a. a controlled experiment) always includes at least one control group that doesn’t receive the experimental treatment.
However, some experiments use a within-subjects design to test treatments without a control group. In these designs, you usually compare one group’s outcomes before and after a treatment (instead of comparing outcomes between different groups).
For strong internal validity , it’s usually best to include a control group if possible. Without a control group, it’s harder to be certain that the outcome was caused by the experimental treatment and not by other variables.
Individual Likert-type questions are generally considered ordinal data , because the items have clear rank order, but don’t have an even distribution.
Overall Likert scale scores are sometimes treated as interval data. These scores are considered to have directionality and even spacing between them.
The type of data determines what statistical tests you should use to analyze your data.
A Likert scale is a rating scale that quantitatively assesses opinions, attitudes, or behaviors. It is made up of 4 or more questions that measure a single attitude or trait when response scores are combined.
To use a Likert scale in a survey , you present participants with Likert-type questions or statements, and a continuum of items, usually with 5 or 7 possible responses, to capture their degree of agreement.
In scientific research, concepts are the abstract ideas or phenomena that are being studied (e.g., educational achievement). Variables are properties or characteristics of the concept (e.g., performance at school), while indicators are ways of measuring or quantifying variables (e.g., yearly grade reports).
The process of turning abstract concepts into measurable variables and indicators is called operationalization .
There are various approaches to qualitative data analysis , but they all share five steps in common:
- Prepare and organize your data.
- Review and explore your data.
- Develop a data coding system.
- Assign codes to the data.
- Identify recurring themes.
The specifics of each step depend on the focus of the analysis. Some common approaches include textual analysis , thematic analysis , and discourse analysis .
There are five common approaches to qualitative research :
- Grounded theory involves collecting data in order to develop new theories.
- Ethnography involves immersing yourself in a group or organization to understand its culture.
- Narrative research involves interpreting stories to understand how people make sense of their experiences and perceptions.
- Phenomenological research involves investigating phenomena through people’s lived experiences.
- Action research links theory and practice in several cycles to drive innovative changes.
Hypothesis testing is a formal procedure for investigating our ideas about the world using statistics. It is used by scientists to test specific predictions, called hypotheses , by calculating how likely it is that a pattern or relationship between variables could have arisen by chance.
Operationalization means turning abstract conceptual ideas into measurable observations.
For example, the concept of social anxiety isn’t directly observable, but it can be operationally defined in terms of self-rating scores, behavioral avoidance of crowded places, or physical anxiety symptoms in social situations.
Before collecting data , it’s important to consider how you will operationalize the variables that you want to measure.
When conducting research, collecting original data has significant advantages:
- You can tailor data collection to your specific research aims (e.g. understanding the needs of your consumers or user testing your website)
- You can control and standardize the process for high reliability and validity (e.g. choosing appropriate measurements and sampling methods )
However, there are also some drawbacks: data collection can be time-consuming, labor-intensive and expensive. In some cases, it’s more efficient to use secondary data that has already been collected by someone else, but the data might be less reliable.
Data collection is the systematic process by which observations or measurements are gathered in research. It is used in many different contexts by academics, governments, businesses, and other organizations.
There are several methods you can use to decrease the impact of confounding variables on your research: restriction, matching, statistical control and randomization.
In restriction , you restrict your sample by only including certain subjects that have the same values of potential confounding variables.
In matching , you match each of the subjects in your treatment group with a counterpart in the comparison group. The matched subjects have the same values on any potential confounding variables, and only differ in the independent variable .
In statistical control , you include potential confounders as variables in your regression .
In randomization , you randomly assign the treatment (or independent variable) in your study to a sufficiently large number of subjects, which allows you to control for all potential confounding variables.
A confounding variable is closely related to both the independent and dependent variables in a study. An independent variable represents the supposed cause , while the dependent variable is the supposed effect . A confounding variable is a third variable that influences both the independent and dependent variables.
Failing to account for confounding variables can cause you to wrongly estimate the relationship between your independent and dependent variables.
To ensure the internal validity of your research, you must consider the impact of confounding variables. If you fail to account for them, you might over- or underestimate the causal relationship between your independent and dependent variables , or even find a causal relationship where none exists.
Yes, but including more than one of either type requires multiple research questions .
For example, if you are interested in the effect of a diet on health, you can use multiple measures of health: blood sugar, blood pressure, weight, pulse, and many more. Each of these is its own dependent variable with its own research question.
You could also choose to look at the effect of exercise levels as well as diet, or even the additional effect of the two combined. Each of these is a separate independent variable .
To ensure the internal validity of an experiment , you should only change one independent variable at a time.
No. The value of a dependent variable depends on an independent variable, so a variable cannot be both independent and dependent at the same time. It must be either the cause or the effect, not both!
You want to find out how blood sugar levels are affected by drinking diet soda and regular soda, so you conduct an experiment .
- The type of soda – diet or regular – is the independent variable .
- The level of blood sugar that you measure is the dependent variable – it changes depending on the type of soda.
Determining cause and effect is one of the most important parts of scientific research. It’s essential to know which is the cause – the independent variable – and which is the effect – the dependent variable.
In non-probability sampling , the sample is selected based on non-random criteria, and not every member of the population has a chance of being included.
Common non-probability sampling methods include convenience sampling , voluntary response sampling, purposive sampling , snowball sampling, and quota sampling .
Probability sampling means that every member of the target population has a known chance of being included in the sample.
Probability sampling methods include simple random sampling , systematic sampling , stratified sampling , and cluster sampling .
Using careful research design and sampling procedures can help you avoid sampling bias . Oversampling can be used to correct undercoverage bias .
Some common types of sampling bias include self-selection bias , nonresponse bias , undercoverage bias , survivorship bias , pre-screening or advertising bias, and healthy user bias.
Sampling bias is a threat to external validity – it limits the generalizability of your findings to a broader group of people.
A sampling error is the difference between a population parameter and a sample statistic .
A statistic refers to measures about the sample , while a parameter refers to measures about the population .
Populations are used when a research question requires data from every member of the population. This is usually only feasible when the population is small and easily accessible.
Samples are used to make inferences about populations . Samples are easier to collect data from because they are practical, cost-effective, convenient, and manageable.
There are seven threats to external validity : selection bias , history, experimenter effect, Hawthorne effect , testing effect, aptitude-treatment and situation effect.
The two types of external validity are population validity (whether you can generalize to other groups of people) and ecological validity (whether you can generalize to other situations and settings).
The external validity of a study is the extent to which you can generalize your findings to different groups of people, situations, and measures.
Cross-sectional studies cannot establish a cause-and-effect relationship or analyze behavior over a period of time. To investigate cause and effect, you need to do a longitudinal study or an experimental study .
Cross-sectional studies are less expensive and time-consuming than many other types of study. They can provide useful insights into a population’s characteristics and identify correlations for further research.
Sometimes only cross-sectional data is available for analysis; other times your research question may only require a cross-sectional study to answer it.
Longitudinal studies can last anywhere from weeks to decades, although they tend to be at least a year long.
The 1970 British Cohort Study , which has collected data on the lives of 17,000 Brits since their births in 1970, is one well-known example of a longitudinal study .
Longitudinal studies are better to establish the correct sequence of events, identify changes over time, and provide insight into cause-and-effect relationships, but they also tend to be more expensive and time-consuming than other types of studies.
Longitudinal studies and cross-sectional studies are two different types of research design . In a cross-sectional study you collect data from a population at a specific point in time; in a longitudinal study you repeatedly collect data from the same sample over an extended period of time.
| Longitudinal study | Cross-sectional study |
|---|---|
| observations | Observations at a in time |
| Observes the multiple times | Observes (a “cross-section”) in the population |
| Follows in participants over time | Provides of society at a given point |
There are eight threats to internal validity : history, maturation, instrumentation, testing, selection bias , regression to the mean, social interaction and attrition .
Internal validity is the extent to which you can be confident that a cause-and-effect relationship established in a study cannot be explained by other factors.
In mixed methods research , you use both qualitative and quantitative data collection and analysis methods to answer your research question .
The research methods you use depend on the type of data you need to answer your research question .
- If you want to measure something or test a hypothesis , use quantitative methods . If you want to explore ideas, thoughts and meanings, use qualitative methods .
- If you want to analyze a large amount of readily-available data, use secondary data. If you want data specific to your purposes with control over how it is generated, collect primary data.
- If you want to establish cause-and-effect relationships between variables , use experimental methods. If you want to understand the characteristics of a research subject, use descriptive methods.
A confounding variable , also called a confounder or confounding factor, is a third variable in a study examining a potential cause-and-effect relationship.
A confounding variable is related to both the supposed cause and the supposed effect of the study. It can be difficult to separate the true effect of the independent variable from the effect of the confounding variable.
In your research design , it’s important to identify potential confounding variables and plan how you will reduce their impact.
Discrete and continuous variables are two types of quantitative variables :
- Discrete variables represent counts (e.g. the number of objects in a collection).
- Continuous variables represent measurable amounts (e.g. water volume or weight).
Quantitative variables are any variables where the data represent amounts (e.g. height, weight, or age).
Categorical variables are any variables where the data represent groups. This includes rankings (e.g. finishing places in a race), classifications (e.g. brands of cereal), and binary outcomes (e.g. coin flips).
You need to know what type of variables you are working with to choose the right statistical test for your data and interpret your results .
You can think of independent and dependent variables in terms of cause and effect: an independent variable is the variable you think is the cause , while a dependent variable is the effect .
In an experiment, you manipulate the independent variable and measure the outcome in the dependent variable. For example, in an experiment about the effect of nutrients on crop growth:
- The independent variable is the amount of nutrients added to the crop field.
- The dependent variable is the biomass of the crops at harvest time.
Defining your variables, and deciding how you will manipulate and measure them, is an important part of experimental design .
Experimental design means planning a set of procedures to investigate a relationship between variables . To design a controlled experiment, you need:
- A testable hypothesis
- At least one independent variable that can be precisely manipulated
- At least one dependent variable that can be precisely measured
When designing the experiment, you decide:
- How you will manipulate the variable(s)
- How you will control for any potential confounding variables
- How many subjects or samples will be included in the study
- How subjects will be assigned to treatment levels
Experimental design is essential to the internal and external validity of your experiment.
I nternal validity is the degree of confidence that the causal relationship you are testing is not influenced by other factors or variables .
External validity is the extent to which your results can be generalized to other contexts.
The validity of your experiment depends on your experimental design .
Reliability and validity are both about how well a method measures something:
- Reliability refers to the consistency of a measure (whether the results can be reproduced under the same conditions).
- Validity refers to the accuracy of a measure (whether the results really do represent what they are supposed to measure).
If you are doing experimental research, you also have to consider the internal and external validity of your experiment.
A sample is a subset of individuals from a larger population . Sampling means selecting the group that you will actually collect data from in your research. For example, if you are researching the opinions of students in your university, you could survey a sample of 100 students.
In statistics, sampling allows you to test a hypothesis about the characteristics of a population.
Quantitative research deals with numbers and statistics, while qualitative research deals with words and meanings.
Quantitative methods allow you to systematically measure variables and test hypotheses . Qualitative methods allow you to explore concepts and experiences in more detail.
Methodology refers to the overarching strategy and rationale of your research project . It involves studying the methods used in your field and the theories or principles behind them, in order to develop an approach that matches your objectives.
Methods are the specific tools and procedures you use to collect and analyze data (for example, experiments, surveys , and statistical tests ).
In shorter scientific papers, where the aim is to report the findings of a specific study, you might simply describe what you did in a methods section .
In a longer or more complex research project, such as a thesis or dissertation , you will probably include a methodology section , where you explain your approach to answering the research questions and cite relevant sources to support your choice of methods.
Ask our team
Want to contact us directly? No problem. We are always here for you.
- Email [email protected]
- Start live chat
- Call +1 (510) 822-8066
- WhatsApp +31 20 261 6040

Our team helps students graduate by offering:
- A world-class citation generator
- Plagiarism Checker software powered by Turnitin
- Innovative Citation Checker software
- Professional proofreading services
- Over 300 helpful articles about academic writing, citing sources, plagiarism, and more
Scribbr specializes in editing study-related documents . We proofread:
- PhD dissertations
- Research proposals
- Personal statements
- Admission essays
- Motivation letters
- Reflection papers
- Journal articles
- Capstone projects
Scribbr’s Plagiarism Checker is powered by elements of Turnitin’s Similarity Checker , namely the plagiarism detection software and the Internet Archive and Premium Scholarly Publications content databases .
The add-on AI detector is powered by Scribbr’s proprietary software.
The Scribbr Citation Generator is developed using the open-source Citation Style Language (CSL) project and Frank Bennett’s citeproc-js . It’s the same technology used by dozens of other popular citation tools, including Mendeley and Zotero.
You can find all the citation styles and locales used in the Scribbr Citation Generator in our publicly accessible repository on Github .
Major Differences
5 differences between control group and experimental group with example.
| It increases the reliability and validity of experimental results | |
You might like
Post a comment.
We Love to hear from U :) Leave us a Comment to improve this site Thanks for Visiting.....
Contact Form
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Open access
- Published: 13 September 2024
Therapeutic efficacy of intracerebral hematopoietic stem cell gene therapy in an Alzheimer’s disease mouse model
- Rita Milazzo 1 , 2 ,
- Annita Montepeloso ORCID: orcid.org/0000-0003-3299-476X 3 ,
- Rajesh Kumar 3 ,
- Francesca Ferro 2 , 3 ,
- Eleonora Cavalca 2 , 3 ,
- Pietro Rigoni ORCID: orcid.org/0009-0007-1653-126X 1 ,
- Paolo Cabras 4 ,
- Yuri Ciervo ORCID: orcid.org/0000-0001-9546-7532 1 ,
- Sabyasachi Das 3 ,
- Alessia Capotondo 2 ,
- Danilo Pellin ORCID: orcid.org/0000-0002-2647-0508 3 ,
- Marco Peviani 3 , 4 &
- Alessandra Biffi ORCID: orcid.org/0000-0002-4610-0870 1 , 2 , 3
Nature Communications volume 15 , Article number: 8024 ( 2024 ) Cite this article
Metrics details
- Alzheimer's disease
- Glial biology
- Haematopoietic stem cells
The conditions supporting the generation of microglia-like cells in the central nervous system (CNS) after transplantation of hematopoietic stem/progenitor cells (HSPC) have been studied to advance the treatment of neurodegenerative disorders. Here, we explored the transplantation efficacy of different cell subsets and delivery routes with the goal of favoring the establishment of a stable and exclusive engraftment of HSPCs and their progeny in the CNS of female mice. In this setting, we show that the CNS environment drives the expansion, distribution and myeloid differentiation of the locally transplanted cells towards a microglia-like phenotype. Intra-CNS transplantation of HSPCs engineered to overexpress TREM2 decreased neuroinflammation, Aβ aggregation and improved memory in 5xFAD female mice. Our proof of concept study demonstrates the therapeutic potential of HSPC gene therapy for Alzheimer’s disease.
Similar content being viewed by others

Blood cell-produced amyloid-β induces cerebral Alzheimer-type pathologies and behavioral deficits
Neural stem cell transplantation in patients with progressive multiple sclerosis: an open-label, phase 1 study
CNS-wide repopulation by hematopoietic-derived microglia-like cells corrects progranulin deficiency in mice
Introduction.
Early during development, a group of myeloid hematopoietic progenitors integrates into the central nervous system (CNS) to provide life-long support as microglia 1 , 2 . This cell population exerts crucial functions for the maintenance of brain tissue homeostasis during development as well as in adulthood, both in physiological and pathological conditions 3 . In fact, microglia cells actively define and reshape the neuronal network by surveying the neuronal microenvironment, pruning synaptic spines, and functionally contributing to neuroinflammation 4 , 5 . Adult microglia is a self-maintained population, but following discrete myeloablation and infusion of hematopoietic stem and progenitor cells (HSPCs), brain-resident microglia leaves space for transplant-derived myeloid cells that can, in appropriate conditions, arise and settle in the CNS, progressively acquiring morphologic, antigenic and functional features that render them similar to microglia 6 , 7 , 8 , 9 , 10 .
During the last decade, the contribution of HSPCs to CNS-associated myeloid cell populations upon transplantation has been deeply investigated and exploited for therapeutic purposes, demonstrating unprecedented therapeutic potential in treating monogenic neurometabolic disorders in gene therapy proof of concept preclinical studies and clinical trials 11 , 12 , 13 , 14 , 15 , 16 , 17 , 18 , 19 , 20 , 21 , 22 , 23 , 24 , 25 , 26 . In these settings, the engineered microglia-like cells progressively appearing in the CNS of transplant recipients act as vehicle for disease‐modulating therapeutics across the blood brain barrier (BBB) and contribute, with scavenging and immune surveillance functions, to the modulation of the CNS neurotoxic environment towards neuroprotection 27 , 28 , 29 . However, the benefit of the procedure is still limited by the slow pace and extent at which cell repopulation in different CNS regions occur. To address these limitations, the mechanisms and the conditions favoring the engraftment and generation of microglia-like cells in the CNS have been extensively studied.
Others and us demonstrated that the engraftment of transplant-derived cells in the CNS is highly favored by and strictly dependent on the recipient’s pre-transplant exposure to a myeloablative regimen based on the alkylating agent busulfan (BU) that alters the brain environment, creating space and opportunity for the local engraftment and proliferation of the transplanted cells and their progeny 7 , 30 , 31 . We also showed that a fraction of the HSPCs that are intravenously (IV) injected in BU-treated recipients enters the CNS shortly after transplant and proliferates locally, possibly contributing to long-term CNS hematopoietic donor chimerism 7 . This process is enhanced and favored when HSPCs are directly infused in the brain by intracerebroventricular injection (ICV) 6 .
Here, we challenge further the standard hematopoietic transplantation paradigm by exploiting and combining different HSPC subsets differentially enriched in long-term hematopoietic reconstituting potential, and by exploring different cell-infusion routes to eventually favor the establishment of a robust and exclusive donor engraftment in the CNS of recipients. In this setting, we unveil a key role exerted by the CNS environment in uniquely driving expansion, distribution and myeloid differentiation of the locally transplanted HSPCs towards a microglia-like phenotype. Importantly, we then test the therapeutic potential of engineered HSPC transplantation in the CNS in an established murine model of Alzheimer’s disease (AD) obtaining a clear example of efficacy of HSPC gene therapy in an adult onset, multifactorial neurodegenerative disease, and a proof of benefit associated with an innovative approach that determines a CNS-restricted engraftment of the genetically modified transplant progeny cells.
Competitive engraftment potential of intra-CNS transplanted HSPCs
Administration of engineered hematopoietic stem and progenitor cells (HSPCs) in the lateral cerebral ventricles (ICV) of lethally myeloablated (BU-based regimen) mice can result in robust CNS-restricted myeloid engraftment and generation of microglia-like cells 6 . To study the mechanisms leading to the engraftment and differentiation of the CNS-transplanted HSPCs, we performed competitive transplantation experiments combining the ICV delivery of CD34 + -equivalent lineage negative (Lin - ) murine HSPCs with the simultaneous or delayed (after 5 days) intravenous (IV) administration of hematopoietic cell subsets that are differentially enriched in long-term hematopoietic reconstituting activity, namely total bone marrow (BM), Lin - cells and c-kit + Sca-1 + stem cell-enriched Lin - (KSL) cells. Moreover, we explored a new intra-CNS delivery approach in the intrathecal lumbar (ITL) space as alternative to the ICV route. To discriminate the differential contribution of the transplanted and recipient cells, map their progeny and obtain relevant insights on the mechanism of HSPC engraftment and differentiation in the CNS, we took advantage of the CD45.1 (donor)/CD45.2 (recipient) allele mismatch and we differentially labeled the donor cells according to the route of administration, by using lentiviral vectors (LVs) encoding for different reporter genes (GFP for CNS-delivered cells and ∆NGFR or Cherry for IV-delivered cells). Wild type CD45.2 C57BL/6 recipients were pre-treated with BU used at myeloablative dose (25 mg/kg, from days −4 to days −1) to promote myeloid cell turnover with donor cells in the CNS 7 . Transplanted mice were then euthanized at different time points after the transplant and the engraftment of the donor cells in the hematopoietic tissues and CNS was assessed by flow cytometry (FC) and immunofluorescence (IF) (Fig. S1a, b ). For each time point, BU-treated mice transplanted IV with ∆NGFR/Cherry Lin - cells were used as term of comparison.
Interestingly, in all the combinations tested, we observed a high competition (in terms of extent of repopulation of the CNS compartment) between the progenies of the HSPCs transplanted through the intra-CNS versus the IV route (Fig. 1a–c and Tables S1 , S2 ; Fig. S1c, e and f and Tables S3 , S4 ). In particular, the contribution of the CNS-injected HSPCs exceeded that of the IV transplanted cells in all the tested conditions, although the number of HSPCs injected in CNS was substantially lower than those administered IV (0.3 × 10 6 vs 1 × 10 6 , respectively) (Fig. 1a–c and Tables S1 , S2 ; Fig. S1c, e and f and Table S1 , S2 ). The highest competition between the IV and the intra-CNS transplanted cells was observed when Lin - cells were employed at both sites of injection, highlighting the ability of this cell population to efficiently occupy CNS niches upon both systemic and intra-CNS delivery (Fig. 1a–c and Tables S1 , S2 and Fig. S1c, e and Tables S3 , S4 ). Similar findings were observed in the spinal cord (Fig. 1a–c and Tables S1 , S2 ; Fig. S1c, e and Tables S3 , S4 ) with greater representation of the progeny of CNS-delivered HSPCs versus the IV-transplanted counterpart, particularly at 45 days post-transplant (post-Tx) and especially when ITL was used for intra-CNS delivery (Fig. 1a ). Notably, and at odds with recent reports 32 , the combination of intra-CNS and IV HSPC transplantation resulted in an overall enhancement of the myeloid donor chimerism in the brain and in the spinal cord of transplant recipients, as compared to the chimerism achieved upon IV-only cell administration (Fig. 1a, b and Fig. S1e, f ). This observation was confirmed by employing both the ICV and ITL routes for HSPCs delivery (Fig. 1a–c ). The increased chimerism was observed as early as 45 days post-Tx and up to 90 days post-Tx, independently from the time of IV cell delivery (simultaneous vs delayed) and the cell population employed for IV (Fig. 1a, b and Fig. S1c, e and f ). The difference was no longer observed at the longest tested time point, 240 days post-Tx, when the chimerism of the transplanted cell progeny reached a plateau (Fig. 1c and S1e,f ). The competitive advantage of intra-CNS transplanted cells versus their IV infused counterpart, and the increased chimerism associated to combined intra-CNS and IV cell delivery were observed within both immature, transiently amplifying, CD45 hi CD11b + microglia-like cells (TAµ) and, even more significantly, within CD45 low CD11b + cells (previously defined microglia like, µ) that according to our previously work share a large portion of the phenotypic and transcriptional features of mature microglia 7 (Figs. 1d , S1d and Tables S3 , S4 ).
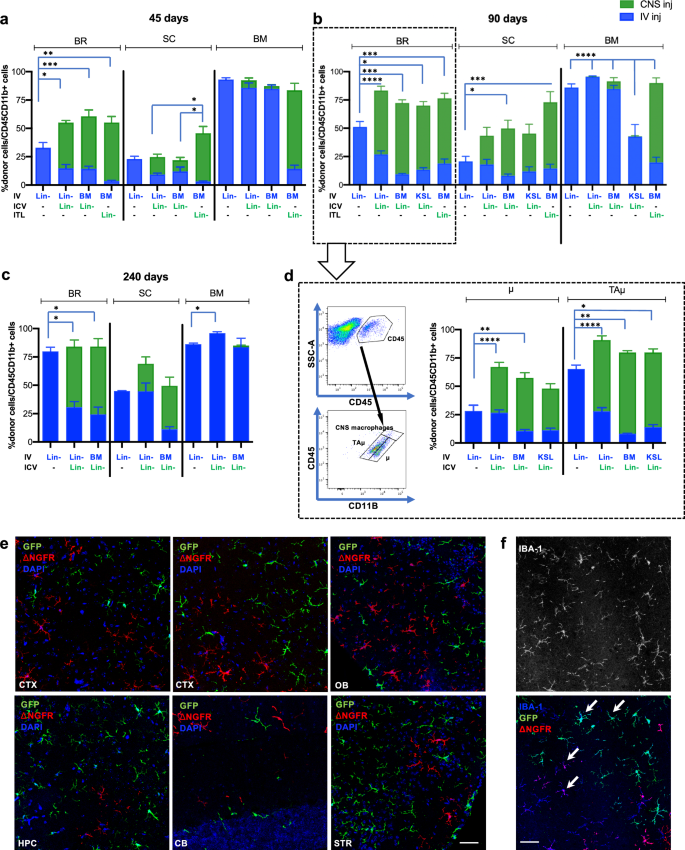
Histograms showing the donor-cell chimerism within the CD45 + /CD11b + cell population in the Brain [BR], Spinal Cord [SC] and Bone marrow [BM] at 45 ( a ), 90 ( b ), and 240 ( c ) days post transplant (pTX). The progeny of intra-CNS transplanted cells (CNS Inj.) either ICV or ITL (as indicated), is shown in green, whereas the progeny of IV administered cells (IV inj.) are shown in blue. On the x-axis different hematopoietic subpopulations used in each combinatorial setting are shown: BM = Bone Marrow; Lin = Lineage negative cells; KSL = c-kit + , Sca-1 + Lin - cells. d Frequency of ICV (green) and IV delivered cells (blue) within the indicated brain subpopulations (μ and TAμ) at 90 days pTX. Representative dot plots showing the gating strategy used to identify μ and TAμ subpopulations are shown on the left. Histograms ( a – d ) show the mean ± SEM of mice at 45 days pTX [ n = 8 IV Lin, n = 9 ICV Lin IV Lin, n = 7 ICV Lin IV BM, n = 9 ITL Lin IV BM mice, n = 3 experimental replicates], 90 days [ n = 11 IV Lin, n = 10 ICV Lin IV Lin and ICV Lin IV BM, n = 7 ICV Lin IV KSL, n = 5 ITL Lin IV BM mice, n = 4 experimental replicates] and 240 days [ n = 4 mice/group, n = 2 experimental replicates]. Statistics for a – d : 1-way ANOVA followed by Tukey post-hoc test for comparisons between the contribution of all donor-derived cells (CNS inj. + IV inj.) among the transplant settings; for additional statistical information refer to Tables S1 and S2 . Source data ( a – d ) are provided as a Source Data file. e , f Representative laser scanning confocal microscope microphotographs of IF-stained brain sections from mice co-transplanted with Lin- both ICV and IV, at 90 days pTX [ n = 5 slices from n = 3 mice]. ICV-derived cells (green signal) are stained with ( e ) anti-GFP antibody, IV-derived cells (red signal) with anti- ΔNGFR antibody, DAPI for nuclei (blue), and ( f ) Iba1 for microglia cells (arrows indicate representative co-localizations of Iba1 and GFP/ΔNGFR signals). CTX cortex, OB Olfactory Bulb, STR striatum, HPC hippocampus, CB Cerebellum. Magnification 40x. M= merge. Images were acquired at Leika SP2 confocal microscope. Scale bar ( e , f ) = 50 µm.
Interestingly, while both (ICV and ITL) of the intra-CNS routes of HSPC administration similarly enhanced CNS donor chimerism, they differentially contributed to marrow hematopoiesis. Indeed, while ICV injected cells did not contribute to the reconstitution of marrow hematopoiesis, as previously described 6 (Figs. 1a–c and S1c ), the ITL-delivered HSPCs robustly engrafted in the marrow of ITL + IV co-transplanted mice (Fig. 1a, b ) with relevant translational implications.
Importantly, independently from the cell source and the setting applied, transplant-derived cells extensively colonized the brain and showed morphological and antigenic features reminiscent of endogenous microglia at confocal analysis, as documented in representative pictures at 90 days post transplant, by the presence of thin ramifications and by the expression of microglia antigens (Fig. 1e, f ).
Route-specific cell distribution in the CNS after HSPC trasplantation
Based on the evidence that intra-CNS administration of HSPCs favors the medium to long-term CNS engraftment of their progeny, we next assessed whether the route of HSPC administration could also affect the pattern of distribution of their cell progeny in the CNS. To this goal, we first performed a short-term monitoring of cell biodistribution in the hematopoietic and CNS compartments of busulfan-treated wild type mice transplanted IV, ICV or ITL with GFP-labeled HSPCs and sacrified at 1, 5 and 14 days post injection ( N = 3 mice/time point/route). Bone marrow, brain and spinal cord were collected and processed for FC and IF analysis (CNS only)(Fig. 2a ). At the earliest time points ( + 1 and +5 days), the donor cells were clearly measurable only in the brain of ICV transplanted mice, while by day 14 discrete levels of CNS engraftment were observed in all the tested conditions. Based on these data, day 14 was chosen as the earliest timepoint to comparatively study the biodistribution of the CNS engrafted cells in the mice transplanted with the different routes by IF analysis. To this scope, we also analyzed animals at +30, 45 and 90 days post-transplant to follow the cell distribution over time, with the animals examined at >30 days post-transplant having received also Cherry_LV-labeled HSPCs IV, in addition to the GFP-labeled counterpart administered ICV or ITL, for assuring long-term survival after myeloablation (Fig. 2b–e ). In the latter setting, the biodistribution data were thus obtained in the more challenging competitive setting.
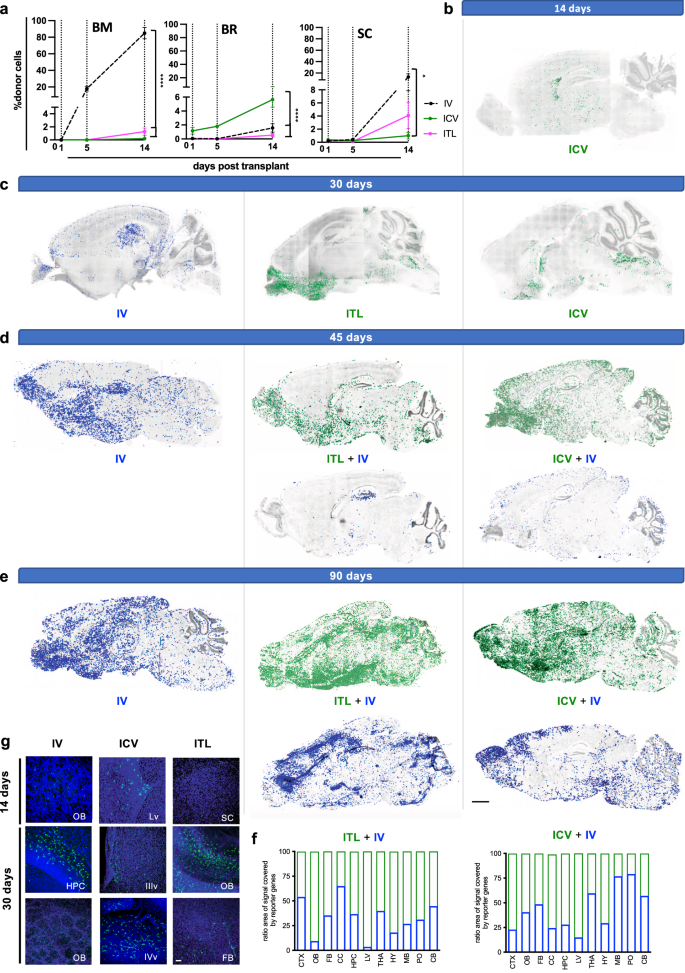
a Short-term HSPC biodistribution in the bone marrow (BM), brain (BR) and spinal cord (SC) at 1, 5 and 14 days post-transplant (pTX) in IV (black), ICV (green) and ITL ( pink) transplanted mice as measured by flow cytometry [ n = 3 mice/group across n = 2 experimental replicates]. Data are shown as mean ± SEM. 2-way ANOVA followed by Tukey post-hoc test pTX ( p * = 0.0351, p **** < 0.0001). Source data ( a ) are provided as a Source Data file. Distribution of GFP + donor-derived cells analyzed at 14 days ( b ) and 30 days pTX ( c ) in the brain of representative IV, ICV or ITL transplanted mice (single transplantations) [ n = 4 slices from n = 3 mice/group]. Images were acquired at 25x magnification using Leica SP5 confocal microscopy after tissues clearing. Distribution of donor-derived cells, labeled with GFP (green signal) or with ΔNGFR/Cherry (blue signal), in representative mice transplanted with Lin - HSPCs via ICV + IV or ITL + IV or IV only delivery (as indicated) and analyzed at 45 ( d ) [ n = 4 slices from n = 3 mice/group] or 90 days pTX ( e ) [ n = 3 slices from n = 4 mice/group]. Images were acquired at Delta Vision Olympus Ix70 for brain reconstruction. The corresponding masks ( b – e ) were generated accordingly using Fiji software. Scale bar = 1000 µm. f Graphs showing the proportion of the total area covered by the progeny of IV injected cells, labeled with ΔNGFR or Cherry (blue bars), vs the progeny of cells injected ICV or ITL, and labeled with GFP of mice sacrified at 90 days pTX [ n = 4 mice/group]. g High resolution confocal z-stacks of selected brain regions (with 5 µm step size) of IV, ICV or ITL transplanted mice at the indicated time points [ n = 3 slices from n = 2 mice/group]. GFP-labeled cells are shown in green, DAPI nuclear stain is shown in blue. Images were acquired at 25x magnification and processed using Fiji open source software. Scale bar = 50 µm. CTX cortex, OB olfactory bulb, FB forebrain, CC corpus callosum, HPC hippocampus, Lv lateral ventricle, THA thalamus, HY hypothalamus, MB midbrain, PO pons, CB cerebellum, IIIv third ventricle, IVv fourth ventricle.
Interestingly, depending on the route of cell administration, we observed a differential distribution of the GFP + cells in the analyzed CNS tissues. At day 14, the progeny of the ICV-transplanted HSPCs was mostly detectable in the close proximity of the ventricular structure, in the septum and projecting rostrally and towards the basal forebrain, and a few additional clustered cells were observed in the midbrain and pons (Fig. 2b ). Although the time point of observation was very precoucious, already discrete branchings of the GFP + cells were visible suggesting an actively occurring differentiation process (Fig. 2g ). At day 30, the GFP + cells exhibited a preferential distribution across brain regions surrounding the cerebral ventricular system, exploiting a trajectory which may suggest proximity to the rostral migratory stream, and confirming the pattern of distribution observed at 14 days. Indeed, clustered cells were also observed in the proximity of the third and fourth ventricles, in the diencephalon and in the hindbrain, from which they distributed into nearby regions, colonizing the striatum, the anterior olfactory area and the brainstem (Fig. 2c ). At these early time points, the distribution of the GFP + cells in the ITL setting was substantially different. At the initial time point ( + 14 days), only a limited quantity of donor cells was documented in the brain by FC and, similarly, only a few and scattered GFP + cells were identified by IF, with no evidence of clustering. In the spinal cord, we failed to identify a pattern, as the few detected GFP + cells were found in different locations without any obvious clustering. By day 30, the GFP + cells were mostly localized in the olfactory bulb and forebrain regions (Fig. 2c ). A few clusters of cells could also be observed in the brainstem, consistent with its proximity to the spinal cord. Unlike the ICV route, no cells were retrieved in the subventricular zone and lateral ventricle regions. In the IV transplant setting, at day 14, very few donor/GFP + cells were detected by FC analysis (Fig. 2a ) and IF (Fig. 2g ) in the brain and spinal cord of transplant recipients. These few cells showed no specific clustering and were mostly localized in the proximity of meninges and blood vessels. Unlike what was observed in ICV-transplanted mice, the GFP + cells here displayed a round shape and no branchings, a phenotype likely consistent with immature cells (Fig. 2g ). By day 30, clusters of GFP + cells could be observed in the hippocampus, suggesting a specific recruitment of IV injected cells in this region (Fig. 2c ). A few cells were also detected in the forebrain region and in the brainstem, likely infiltrating throughout the brain parenchyma from the meninges and blood vessels (Fig. 2c ). The cells started to present a ramified morphology at this time point (Fig. 2g ).
The analysis at later time points (i.e. 45 days post-transplant) demonstrated the presence of an abundant donor cell progeny in the brains of the transplanted animals. This suggests a robust expansion of the graft that continued up to 90 days, independently of the transplantation route applied. In the competitive settings, there was an overall relative higher frequency of the intra-CNS versus IV transplanted cell progeny in the brain (Figs. 2d, e and S2a ). Interestingly, a route-specific cell distribution was confirmed also in this case. Indeed, at 45 days, we again observed that the ICV cell progeny in the brain was spreading rostrally, in a trajectory in the proximity of the rostral migratory stream, with many cells localized particularly in the forebrain and olfactory region. Notably, GFP + cells expanded in regions that were poorly colonized at the shorter time points, as the cortex and the cerebellum, unlike IV injected Cherry + cells whose contribution remained negligible in these areas (Figs. 2d and S2a, f ). At +90 days post-transplant, the IV-transplanted cell progeny mostly localized in the thalamus, midbrain, pons and cerebellum, whereas the ICV cell progeny expanded across all the other regions, with still a preference in colonizing the cortex (as at +45 days), the hippocampus, the corpus callosum, and the olfactory bulbs (Figs. 2e and S2a ). A unique distribution pattern of the IV versus intra-CNS transplanted cell progeny was observed also in the ITL + IV setting (Figs. 2c–e and S2a ). While the Cherry + progeny of the IV-transplanted cells expanded starting from the hippocampus to the cortex and the olfactory bulb, confirming the observations at the earlier time points, the ITL cell progeny preferentially colonized the hypothalamus, the striatum, the thalamus, the midbrain regions and the pons, adopting a mutually exclusive distribution compared to their IV-injected counterparts. When HSPCs were transplanted only IV, with no intra-CNS HSPC co-delivery, their progeny distributed also across regions occupied, in the co-transplanted mice, by the ICV-/ITL-transplanted cell progeny, as the forebrain, the corpus callosum, the hippocampus and the hypothalamus, indicating the physiological tendency of cells to migrate also in these regions upon transplantation (Fig. 2c–e and S2b ). Notably, these findings were confirmed independently from the timing of IV cell transplant and the combination of cell source adopted, indicating that the route of cell administration is the most relevant factor affecting the regional distribution of the transplant-derived cells.
To quantify and estimate relative cell abundance and distribution, and precisely map the distribution of the intra-CNS- and IV-delivered cell progeny in different brain regions at the latest time points, we acquired and quantified the signal of each reporter gene (in terms of total area covered by the reporter positive signal divided by the total area of the corresponding CNS region analyzed) (Figs. 2f and S2c,f ). The accuracy of this measurement was assessed by estimating relative abundance of the cells expressing the different reporters (intra-CNS to IV ratio) and comparing it to the data obtained by FC analysis (Fig. S2d,e ), showing a good correlation between the two measures (correlation coefficient R 2 : 0.79). Image analysis confirmed the higher donor chimerism in mice receiving HSPCs both IV and in the CNS versus IV-only recipients, and the greater frequency of CNS-administered HSPC progeny versus IV-derived ones in the animals that received the combined transplant (IV + ICV or IV + ITL) (Figs. 2d, e and S2a, b ).
Route-specific differentiation of HSPC progeny cells in the brain
We then investigated whether the route of cell administration could affect not only the engraftment, expansion and distribution of the transplanted cells and their progeny in the brain, but also their maturation and acquisition of a microglia-like phenotype. To this goal, we conducted a morphological branching analysis of the transplant-derived cells at 45 days post transplant, distinguishing between the progeny of the IV-versus intra-CNS-transplanted HSPCs in the co-transplant setting. We analyzed microglia-specific parameters (see methods for details) as per literature 33 and extrapolated two criteria to provide a morphological description of the transplanted cell progeny differentiation within the complex environment of the CNS: the total length of ramifications (Sum Length) and the complexity index (CI), defined as the ratio between the total number of branches of each cell (where a branch is the segment between two nodes), and the number of primary ramifications (defined as the segments connected to the cell body) (Fig. 3a ). We also performed an automated Sholl analysis 34 , to study the radial distribution of branches around the cell body by a quantitative method and gain information on the spatial distribution and the complexity of the cell arborization pattern (Fig. 3a ). The analysis was performed on several individual cells from different brain regions based on reporter gene expression. The branching analysis revealed significant differences between the progeny of the IV versus intra-CNS transplanted HSPC (the ICV- and ITL-transplanted HSPC progeny cells shared similar features). Indeed, the progeny of the ICV- and ITL-injected cells showed a higher sum length of ramifications as compared to cells derived from IV-transplanted HSPCs in most of the brain regions analyzed (Fig. 3b and Table S5 ). The results of the CI analysis showed a similar tendency for the progeny of the intra-CNS transplanted cells to exceed the morphological complexity of the IV-transplanted cell progeny in the same regions (Fig. 3c and Table S5 ). Interestingly, the olfactory bulb, which is the region more abundantly and more rapidly reconstituted by the progeny of the transplanted cells, represents an exception as we observed an equal complexity of ICV vs IV co-injected cells (Fig. 3c and Table S5 ). Additional parameters were evaluated with the Sholl analysis to describe the morphology of intra-CNS and IV-transplanted progeny cells. We selected the Sum intersections and number of intersecting radii to characterize transplant-derived cells as per complexity and spatial extension of the cell arborizations, respectively. By analyzing the relationship between these two parameters, we observed that the population covering the greater surface and displaying more arborizations (and occupying the upper right—UR—quadrant in the graphs of Fig. 3d ) was the progeny of the intra-CNS transplanted cells. In particular, these cells showed a structurally mature phenotype in different regions, such as the cortex and the hippocampus, as compared to the cells derived from IV-transplanted HSPCs in the co-transplanted mice (Fig. 3d, e ). Overall, this analysis confirmed the results obtained with the branching analysis and suggested that HSPC administration directly in the CNS favors the maturation of the transplanted cell progeny and its differentiation towards highly ramified microglia-like cells.
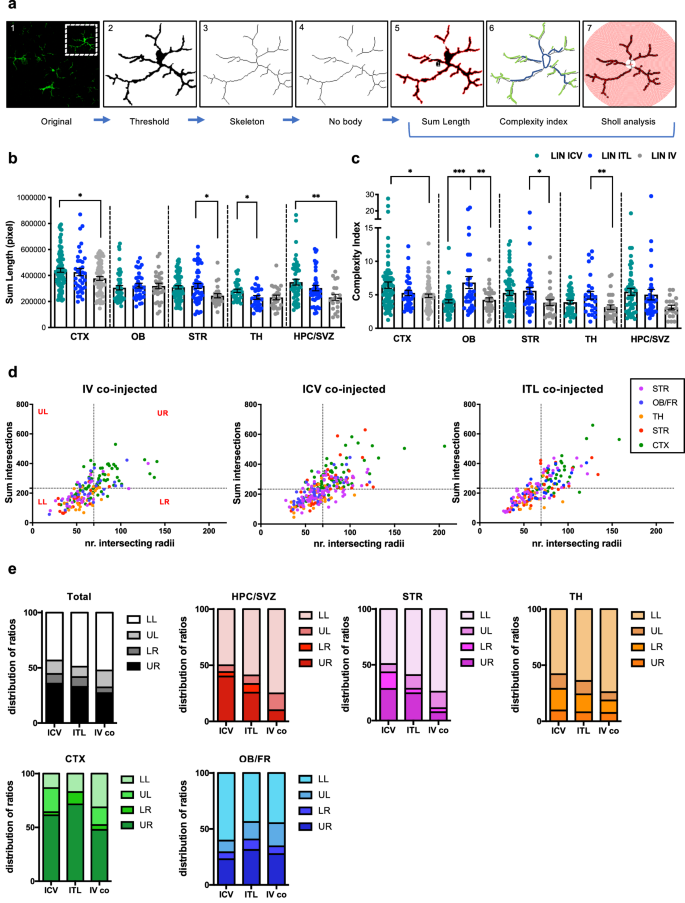
a Workflow adopted for branching analysis. Confocal images after maximum intensity projection (1) are used to select individual microglia cells based on the expression of fluorescence reporter genes (dotted box). Thresholded image (2) is used to generate a skeletonized image (3). Cell body is manually removed from the image (4). Then, the following morphological parameters are extracted: sum length of all branches (highlighted in red in image (5); complexity index expressed as ratio between the total number of branches and the number of primary ramifications highlighted in blue in image (6); Sholl analysis (7). Branching analysis on microglia-like cells engrafted in the brain of co-transplanted injected mice. Total length of ramifications (Sum Length) ( b ) and complexity index ( c ) on multiple individual microglia-like cells derived from Lin - cells transplanted ICV, ITL or from HSPCs co-transplanted IV are shown within the indicated brain region. Data from n ° cells/group comprised between 20 and 80 analyzed in n = 4 mice per group across n = 3 experimental replicates, are summarized as mean ± SEM. Source data ( b , c ) are provided as a Source Data file. Statistics for b , c : 1-way ANOVA followed by Tukey post-hoc test. For additional statistical information, refer to Table S5 . d Correlation between the number intersection radii and the sum intersections for microglia-like cells derived from HSPCs co-transplanted in the CNS (ICV or ITL) and IV, and retrieved in the indicated brain regions. The vertical and horizontal dashed lines divide the graphs in four quadrants, to highlight subpopulations with different complexity, i.e.: UR = upper right quadrant, for highly complex cells characterized by high sum of intersections and high number of intersecting radii; LL = lower left quadrant for cells with low complexity; UL (upper left) and LR (lower right) quadrants for cells displaying intermediate complexity between the LL and UR quadrant. e Histograms displaying the percentage of cells retrieved in each of the four quadrants highlighted in d . Ratios are reported for ICV, ITL and IV co-injected cells in the total and indicated regions. CTX cortex, OB/FR olfactory bulb/front, STR striatum, TH thalamus, HPC/SVZ Hippocampus/subventricular zone.
Gene expression profile of HSPC progeny cells
To investigate further the role of the CNS environment in driving the differentiation of transplant-derived cells, we analyzed by real time PCR the expression of microglia genes, including homeostatic genes (Tmem119, Tgfbr1, P2ry13, Mertk, and Olfml3, CX3CR1, CSF1R) 35 , 36 , 37 , 38 , 39 and transcriptional factors (Jun, Mafb, Mef2a) 40 from isolated transplant-progeny cells (Fig. 4 ). To this goal, we FACS-sorted transplant-derived brain subpopulations according to CD45 and CD11b expression, distinguishing CD45 high CD11b low TAμ and CD45 + / low CD11b high μ subsets from single IV-injected and co-transplanted (ICV/ITL + IV) mice at 45 days post-transplant. Within TAμ and μ, GFP + (from intra-CNS delivered HSPCs) and ∆NGFR + /Cherry + (from IV-injected HSPCs) cells were FACS-sorted separately. We selected an early post-transplant time point (45days) to increase our ability to identify early patterns of cell differentiation possibly leading to the morphometric differences described at 90 days post-transplant. Indeed, at this time point we observed the greatest difference between TAµ and µ frequency in the tested transplant conditions and the analysis of these two subsets at this time point could enhance our abilty to capture information about the process of differentiation and maturation of donor-derived myeloid cells in the CNS towards functionally equivalent microglia cells. Myeloid cells sorted from the brain of naïve adults and pups (post-natal day 1- P1) were used as reference, and values were expressed as fold change to adult microglia cells. Consistently with our previous data, the CD45 + CD11b + myeloid progeny of the transplanted cells, independently from the setting applied, displayed a microglia-enriched signature comparable to that of the adult naïve microglia (Fig. 4a and Table S6 ). Interestingly, we observed differences in the expression of the microglia related genes depending on the route of cell injection employed. Indeed, a higher expression of both homeostatic genes and transcriptional factors were observed in the progeny of the ICV transplanted HSPCs, as compared to the progeny of ITL and IV transplanted HSPCs (Fig. 4a, b and f and Tables S6 , S8 ). These observations were also confirmed within the µ and TAµ fractions (Fig. 4 c, d and Table S7 ). Indeed, only the two GFP + ICV-derived subpopulations expressed microglia-enriched homeostatic genes and transcriptional factors at levels overlapping those of the reference adult naïve microglia, indicating a higher maturation state reached in this transplant setting as compared to the others (IV and ITL). An intermediate level of expression of the microglia genes was observed in the ITL-progeny cells. Notably, transcriptional factors were expressed at above normal level in the ICV cell progeny, which also showed the highest expression of microglia specific genes, suggesting that the enhanced expression of the microglia associated transcriptional factors could have determined an early boosting phase driving the differentiation of transplant-derived cells (Fig. 4c, d and f and Tables S7 , S8 ).
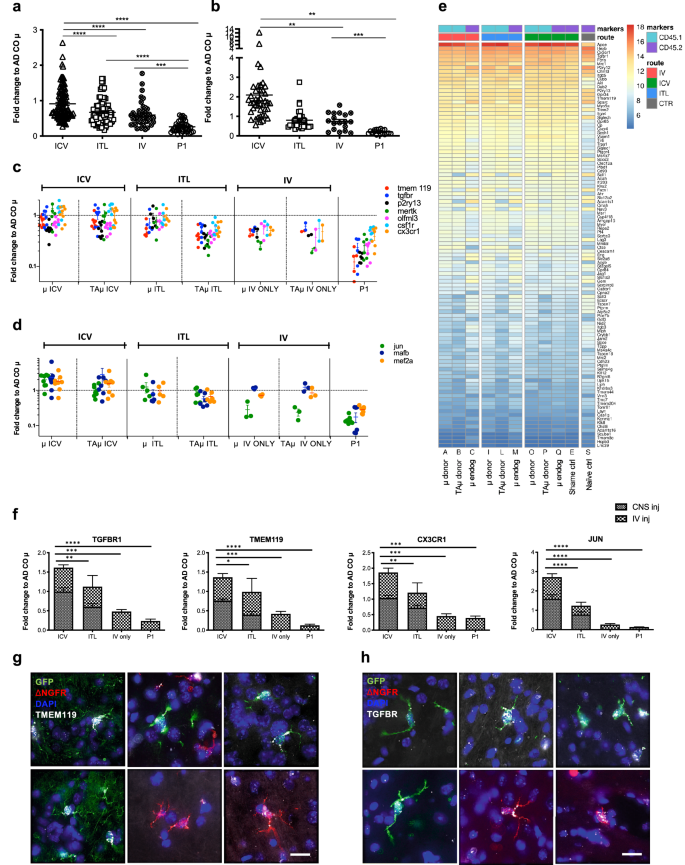
Expression of a pool of selected microglia genes ( a ) and transcription factors (TF) ( b ) obtained by qPCR in microglia cells FACS-sorted from the brain of IV only, co-transplanted animals sacrified at 45 days post-transplant (pTX) and pups (P1) [ N = 3 mice/group]. Data are shown as mean ± SEM. Statistics for A-B: 1-way ANOVA followed by Tukey post-hoc. c - d Microglia enriched genes ( c ) and TF ( d ) shown in a , b reported as individual values in µ and TAµ subpopulations. Data are shown as fold change (calculated as 2 − DDCT) on adult control microglia. P1 mice are used as control of immature microglia. Mean values ± SEM are shown. Statistics for c , d : 1-way ANOVA followed by Tukey post-hoc test. e Heatmap of normalized expression values for microglia genes, TF and F4/80 low/high signature genes from Bruttger et al. Genes (rows) are sorted according to the mean gene’s expression. RNA sequencing was performed on a pool of animals ( n = 3 mice/group) analyzed at 45 days pTX upon sorting of µ and Taµ subpopulations. Endogenous microglia from untreated or busulfan-treated mice injected ICV with PBS (sham-control) were used as controls. Statistical analysis is performed in R, using DEseq2 package. f Expression of TGFBR1, Tmem119, CX3CR1 and JUN genes obtained by qPCR on GFP (ICV or ITL) or ΔNGFR (IV) labeled microglia-like cells FACS-sorted from the brain of intra-CNS and IV only transplanted mice [ n = 3 mice/group]. Data are shown as fold change (calculated as 2 − DDCT) on adult control microglia. Mean values ± SEM are shown. Statistics for f : 1-way ANOVA followed by Tukey post-hoc test. Source data ( a – d and f ) are provided as a Source Data file and additional statistical information are shown in Tables S6 – 8 . g , h Representative laser scanning confocal microscope microphotographs of brain sections showing the expression of selected microglia genes in the brain of intra-CNS co-transplanted mice [ n = 5 slices from n = 2 mice/group] detected with RNAscope. Tissues were hybridized with probes for Tmem 119 ( g ) or TGFBR ( h ) gene (white dots) and their expression was analyzed within CNS (green) or IV co-injected cells (red). Nuclei were counterstained with DAPI (blue). Scale bar = 50 μm.
To provide a more complete characterization of the transcriptomic differences between the different myeloid brain subpopulations retrieved from the transplanted mice we performed a genome-wide expression analysis by means of an Illumina RNA sequencing (RNA-seq) platform on a pool of sorted µ and TAµ subpopulations from mice that were IV, ITL or ICV transplanted 45 days earlier with LV_GFP CD45.1 HSPCs (N = 2–3 animals/group) (Fig. 4e ). Donor-derived and recipient myeloid cells were identified and sorted separately according to the expression of CD45.1 and CD45.2, respectively, within the CD11b brain compartment and characterized for the expression of microglia signature enriched genes and transcription factors commonly expressed by microglia. To characterize host microglia and newly formed microglia-like cells (µ and TAµ) in the different settings, we further extended the analysis to a published gene set that was previously used for studying microglia and engrafting myeloid cells across several models of microglia depletion 41 , 42 , 43 , 44 . Overall, we observed a very similar expression pattern between resident microglia and corresponding donor-derived µ and TAµ myeloid subpopulations retrieved from each of transplant settings. A lower expression of Sall1, commonly associated to yolk sac-derived microglia, was noticed in transplant-derived cells compared to controls, likely due to the different ontogeny 42 . Sall1 expression was lower in TAµ versus µ cells, further supporting the hypothesis of a differential state of maturation of the two subpopulations. As an additional control, we analyzed endogenous microglia retrieved from busulfan-treated mice injected ICV with PBS (sham control), to exclude with the reported results that the procedure per se could significantly affect the gene expression profile of resident microglia. In this setting we observed some differences that might be associated with a response to the conditioning regimen, this being the only difference when comparing endogenous microglia from untreated vs transplanted animals.
Notably, in situ RNA analysis of selected genes on cryostatic brain sections by RNAscope assay confirmed the expression of representative above-mentioned genes by the ICV/ITL derived cell progeny (Fig. 4g, h ).
Overall, this data indicate that, independently from the route, we generated microglia-like cells with a signature reminiscent of microglia.
Therapeutic benefit in an Alzheimer’s disease animal model
Given the exquisite neurodegenerative nature of Alzheimer Disease (AD), we considered AD as an ideal setting to assess the therapeutic potential of our CNS-restricted HSPCs transplantation approach. In particular, we conducted a proof of concept (PoC) of efficacy study selecting the optimized protocol for ICV delivery of engineered HSPCs in a severe animal model of AD that requires timely intervention. For HSPC engineering we focused on TREM2 (Triggering receptor expressed on myeloid cells 2), a microglia cell-surface receptor required for microglial responses to neurodegeneration. TREM2 is needed to enhance signaling and to boost the metabolic capacity of microglia 45 and its overexpression through BAC-mediated transgenesis showed encouraging results in enhancing the scavenging function of microglia and pathological phenotypes in AD murine models 46 . Importantly, elevated risk of developing AD is associated with hypomorphic variants of TREM2 47 . We produced 3rd generation LVs expressing the human TREM2 cDNA under the control of the human phosphoglycerate kinase (hPGK) promoter, which has a ubiquitous robust expression, and confirmed vector-mediated TREM2 expression in transduced 293 T cells and BV2 murine microglia cell lines by Western blot (WB) (untrasduced cells were used as negative control) (Fig. S3a ). We then proved transgene functionality by comparing the ability to engulf Aβ of mock treated versus TREM2-LV transduced BV2 microglia cells by a fluorescent imaging system. As expected, Aβ oligomer engulfment was increased in TREM2-LV transduced BV2 cells as compared to untransduced control cells (Fig. S3b ). We then challenged our approach in transgenic mice with Five Familial Alzheimer’s Disease mutations (5xFAD), an established model of AD that express human APP and PSEN1 transgenes with a total of five AD-linked mutations leading to overproduction of Aβ−42 with an accelerated amyloid plaque pathology similar to that found in patients 48 . These mice develop early onset AD disease at approximately 6 weeks of age, with survival reduced after 10 months. Lin - HSPCs were purified from the BM of young adult 5xFAD donor mice and transduced with the TREM2-encoding LV in standardized conditions (see methods for details). The transduced Lin - cells (0.3−0.5 × 10 6 /mouse) were injected ICV in BU myeloablated (25 mg/kg/day for 4 consecutive days), 2 months-old 5xFAD recipient mice, which then received an unmanipulated BM support administered IV 5 days post-transplant to rescue peripheral hematopoiesis (2 × 10 6 total bone marrow cells from 5xFAD donors). Controls included 5xFAD mice transplanted with mock- or GFP-transduced 5xFAD Lin - cells, and wild type animals transplanted with mock- or GFP-transduced wild type Lin - cells, age matched to treated animals (Fig. 5a ). We performed lentiviral Vector Copy Number (VCN) analysis on the liquid culture (LC) progeny of the pre-infused cell products and confirmed a similar LV content in the different settings. In particular, we measured VCN values of 8.45 ± 1.34 LV copies/genome in the LC progeny of WT Lin - cells transduced with the GFP_LV, and of 8.54 ± 1.09 and 10.09 ± 1.52 copies/genome in the LC progeny of 5XFAD Lin- cells transduced with the GFP_LV and hTREM2_LV, respectively. Phenotypic assessment and behavioral tests were then performed on the treated and control animals every month, starting at 4 months until they reached 12 months of age. Indeed, the 5xFAD mouse model develops an age-dependent motor phenotype in addition to working memory deficits in an alternation task, and reduced anxiety levels, as revealed in the elevated plus maze (EPM) task 49 , 50 . The EPM test, employed for the assessment of anxiety, demonstrated a clear disease progression of AD in control animals (mock/GFP-transplanted 5xFAD mice), while an ameliorated phenotype was observed in the TREM2-treated group, as shown at the 12 months-time point (Fig. 5b ). For the analysis, data from mock and GFP transplanted mice were combined, to demonstrate the consistency across experimental conditions of animals that received identical treatment, consisting of four doses of Busulfan (25 mg/kg) and HSPC ICV transplantation.
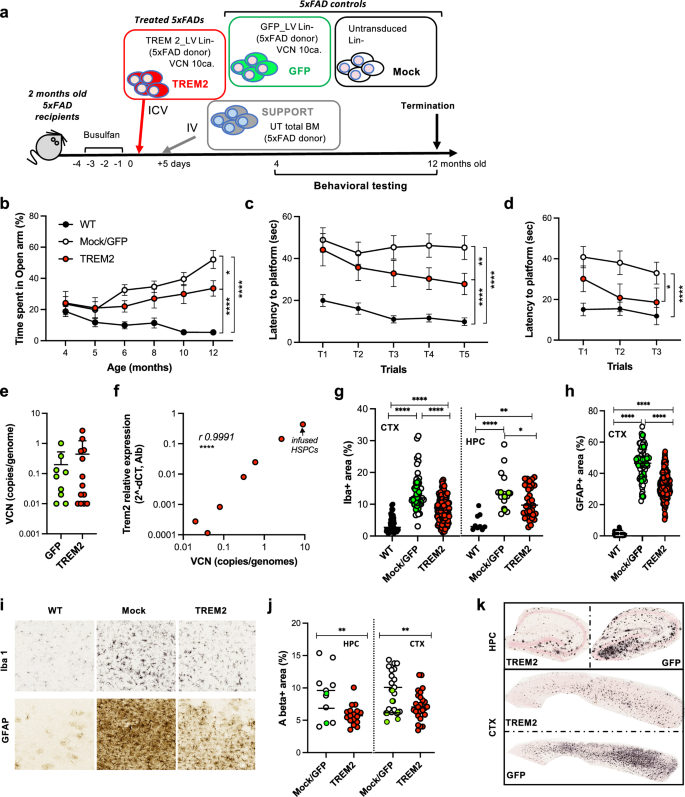
a Experimental scheme of TREM2_LV HSPCs transplantation in 5xFAD mice. Performance of TREM2, mock/GFP transplanted 5xFAD mice and WT controls in the elevated plus maze (EPM) ( b ), analyzed monthly from 4 to 12 months and in the Morris Water Maze (MWM) test, in the hidden platform ( c ) and reversal platform trial ( d ), at 12 months ( n ° mice/group/time point b: N ≥ 7; c : N ≥ 12; d : N ≥ 9). Data values ( b – d ) are shown as MEAN ± SEM [ n = 4 experimental replicates]. Statistics ( b – d ): 2-way ANOVA, Tukey post-hoc test [( b , p * = 0.0272 p **** < 0.0001), ( c , p ** = 0.0039, p **** < 0.0001), ( d , p * = 0.0231, p ****<0.0001)]. e VCN calculated by ddPCR on DNA from the brain of 5xFAD treated animals [GFP: n = 9, TREM2: n = 13, n = 4 experimental replicates]. Statistics: unpaired T test: not significant ( P -value: 0.3881). f Correlation between VCN and TREM2 expression measured by SYBR Green qPCR on brain from transplanted mice [ n = 6] and on the liquid culture progeny of the infused HSPCs (reference). Correlation analysis: Pearson r = 0.9991, R 2 = 0.9981, p < 0.0001. Signal quantification for Iba1 ( g —cortex, CTX and hippocampus, HPC) and GFAP ( h —CTX), on brain from treated 5xFAD mice (Mock/GFP and TREM2) and controls (WT) at >12months [ n ° slices/mouse: g , h CTX: N > 15 slices/ n = 3 WT, n = 2 Mock/GFP, n = 10 TREM2; g , HPC: N > 3 slices/ n = 3 WT, n = 3 Mock/GFP, n = 6 TREM2]. Statistics ( g , h ): 1-way ANOVA, Tukey post-hoc test ( p * = 0.416, p ** = 0.0032, p **** < 0.0001). Data from mock and GFP mice are indicated by clear versus green dots, respectively. i IHC on brain slices showing the extent of neuroinflammation (Iba1 and GFAP staining) retrieved in mock vs TREM2 5xFAD transplanted mice at 12 months. j Quantification of Aβ deposits in the CTX and HPC of TREM2 transplanted animals (brain VCN > 0.5) compared to GFP/Mock animals [ n > 3 slices/mouse, TREM2 n = 4, Mock/GFP n = 4]. Mean ± SEM, asterisks indicate significance at unpaired t -test (CTX p ** = 0.0012; HPC p ** = 0.0016). Source data ( b – h , j ) are provided as a Source Data file. k IHC on brain slices showing the extent of Aβ deposits in the HPC and CTX of TREM2 and mock/GFP Tx 5xFAD mice at 12 months. IHC in i and k [ n > 8 slices/mouse, n = 3 mice/group].
Similar results were observed with the Morris Water Maze (MWM) test (Fig. 5c, d ), here used to document cognitive deficits at late stage of disease (12 months). Indeed, MWM revealed a different ability of controls vs TREM2-treated animals to travel in the target quadrant (NW)(probe trial) and to reach a hidden platform over multiple trials (Figs. 5c, d and S3c ). In particular, TREM2-treated animals showed a lower latency in reaching the platform and covered a significantly increased distance in the target quadrant (NW) as compared to control mock-treated 5xFAD animals, with a performance similar to wild type controls (mock/GFP transplanted wild type mice), indicating a preserved memory retention or recall as compared to 5xFAD controls (Fig. S3c ). Moreover, TREM2-treated animals showed a significant improvement in the latency to find the hidden platform after 5 trials as compared to controls and a prevention of the deficit of cognitive flexibility observed in 5xFAD during reversal learning (Fig. 5c, d ). After completing behavioral testing, the study was ended when treated and control mice reached 12 months of age, with euthanasia and tissue collection for molecular and histological analyses. Quantification of lentiviral VCN on the whole brain tissue documented a uniform engraftment of the engineered cell progeny in both treated and control animals (Fig. 5e ). In addition, hTREM2 expression was measured on the brain of the treated animals by RT-qPCR, showing a strong correlation (R 2 = 0.99) between protein expression and LV-hTREM2 content measured as VCN (Fig. 5f ). To further measure disease modifying effects of the transplant, histology was performed on brain sections from the experimental animals. The extent of neuroinflammation was quantified by Iba1 and GFAP staining on brain tissue slices (Figs. 5g–i and S3d ). Overall, the expression of Iba1 (Fig. 5g ) and GFAP (Figs. 5h and S3d ) was higher in control 5xFAD compared to wild type animals indicating the presence of both microglia and astrocyte activation. Instead, TREM2-treated animals showed a significant reduction of microgliosis (Fig. 5g ), particularly in the cortex and hippocampus region. Moreover, measurement of Aβ accumulation was performed (Figs. 5j and S3d ). Interestingly, the treated animals showed an overall significant reduction in Aβ deposition levels throughout the brain (Fig. S3d ), that was particularly evident in the cortex and hippocampus (Fig. 5j ) with dose-dependency: higher rates of Aβ reduction were observed in animals showing a VCN in the brain tissue > 0.5, average value of the study cohorts.
To assess whether therapeutic benefit was indeed related to an interaction of the TREM2 expressing cells with Aβ plaques, as hypothetized, we performed an in-situ hybridization (ISH) with a probe directed against the Woodchuck Hepatitis Virus (WHV) post-transcriptional regulatory element (WPRE) 51 , as a strategy to specifically identify the LV-transduced TREM2 expressing cells. Indeed, since WPRE sequence is not present in the mouse genome, ISH for WPRE allows to detect specifically the distribution of LV-transduced cells in the tissue 52 ; moreover, as the WPRE sequence was added after the coding sequence o TREM2 in the LV expression cassette to stabilize the therapeutic transcript, the WPRE signal at in-situ corresponds to TREM2 expression. The ISH was combined with IF staining with an anti-A β antibody. Through this approach, we could detect abundant TREM2-expressing cells in the brain of the treated 5xFAD mice; these cells, which were distributed throught the brain parenchyma, were particularly abundant in the cortex and hippocampal regions, which are the brain areas where A β plaques were mostly detected (Fig. 6a ). Interestingly, the TREM2 expressing cells were detected in the close proximity of the A β signal (Fig. 6b ) and not in other regions as the cerebellum, where A β plaques were not retrieved (Fig. S3e ). To elucidate the contribution of these cells to amyloid plaque phagocytosis we employed activation-associated markers such as CD68 for CNS tissue stainings on treated animals. Interestingly, the donor-derived microglia like cells identified by WPRE ISH probe positivity and Iba1 staining in the proximity of amyloid plaques showed an activated morphology (increased soma size and thickening of processes) and robust expression of CD68, allowing to hypothesize for them an active role in reducing the amyloid burden (Fig. 6c–g ). On the contrary, microglia distant from plaques showed in general lower CD68 expression and showed morphological features characteristic of a more homeostatic/resting state.
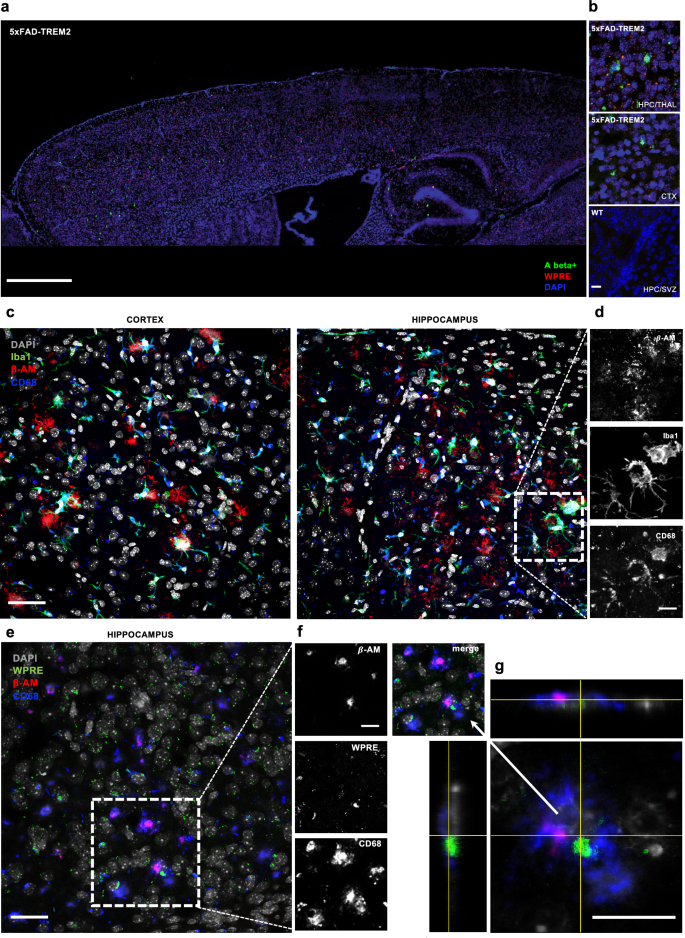
a Representative epifluorescence microscope image reconstruction of a LV_TREM2 transplanted 5xFAD mouse showing the enrichment of transduced cells (identified through RNAscope with WPRE-specific probe, in red) in the cortex and hippocampus, in proximity to Aβ deposits, highlighted by IHC (in green). DAPI nuclear stain is shown in blue. Images were captured at a 20x magnification. Scale bar = 1 mm. N = 5 brain slices from n = 2 mice were analyzed. b Representative laser scanning confocal microphotographs of the cortex and hippocampal region from the brain of 5xFAD TREM2 transplanted mice (top and central figure) analyzed in a or in a WT mouse (bottom) used as negative control to demonstrate the specificity of Aβ amyloid and WPRE. Images were captured at a 40x magnification with a 1.5x digital zoom using a Leica SP5 scanning confocal microscope. CTX cortex, HPC hippocampus, THA thalamus, SVZ sub ventricular zone. Scale bar = 50 µm. c Representative laser scanning confocal microphotographs of the cortex and hippocampal region from the brain of a representative TREM2 transplanted mouse showing that reactive Iba1 + microglia/myeloid cells localized in close proximity to beta amyloid plaques are immunopositive for CD68. Images were captured at a 40x magnification with a 1.5x digital zoom using a Leica SP5 scanning confocal microscope. Scale bar = 50 µm. d Single channels of the ROI highlighted in the dashed square in c . Scale bar = 25 µm. e Representative laser scanning confocal microphotographs of the hippocampal region from the brain of 5xFAD TREM2 transplanted mice showing the presence of WPRE probe positive signal in CD68 positive cells in proximity of beta amyloid plaques. Scale bar = 50 µm. f Single channels of the ROI highlighted in the dashed squares in e . Scale bar = 25 µm. g Orthogonal view of the cell highlighted by the arrow in f . Scale bar = 25 µm. Images in c - g were analyzed in N = 4 brain slices from n = 3 mice.
Overall, these data provide clear proof of the therapeutic potential of HSPC gene therapy in a pure neurodegenerative condition as well as evidence of therapeutic benefit associated to a CNS-restricted engraftment of engineered hematopoietic cells.
The therapeutic potential of engineered HSPC transplantation in benefiting selected monogenic neurodegenerative conditions has been extensively proven in mice and humans 53 . The engineered HSPCs exert therapeutic benefit through their mature progeny that represents a healthy, metabolically competent myeloid population, which acquires microglia-like features in the CNS, capable of protein delivery and restoration of the scavenging and homeostatic functions 54 . Therefore, enhancing the engraftment, distribution in the CNS and maturation of the transplanted cells/their progeny towards a microglia-like phenotype, as well as fine tuning the contribution of the transplant solely to the CNS could allow to further improve the efficacy of HSPC-based gene therapy protocols for CNS disorders. To this goal, we previously proposed the direct CNS-delivery of HSPCs in the lateral ventricles (ICV) as an approach for favoring the turnover of endogenous microglia with donor cells 6 . Confirming the interest in this approach, other groups have recently exploited the intra-CNS route of cell administration by conducting biodistribution studies on differentially transplanted animal model 32 , 55 . Intra-CNS transplantation was used to evaluate the capacity of microglia or peripheral immune cells to engraft in the brain parenchima of CSF1R -/- mice that lack microglia 43 , 56 . Interesting data were also obtained by transplanting hPSC-derived primitive macrophage progenitors via intra-CNS injection (i.e intracranial, transnasal injection) 57 , 58 .
In our work, by challenging further the hematopoietic cell transplantation paradigm we unveiled a key role exerted by the CNS environment in uniquely driving expansion, distribution and myeloid differentiation of the locally transplanted HSPCs towards a microglia-like phenotype. Importantly, we also provided a clear example of the therapeutic potential of this approach that determines a CNS-restricted engraftment of the genetically modified transplant progeny cells, in a multifactorial neurodegenerative condition, namely in a severe murine model of AD.
Firstly, we showed that intra-CNS delivery favors the transplanted HSPC engraftment over IV-delivered cells in a competitive setting. This was demonstrated by employing two different intra-CNS delivery routes, ICV and ITL. Both routes are clinically viable. Indeed, despite the ITL route, here employed for the first time for HSPC delivery, is by definition a very feasible and poorly invasive intra-CNS delivery route, protocols allowing the safe administration of drugs, proteins or cells in the cerebrospinal fluid through ICV delivery are also available in clinical practice 59 , 60 , 61 . Interestingly, the two intra-CNS routes equally favored CNS colonization by transplant progeny cells. Importantly, with both ICV and ITL HSPC delivery, the intra-CNS injected HSPCs exceeded the IV co-transplanted cells in contributing to the donor-cell chimerism in the brain in all the tested conditions, despite the number of cells delivered intra-CNS was substantially lower that the IV dose. This advantage was particularly evident at the earliest time points and up to 90 days post-transplant, independently from the setting employed, with strong clinical implications for diseases in which a timely and sustained therapeutic delivery to the brain is essential for an effective benefit. However, differently from the ICV injected cells that do not contribute to marrow hematopoietic reconstitution 6 , ITL-delivered HSPCs also robustly engrafted in the marrow of transplanted mice. This may be attributed to differences in the BBB architecture in the brain as compared to the spinal cord 62 . Indeed, despite the blood-spinal cord barrier (BSCB) has a similar cellular and molecular structure as the BBB, the lower number of pericytes and reduced expression of junctional proteins retrieved in the BSCB result in an increased permeability as compared to the BBB. In accordance, murine spinal cord microvascular endothelial cells in culture were shown to express reduced amounts of ZO-1, occludin, b-catenin, VE-cadherin and P-glycoprotein than brain endothelial cells 63 . Thus, the characteristics of the two barrier systems, i.e. the BBB and the BSCB, might be responsible for some of the differences observed between the two routes in the colonization potential of the hematopoietic organs. Notably, and in contrast to recent works 32 , we observed significantly higher donor engraftment when a combination of intra-CNS and IV routes were adopted compared to single IV transplantation up to 3 months post-transplant, indicating a faster early seeding of progeny cells achieved in the co-transplant setting. These results point out the clinical relevance of the combination of different HSC transplant routes, particularly for those diseases where anticipating the time of clinical benefit is crucial. Different outcomes 7 , 30 , 31 could be explained by different methodologies applied, i.e. for the administration of the conditioning protocol, which is essential to favor robust CNS-engraftment of the transplant progeny cells, or for HSPC manipulation and transplantation.
Interestingly, we also showed that intra-CNS delivery of HSPC is associated to a specific pattern of distribution of progeny cells in the brain. As early as 14 days after transplant, the GFP + progeny of the transplanted cells was located in the close proximity of the ventricular structure, projecting rostrally and towards the basal forebrain, in a trajectory which may suggest proximity to the rostral migratory stream. Interestingly, these ICV-derived cells already showed discrete branchings indicating an actively occurring differentiation process. Notably, and at odd with these findings, the progeny of the IV transplanted cells had no preferential location and was mostly identified in the proximity of meninges and blood vessels, showing a morphology consistent with immature cells, with a round shape and no branchings. While the former findings (ICV) are suggestive of anatomical proximity leading the cell distribution, the latter (IV) are in line with the emerging view of the meninges as a potential relevant route for cellular infiltration to the brain in physiological 64 and pathological conditions 65 , 66 . This is possible due to the anatomical distribution of the meninges and their unique relationship with blood, lymphatic vessel and CSF. In addition, meninges consist of highly heterogeneous cell populations including endothelial cells, CNS-associated macrophages, blood borne immune cells as well as meningeal neuronal precursors capable to respond from their local niche to external chemokine-mediated stimuli and promptly react and infiltrate the brain parenchima in pathological conditions 67 . The analysis at the subsequent time points post transplant provided additional relevant information. Firstly, the progeny of the ICV-transplanted cells followed a path reminiscent of the rostral migratory stream and likely exploited the cerebral ventricular system, including the lateral, third and fourth ventricles, to diffuse into nearby regions such as the striatum, the anterior olfactory area and the brainstem. On the other hand, the progeny of the IV-administered HSPCs preferentially localized in the hippocampus, as a possible effect of the secretion of key chemokines as stromal cell-derived factor (SDF-1/CXCL12), and monocyte chemoattractant protein-1 (MCP-1/CCL2) from this area, as described in the literature 68 . The pattern of cell distribution adopted by ITL transplanted cells became also more apparent, involving the olfactory bulb and forebrain regions as well as the brainstem, consistently with the proximity to the spinal cord (injection site). Despite the differences, all three routes shared a common early colonization of the olfactory bulb/forebrain area. As neural precursor cells from the subventricular zone have been shown to migrate along the rostral migratory stream to the olfactory bulb 69 , 70 , we could hypothesize that also engrafted HSPC-progeny cells could follow the same route of distribution and rapidly reach this region upon IV and ICV injection. A different way to reach this area could be hypothesized for cells infused via ITL injection, possibly involving a specific vascular network. Interestingly, the observation at 45 and 90 days post-transplant, time points where co-transplantation (intra-CNS and IV) was performed to allow animal survival after myeloablation, allowed also to assess the relative contribution of the co-transplanted cell progeny to microgliosis. Indeed, we noticed that the areas preferentially colonized by the ICV-transplanted cell progeny were those closer to the injection site, from the lateral ventricles to hippocampus, corpus callosum, olfactory bulbs and up to the cortex, while those preferentially colonized by the ITL-transplanted cell progeny were the midbrain, pons, hypothalamus and thalamus. Interestingly, in both settings, the progeny of IV co-transplanted cells colonized alternative regions, with a mutually exclusive pattern. The finding that the transplant cell progeny could adopt a mutually exclusive distribution depending on the route of cell delivery could be interpreted in different ways. The most obvious speculation would be that cells directly injected in the CNS could have an advantage in terms of early occupancy of areas and niches that would no longer be available for brain immigrant cells incoming after intravenous injection and/or BM engraftment. According to the route of administration, cell trafficking into the CNS could be mediated by intrinsic factors or specialized tissue interfaces such as the meningeal vasculature or the choroid plexus structure 71 . Indeed, the choroid plexus, as critical interface between the systemic blood circulation and the CSF, exerts an important role in supporting immunosurveillance and regulation of cell activation and trafficking in response to pathological insults or specific cytokine milieu, due to the presence of specialized cell types like dendritic cells or macrophages, and specific patterns of epithelial adhesion molecule expression that could be recruited in response to conditioning and HSPC transplantation 72 .
Regardless of the route, we confirmed that the progeny of both IV and intra-CNS delivered cells acquires morphological features typical of homeostatic microglia. Branching and Sholl analysis demonstrated that the progeny of intra-CNS transplanted cells has a more complex morphology as compared to IV co-injected cells, with a higher number of ramifications and a more enriched arborization network, particularly in the regions in which they preferentially expanded. The observation that the progeny of the HSPCs transplanted in the CNS has a greater resemblance to endogenous microglia than the progeny of cells transplanted IV suggests that the CNS environment could have a role in favoring and driving their differentiation and maturation. This hipothesis is supported by several works showing the importance of the environment for the acquisition of microglial feature 43 . As described by Bohlen and colleagues, isolated microglia kept in culture in “non-neural” medium drastically reduce the expression of microglial signature genes, while reversing the loss of microglia identity when re-infused into the brain of CSF1R -/- mice 56 . Therefore, if the transcriptome of mature microglia could be affected by CNS signaling, it is plausible to assume that intra-CNS injected HSPCs may differentiate toward microglia faster than upon IV administration. The olfactory bulb was characterized by high but equal presence of both IV and intra-CNS cell progenies in the combinatorial transplants with no morphological differences, supporting the hypothesis that this neurogenic area may play a crucial role in the recruitment of engrafted cells independently from the route of cell injection, as demonstrated by the short term biodistribution study.
Notably, the results of a transcriptome analyses and of independently generated RNAseq data performed on microglia-like cells sorted from the brain of the transplanted mice were in line with our morphological observations, confirming the expression of microglia signature genes by newly formed cells with some differences depending on the transplantation route adopted and the brain subpopulation analyzed. Indeed, the expression of microglia signature genes 35 , 36 , 73 and transcriptional factors such as mafb and jun 40 , required for microglia development correlates with the greater maturation stage of intra-CNS vs IV-transplanted cell origin. The analysis was performed at an early post-Tx time point (45days) to increase our ability to identify early patterns of cell differentiation possibly driving the maturation of transplant-derived cells described at 90 days post-transplant. According to our experience, this could explain the differences observed when comparing our data with those obbtained by other groups 32 , by which a downregulation of canonical genes associated with resident microglia, like Tmem119, was observed at later time points (12–13 months post-transplant).
Clearly, despite our transplant-derived microglia-like cells, and particularly those derived from ICV/ITL transplanted HSPCs, resemble their endogenous counterpart, their gene expression profile does not completely overlap with that of bona fide microglia isolated from adult naïve mice, as also reported by others 32 , 74 , 75 . This finding, which could be related to a different maturation stage between resident and newly formed microglia or to their different ontogeny, is in agreement with what reported by recent works that described how bone marrow-derived cells that colonize the brain parenchyma in response to conditioning or pathology present differences at the trascriptome level compared with adult microglia of embryonic origin, despite the fact that they resemble microglia in morphology and in the expression of many key markers 42 , 43 , 44 , 74 , 76 . Importantly, these differences at the transcriptome level do not necessarily result in functional impairment. On the contrary, it was demostrated that bone marrow-derived cells are even more efficient than yolk sac-derived microglia in certain conditions, mostly associated with aging, such as phagocytosis of amyloid deposits 74 , 77 .
To this regard, recent works have shown the potential of bone marrow derived microglia-like cells as cell-based disease-modyfing therapy for AD 77 , 78 . In line with these interesting findings, we here provided experimental proof of concept of the therapeutic potential of intra-CNS HSPC gene therapy for AD in a severe animal model of the disease. In this setting, we selected TREM2 as a relevant therapeutic transcript specifically intended at enhancing a disease-specific function of transplant-derived microglia-like cells for this study. Importantly, upon ICV transplantation of HSPCs from 5xFAD mutant mice transduced with a LV encoding the human TREM2 cDNA under the control of a moderate-strenght promoter, a CNS-restricted engraftment of the TREM2-expressing cells was observed up to 12 months post-transplant in busulfan myeloablated 5xFAD recipients. This resulted in substantial disease-modifying effects: a significant amelioration of the behavioral and histopathological disease manifestations was observed, with improved performance of the treated versus control mice at the Elevated plus maze and Morris Water Maze tests, as well as reduction of neuroinflammation (astrogliosis and microgliosis).
The phenotypic effects observed were accompained by a LV-dose dependent decrease of Aβ-deposits in treated mice as compared to mock treated controls. Importantly, using a WPRE ISH probe as strategy to specifically identify the LV-transduced cells in brain sections, we documented a preferential distribution of the TREM2 expressing cells in brain regions associated to β amyloid deposits such as the cortex and hippocampal area and in particular a localization of CD68 + TREM2 + cells in an activated state in the close proximity of amyloid plaques.
These evidences thus support the hypothesis that TREM2 microglia could exert a key role in A β clearance, in agreement with previous studies that demonstrated migration of engrafting microglia to amyloid plaques in the CNS of chimeric models, followed by secretion of proteins that in turn promote the Aβ degradation and phagocytosis 79 . Notably, the capability to clear A β was, as reported, in some cases even more prominent than that of the resident microglia 80 .
Of note, despite the proof-of-concept nature of our findings will require proper adaptation of protocols in a clinical development plan, it is noteworty to report that, in the presence of anticonvulsant prophylaxis during conditioning administration, no short- or long-term neurotoxic effects of busulfan were noticed, at least phenotypically as per regular daily observation and at behavioral studies, nor obvious and severe signs of neural damage were observed at histopathological observation of the treated and control mice. Although specific neurotoxic effects of busulfan across epileptogenic and/or other neural circuits in this Alzheimer’s disease models remain undefined at the microscopic evaluation, such effects have not compromised treatment outcomes nor treated and control mice overall performances.
In conclusion, this work unveiled a key role exerted by the CNS environment in uniquely driving expansion, distribution and myeloid differentiation of locally transplanted HSPCs towards a microglia-like phenotype and showed the robust potential of intra-CNS HSPC gene therapy for exerting specific therapeutic effects in the CNS, paving the way for the design of ad hoc gene therapy strategies to treat severe neurodegenerative disorders.
The specific protocols covering the studies described in this paper were performed in compliance with all relevant ethical regulations and were explicitly approved by the Italian Ministry of Health, an internal ethical committee at San Raffaele Hospital (IACUC 573) and the Dana Farber Animal Facility Institutional Animal Care and Use Committee (IACUC 15-031). Experiments performed on wild type C57BL/6 mice or C57BL6/Ly5.1 mice (hereafter called CD45.2 or CD45.1 respectively) were obtained from Jackson Lab or Charles River and maintained at San Raffaele Hospital and at the Dana Farber Animal Facility. Experiments performed on 5xFAD mice (#034840-JAX) were obtained from Jackson Lab and maintained at the ARCH animal Facility at Boston Children’s hospital (approved by the Institutional Animal Care and Use Committee (IACUC 17-06-3455) and at Fondazione Istituto di Ricerca Pediatrica Città della Speranza (IRPCDS) animal facility (IACUC 46/2023-PR).
Animals used in the study were housed within temperature and humidity ranges appropriate for the species, to which they can adapt with minimal stress and physiologic alteration. Light in animal holding rooms provided for both adequate vision and neuroendocrine regulation of diurnal and circadian cycles.
Isolation, transduction and transplantation of murine hematopoietic cells
Seven/eight-week-old wild type mice were euthanized with CO 2 , and the bone marrow (BM) was harvested by crushing bones. After BM lysis, HSPCs were purified by Lineage- (Lin - ) selection using the Miltenyi Biotec Lineage Cell Depletion Kit with Magnetic separation with the autoMACS™ Separator, following manufacturer’s instruction. For the isolation of the KSL fraction (c-kit + sca-1 + ), Lin - cells were then stained with rat APC-eFluor 780 anti-mouse CD117(c-kit) (eBioscience, 1:100) and with rat Pe-cy7 anti-mouse Ly6A/E Sca-1 (Sca-1) (BD Bioscence, 1:100). At the end of the staining the cells were isolated by the cell sorter MOFLO XDP (Becton Dickinson), according to the expression of the selected markers. BM cells, isolated Lin - or KSL were transduced using different Lentiviral Vectors (LVs), for 16 h at Multiplicity of Infection (MOI) 100 81 . The following LVs were used: pCCLsin.cPPT.humanPGK.GreenFluorescentProtein.Wpre for CNS injected cells (GFP-LV) pCCLsin.cPPT.humanPGK.DeletedNerveGrowthFactorReceptor.Wpre (ΔNGFR-LV) or pCCLsin.cPPT.humanPGK.mCherryProtein.Wpre (mCherry-LV) for IV injected cells. A fraction of the transduced cells was cultured for 10 days in vitro in order to assess transgene expression by cytofluorimetric analysis. Transduced cells were injected via the tail vein or directly in the CNS by means of ICV or ITL injection into seven/eight-week-old conditioned C57BL6/J female mice, 24 h after the fourth busulfan dose (25 mg/kg x four days, Merck, cod. B2635), at different concentration according to the different experimental settings. CNS injected cells (Lin - 0.3 × 10 6 /mouse); IV injected cells (Lin - 1 × 10 6 ; BM 3 × 10 6 ; KSL 0.5 × 10 6 ). 8 weeks old 5xFAD mice were transplanted ICV with 0.3 × 10 6 Lin - cells obtained from 5xFAD donors (6–8 weeks old) and transduced at MOI 100 with a TREM2 expressing LV (pCCLsin.cPPT.humanPGK.Trem2.Wpre) as described above. 5 days post transplantation, 2.0 × 10 6 BM cells from 5xFAD donors were provided IV for hematopoietic rescue. Mice were conditioned with 25 mg/kg of busulfan for 4 days prior to transplant and provided with Diazepam to avoid seizures.
Diazepam solution (available at 5 mg/mL) was provided daily in the drinking water starting for a total of 10 days with progressive decreases in drug dose (0.25 mg/kg used for the first 8 days, then dose decreased in the two following days to 0.125 mg/kg −0.063 mg/kg and removed).
5xFAD mice transplanted with 0.3 × 10 6 GFP-transduced Lin - cells ICV were used as control.
Intracerebroventricular injection (ICV)
ICV transplantation was performed by surgery, upon anesthesia (ketamine (100 mg/kg) and xylazine (10 mg/kg). The head of the mouse, shaved and disinfected, was fixed with ear bars in a stereotactic frame and the skin was disclosed longitudinally. Bregma was visualized and coordinates were recorded. From bregma, injection coordinates (1 mm lateral, −0.5 mm anterior) were adjusted before the cranial bone was enclosed under visual control with a drill head of 0.7 mm diameter. Five µl of the cell suspension (0.3 × 10 6 cells) were injected through a 10 µl Hamilton syringe upon insertion into the brain 2.5 mm distal from the cranial bone. Following wound closure, animals received a single dose of atipamezole (1 µl/g) and were maintained in sterile conditions.
Intrathecal lumbar injection (ITL)
ITL transplantation was performed under anaesthesia with isoflurane (4% induction, 2.5% maintenance). A 10 µl Hamilton syringe connected to a 30-gauge needle containing 5 µl of cell suspension was introduced into the intervertebral foramen between L5 and L6 through the intact skin. Slow administration of 0.3 × 10 6 cells/mouse was performed once the proper position of the needle was confirmed by tail flick reflex. At the end of the procedure, animals received a single i.p. injection of atipamezole (1 μl/g) as analgesic and returned to the cage. Prophylactic Antibiotic (Gentamycin Sulfate, 80 mg/250 mL) was administered via the drinking water for 2 weeks following conditioning and transplantation.
Mouse tissue collection and processing for flow cytometry and histology
Mice were euthanized under deep anesthesia by extensive intra-cardiac perfusion with cold PBS for at least 10–15 min/mouse after clumping the femur. The procedure was stopped only when complete liver blanching was observed to avoid contamination with circulating blood cells. Organs were then collected and differentially processed. BM cells were collected from the clumped femur as described. Brain was removed and the two hemispheres were differently processed. For immunofluorescence analysis, one hemisphere was fixed for 24 h in 4% PFA, embedded in OCT compound and stored at −80 °C, after equilibration in sucrose gradients (from 10 to 30%). For flow cytometry analysis, cells from the other hemisphere were mechanically disaggregated to obtain a single cell suspension in 20 ml of GKN/BSA buffer (8 g/L NaCl, 0.4 g/L KCl, 1.42 g/L NaH 2 P0 4 , 0.93 g/L Na2HP0 4 , 2 g/L D+ Glucose, pH 7.4 + 0.002% BSA). The same protocol was used to process the spinal cord. Cells from BM and CNS tissues (brain and spinal cord) were analyzed by FC upon re-suspension in blocking solution (PBS 5%FBS, 1%BSA) and labeling at 4 °C for 15 min with the following specific antibodies: rat PE anti-mouse CD45 (BD Pharmingen) 1:100; rat APC anti-mouse CD45 (BD Biosciences) 1:150; rat Brilliant Violet 510 anti-mouse CD45, (BioLegend) 1:150; rat Pacific Blue antimouse CD45.2 (Bio Legend) 1:100; mouse PE anti-Mouse CD45.1 (BD bioscience) 1:100; rat APC anti-mouse CD11b (eBioscience) 1:100; mouse Alexa Fluor 647 anti-Human CD271 (NGF receptor) (BD Pharmingen) 1:30; rat APC 780 anti-mouse CD11b (eBioscience) 1:100; rat APC 780 anti-mouse CD117(c-kit) (eBioscience) 1:100; rat PE-Cy7 anti- mouse Ly-6A/E Sca-1 (Sca-1) (BD Bioscence) 1:150. 7-AAD (1 mg/ml) (Sigma-Aldrich) or DAPI was added to the cells prior to analysis for dead cell exclusion. Samples were analyzed by LSR Fortessa (Beckton Dickinson).
Immunofluorescence analysis
Brains were serially cut in the sagittal planes on a cryostat in 15 µm sections. Tissue slides were washed twice with PBS, air dried and blocked with 0.3% Triton, 2% BSA, 10% NGS (Vector Laboratories) for 2 h. Then sections were incubated over night with primary antibodies diluted in PBS, 0.1% Triton, 2% BSA, 10% NGS at 4 °C as follows; rabbit anti Iba1 (Wako) 1:100; chicken anti-GFP (Abcam) 1:250; rabbit anti GFP (Invitrogen) 1:100; mouse PE anti-human CD271 (NGF Receptor) (BD Pharmingen) 1:50; rabbit anti-cherry (Abcam) 1:100. The secondary antibodies goat IgG anti-Chicken Alexa Fluor 488, goat IgG anti-Rabbit Alexa Fluor 488, 546 or 633, goat IgG anti-Rat Alexa Fluor 546 or 633, goat IgG anti-Mouse Alexa Fluor 546 (Molecular Probes, Invitrogen) were diluted 1:500 in the same blocking solution used for primary antibodies staining and incubated with sections for 2 h at room temperature. Nuclei were stained with Topro III (Molecular Probes, Invitrogen) 1:1000 in PBS or by DAPI (Roche) 1:30 in PBS. Slices were washed in PBS, air dried and mounted with Fluorsafe Reagent (Calbiochem). Not transplanted mice were used as negative controls for the reporter transgene staining. Incubation with secondary antibody alone was performed in order to exclude the background signal. Samples were analyzed with a confocal microscope (Zeiss and Leica TCS SP2; Leica Microsystems Radiance 2100; Bio-Rad) (λexcitation = 488, 586, 660). Fluorescent signal was processed by Lasersharp 2000 software. Images were imported into Adobe Photoshop CS 8.0 software and processed by using automated level correction. For the reconstruction of brain sections, a fluorescence microscope Delta Vision Olympus Ix70 was used for the acquisition of the images, which were then processed by the Soft Work 3.5.0 software. Images were then imported into the Adobe Photoshop CS 8.0 software and reconstructed.
Branching analysis
The branching analysis was performed with ImageJ software using an automatic Macro, developed in the lab for standardizing the procedure, on multiple individual cells (comprised between 20 and 80) from different brain region of transplanted mice. Cells were identified according to the expression of different fluorescent markers (GFP/Cherry/∆NGFR) and validated reproducing the same analysis on Iba-1+ stained cells. Automated Sholl analysis was performed by applying the same strategy, to study the radial distribution of microglia branches around the cell body. The sum intersections and number of intersection radii were selected as parameters to characterize donor derived cells, in terms of complexity and spatial extension of the cell arborizations, respectively.
Area signal quantification
For area signal characterization, a mask was created from splitted channel images by using Fiji program. The positive signal was then converted in colored spotted signal to differentially mark CNS vs IV transplanted cells. For signal quantification, a deeper analysis was conducted on splitted channel images upon removal of background noise and identification of region.
Tissue clearing procedure, image acquisition and processing
Tissue clearing procedure 82 was applied on 2mm-thick sagittal brain section for the characterization of the distribution pattern of short-term immigrant cells in the CNS of transplanted mice on a whole-mount 2mm-thick sagittal brain section (Tissue Clearing kit,Binaree Inc., Republic of Korea).
In particular tissues were a) soaked in Starting Solution overnight at 4 °C; b) transferred to Solution A at 37 °C for 24 h, followed by three 20-minute washes in distilled water at 4 °C; c) immersed in Solution B at 37 °C for 24 h, followed by four 30-minute washes in distilled water at 4 °C; d) stained with Nuclei staining solution (DAPI 10 µg/ml in distilled water) for 2 h at 4 °C, followed by three 10-minute washes in distilled water at 4 °C; e) placed in Mounting Solution at 37 °C for 24 h and then stored at room temperature for imaging.
A z-stack of 400–500 μm thickness (with 20 μm intervals) was obtained in both the UV and FITC channels to visualize the DAPI-stained nuclei and GFP + cells, respectively. A composite image was generated through stitching. Four partially overlapping acquisitions were performed, focused on four different brain subregions (forebrain, hippocampus/midbrain, cerebellum and brainstem). Full brain was reconstructed by manually merging the four acquisitions (with GIMP v.2.10.8 open-source software.
Selected brain regions of interest were acquired at high-resolution on the z-axis by capturing a z-stack image of 400–500 μm thickness (with 5 μm intervals) at 25x magnification with a Leica SP5 scanning confocal microscope.
In-situ hybridization and immunohistochemistry in combinatorial transplant settings
For the analysis of microglia enriched genes, cryostatic brain sections were processed and stained using the RNAscope 2.0 kit (cat#310095, Advanced Cell Diagnostics, Hayward, CA), following the Fixed-frozen tissue sample preparation and pre-treatment protocol according to manufacturer instructions. Perfusion and post-fixation in paraformaldehyde were performed as described above. After equilibration in 30% phosphate buffered sucrose solution, 14 µm thick brain sections were mounted onto Superfrost Plus glass slides using a cryostat. Detection of Tmem119 and Tgfbr1‐mRNA was performed using the RNAscope® Fluorescent Multiplex Assay with a probe set targeting base pairs 2–1106 of mouse Tmem119‐mRNA (Cat No. 472901-C2, Advanced Cell Diagnostics, Inc., Newark, CA) or base pairs 2257–3252 of mouse Tgfbr1-mRNA (Cat No 406201, Advanced Cell Diagnostics, Inc., Newark, CA). Briefly, the sections were air dried for 20 min, washed in PBS and subsequently immersed in boiling 1x Target Retrieval solution for 5 min. After washing in distilled water and 100% ethanol for 2 min each, the sections were incubated with Protease IV solution for 30 min at 40 °C in a humidified tray. After washing in distilled water for 2 min, the sections were then hybridized with target probes for 2 hr at 40 °C followed by amplification solutions 1 (30 min), 2 (15 min), 3 (30 min), and 4 (15 min) at 40 °C. Between each amplification step, the sections were rinsed in 1x Wash Buffer for 2 min at room temperature. Finally, the sections were counterstained with 4’,6‐Diamidine‐2’‐phenylindole dihydrochloride (DAPI) for 30 s and coverslipped in PVA‐DABCO. Images were acquired at Delta Vision Olympus 10x.
In-situ hybridization and immunohistochemistry in 5xFAD mouse model
Brain samples from 5xFAD mice were processed for immunohistochemistry by Neuroscience Associates, Inc for Iba1, GFAP and β amyloid detection. After equilibration in sucrose gradients (from 10 to 30%) and 24 h treatment with 4% PFA, samples were embedded in a gelatin matrix using MultiBrain®Technology. The blocks were rapidly frozen, after curing by immersion in 2-Methylbutane chilled with crushed dry ice and mounted on a freezing stage of an AO 860 sliding microtome. The MultiBrain® blocks were sectioned coronally with desired micrometer (μ) setting on the microtome. All sections were cut through the entire length of the specimen segment and collected sequentially.
For immunohistochemistry, free floating sections were processedd for IF as per internal protocols. All incubation solutions from the blocking serum onward use Tris buffered saline (TBS) with Triton X-100 as the vehicle; all rinses are with TBS. After a hydrogen peroxide treatment and blocking serum, the sections were immunostained with the primary antibodies (i.e anti-Iba1, anti-GFAP), and incubated overnight at room temperature. Vehicle solutions contained Triton X-100 for permeabilization. Following rinses, a biotinylated secondary antibody was applied. After further rinses Vector Lab’s ABC solution (avidin-biotin-HRP complex) was applied. The sections were again rinsed, then treated with diaminobenzidine tetrahydrochloride (DAB) and hydrogen peroxide to create a visible reaction product. Following further rinses, the sections were mounted on gelatin coated glass slides, air dried. The slides were dehydrated in alcohols, cleared in xylene and coverslipped.
The identification of LV-transduced cells in the tissues of transplanted 5xFAD mice was performed through in-situ hybridization for WPRE on 20 μm brain slices. The protocol was optimized to allow the co-detection of WPRE as well as amyloid beta, CD68 and Iba1 (stained with immunohistochemistry) on the same tissue slice. Firstly, a standard immunohistochemistry protocol was performed. Briefly, brain sections were blocked for 1 h at RT in blocking buffer (NGS 10% or BSA 3%, 0.3% Triton X-100 in PBS pH 7.4). The primary antibodies used were Mouse anti-B-Amyloid (1:500, BioLegend, SIG-39320), Rat anti-CD68 (1:100, BioRad, clone FA-11, MCA1957T) and Rabbit anti-Iba1 (1:500, Fujifilm Wako, 019–19741). After three washes with PBS, the sections were incubated with Biotinylated Goat anti-Mouse IgG (H + L) (1:200, Vector Labs, BA-9200), Biotinylated Goat anti-Rat IgG (H + L) (1:200, Vector Labs) or Biotinylated Donkey anti-Rabbit IgG (H + L) (1:200, Biotium, 20191), respectively, followed by 30’ incubation at RT with HRP-streptavidin conjugate (1:100, AAT Bioquest, 16920) and Cy3 tyramide (1:1000, AAT Bioquest, 11065) or Cy2 tyramide (1:1000, AAT Bioquest, 45100). Before starting the in-situ hybridization protocol, Avidin/Biotin blocking kit (ABCAM, AB64212) was used following manufacturer’s instructions to saturate any available biotin-binding surface in the tissue. Slices were fixed with PFA 4% and left to dry O/N at RT. In-situ hybridization was performed using the RNAscope Probe WPRE-04 (Biotechne, 540341), initially blocking peroxidases through the RNAscope H 2 O 2 followed by protease treatment according to the manufacturer instructions (ACD, Biotechne, 322381) and subsequently amplifying the target signal with the RNAscope Multiplex Fluorescent Detection Kit V2 (ACD, Biotechne, 323110) and associating it with the Cy5 tyramide fluorophore (1:1000, AAT Bioquest,11066). Images were captured at a 40x magnification with a 1.5x digital zoom using a Leica SP5 laser scanning confocal microscope.
Gene expression analysis in sorted population from transplanted mice
Total RNA was extracted with the RNeasy Plus Micro kit (Qiagen) from sorted myeloid brain populations, RNA quantity was determined using QuantiFluor® RNA system and QuantusTM Fluorometer (Promega). cDNA was generated starting from 1 ng up to 100 ng of purified mRNA by using SuperScript VILO Master mix (Thermo Fisher Scientific). cDNA was then pre-amplified using Custom Taqman® PreAmp Pools (Thermo Fisher Scientific). Thermal cycling for cDNA generation and preamplification was performed on T100 Thermal cycler (BIO-RAD) following manufacturer’s instructions. Gene expression analysis was performed using a custom design TaqMan-based microfluidic card gene expression assay (Applied Biosystems). Real time PCR was run in standard mode on Applied Biosystems® ViiATM 7 Real-Time PCR System, using the following thermal cycling conditions: one cycle at 50 °C for 2 min, one cycle at 95 °C for 10 min, 40 cycles at 95 °C for 15 s and 60 °C for 1 min (Applied Biosystems). The ViiATM 7 Software v1.2.2 was used to extract raw data. The difference (dCT) between the threshold cycle (CT) of each gene and that of the reference gene (mean of HPRT and r18S CTs) was used to determine gene expression. Fold change expression of selected microglia genes was calculated by 2 −ddCT method.
The RNA-seq gene expression analysis was performed on additional samples, corresponding to different combinations of route of administration (IV, ICV, ITL and CTRL), brain subpopulation (µ, Taµ, PBS) and markers (CD45.1, CD45.2). The complete design matrix reporting the characteristics of each sample is available as Source data in a Source data file.
Raw sequencing files were investigated for QC and aligned to the reference mouse genome mm39 (Genome assembly GRCm39) using STAR workflow 83 , obtaining, as a result, the gene-based count matrices. Poorly-detected genes with a cumulative reads count smaller than 10 or measured (reads count>0) in less than two samples were filtered out.
Statistical analysis has been performed in R 84 , using DEseq2 package 85 . Gene counts have been modeled using a negative binomial model. Normalized data are used for the heatmap representations and are available as Source Data in a Source data file.
Sybr green quantitative qPCR
hTREM2 expression was detected on brain tissue extracts by qPCR using a SYBR Green Kit with primers and probe from Thermofisher (Catalog number: 4448892, Hs01003898_m1, Product size: 91 bp, Dye: FAM). Primers for hTREM2 were preliminary validated on human THP1 cells, a monocytic cell line that constitutively expresses hTREM2, and on BV2 murine cells transduced with the LV_hTREM2, both used as positive controls for hTREM2 expression. All values were normalized to the housekeeping gene GAPDH. hTREM2 expression was then measured on the brain of animals transplanted ICV with LV-hTREM2 HSCs at sacrifice at 10 months after the transplant.
Droplet Digital™ PCR (ddPCR) for VCN determination
For VCN determination, ddPCR was performed on genomic DNA extracted respectively from the liquid culture grown in vitro for 14 days post transduction and from the brain tissues by using the DNeasy Blood & Tissue kit (Qiagen). ddPCR was ran in duplicate using 20 ng of gDNA as template, ddPCR Supermix for Probes (Bio-Rad Laboratories Inc) and primers specific for HIV Psi region LENTI (FAM fluorochrome, forward primer: 5’-TGAAAGCGAAAGGGAAACCA-3’; Reverse primer 5’-CCGTGCGCGCTTCAG-3’; probe: AGCTCTCTCGACGCAGGACTCGGC) and for the murine RPP30-(HEX fluorochrome, forward primer: 5’-CCAGCTCCGTTTGTGATAGT-3’, Reverse primer 5’-CAAGGCAGAGATGCCCATAA-3’), located on chromosome 19 of murine genome.
Poisson statistical analysis of the numbers of positive and negative droplets yields absolute quantification of the target sequence.
Behavioral testing in Alzheimer’s disease mouse model
For phenotypic assessment, as behavioral tests, the elevated-plus maze (EPM) test was performed on the transplanted 5xFAD animals every month from age 4 months until the age of 12 months and the Morris Water Maze (MWM) test at study termination (12 months of mice age).
The elevated-plus maze (EPM)
The EPM was performed according to previously described procedures 50 , 86 , 87 using a mouse EPM (model ENV-560A) purchased from Med Associates (St. Albans, VT). All four arms are elevated 1 meter from the floor, with the drop-off detectable only in the open arms. The EPM contained two open arms (35.5 cm×6 cm) and two closed arms (35.5 cm × 6 cm) radiating from a central area (6 cm × 6 cm). A 0.5 cm high lip surrounded the edges of the open arms, whereas the closed arms were surrounded by 20 cm high walls. The EPM was cleaned with Peroxigard before the beginning of the first test session and after each subject mouse was tested, with sufficient time for the ethanol odor to dissipate before the start of the next test session. Room illumination was ~30 lux. To begin the test, the mouse was placed in the central area facing the open arm. The mouse was allowed to freely explore for 5 min. Trials were videotaped and scored with EthoVision XT video tracking software (version 12.0, Noldus Information Technologies, Leesburg, VA).
Morris Water Maze (MWM)
A white, opaque, circular tub (60 cm depth x 83 cm diameter) was filled to 29 cm deep with water that was approximately 25 °C. Four visible, distinct shapes were places in each of the four quadrants inner walls of the tub to form distinct quadrants. Trials were videotaped and scored with EthoVision XT video tracking software (version 12.0, Noldus Information Technologies, Leesburg, VA). Acquisition training (day 1) consisted of 8 trials per mouse with a white platform (10 cm diameter) 0.5 cm above the surface of the water and marked with the red reflector. Each training trial began by lowering the mouse into the water close to the pool edge. The start location for each trial was alternated in a semirandom order for each mouse. Each mouse started in each of the quadrants twice. Hidden training (day 2–3) consisted of 20 trials per mouse, 12 on day 2 and 8 on day 3. The platform was placed in a new quadrant, 1 cm below the water. Each mouse started in each of the quadrants five times. For probe testing (day 4) the platform was removed and mice started opposite the position of where the hidden platform had been located. Mice were allowed to swim for 90 s. Reversal training (day 5 consisted of 1 trials per mouse, 3 from each starting quadrant. The platform was placed in a new quadrant, 1 cm below the water. Mice were allowed a maximum of 90 s to reach the platform. During visible, hidden and reversal trials, a mouse that failed to reach the platform in 60 s was guided to the platform by the experimenter. Mice were left on the platform for 5 s before being removed. After each trial, the subject was placed in a cage lined with absorbent paper towels and allowed to rest. Time spent in each quadrant and number of crossings over the trained platform location was used to analyze probe trial performance.
Statistics and reproducibility
For the comparison of two groups, we used Unpaired T -test with Welch’s correction.
For comparisons with more than two groups, one-way ANOVA followed by Tukey post-hoc test was used; for multiple variable comparisons, two-way ANOVA followed by Sidak post-hoc test was applied. Details on the exact statistical test used as well as the number of subjects and samples are included in the respective figure legends. P-values are reported in the respective figure legends whenever possible or are available as Supplementary information.
No statistical method was used to predetermine sample size. A biological triplicate (independent experiment) was guaranteed in both in vitro and in vivo experiments, except where specifically indicated.
Blinding analysis was adopted for behavioral testing in AD mice.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
All data needed to evaluate the conclusions in the paper are present in the paper and/or are reported as Supplementary information. Raw data will be provided upon request to the corresponding authors. The raw RNA-seq data are deposited in GEO under the accession code GSE275808 , and the RNA-seq and samples’ annotation data are available in the Zenodo database under accession code 12793606 [ https://doi.org/10.5281/zenodo.12793606 ]. Source data are provided with this paper.
Gomez Perdiguero, E., Schulz, C. & Geissmann, F. Development and homeostasis of “resident” myeloid cells: the case of the microglia. Glia 61 , 112–120 (2013).
Article PubMed Google Scholar
Ginhoux, F. et al. Fate mapping analysis reveals that adult microglia derive from primitive macrophages. Science 330 , 841–845 (2010).
Article ADS CAS PubMed PubMed Central Google Scholar
Wolf, S. A., Boddeke, H. W. & Kettenmann, H. Microglia in Physiology and Disease. Annu Rev. Physiol. 79 , 619–643 (2017).
Article CAS PubMed Google Scholar
Wu, Y., Dissing-Olesen, L., MacVicar, B. A. & Stevens, B. Microglia: Dynamic Mediators of Synapse Development and Plasticity. Trends Immunol. 36 , 605–613 (2015).
Article PubMed PubMed Central Google Scholar
Paolicelli, R. C. et al. Synaptic pruning by microglia is necessary for normal brain development. Science 333 , 1456–1458 (2011).
Article ADS CAS PubMed Google Scholar
Capotondo, A. et al. Intracerebroventricular delivery of hematopoietic progenitors results in rapid and robust engraftment of microglia-like cells. Sci. Adv. 3 , e1701211 (2017).
Article ADS PubMed PubMed Central Google Scholar
Capotondo, A. et al. Brain conditioning is instrumental for successful microglia reconstitution following hematopoietic stem cell transplantation. Proc. Natl Acad. Sci. USA 109 , 15018–15023 (2012).
Corti, S. et al. Wild-type bone marrow cells ameliorate the phenotype of SOD1-G93A ALS mice and contribute to CNS, heart and skeletal muscle tissues. Brain 127 , 2518–2532 (2004).
Beam, D. et al. Outcomes of unrelated umbilical cord blood transplantation for X-linked adrenoleukodystrophy. Biol. Blood Marrow Transpl. 13 , 665–674 (2007).
Article Google Scholar
Mildner, A. et al. Microglia in the adult brain arise from Ly-6ChiCCR2+ monocytes only under defined host conditions. Nat. Neurosci. 10 , 1544–1553 (2007).
Peterson, C. D. et al. AAV-Mediated Gene Delivery to the Spinal Cord by Intrathecal Injection. Methods Mol. Biol. 1950 , 199–207 (2019).
Eichler, F. et al. Hematopoietic Stem-Cell Gene Therapy for Cerebral Adrenoleukodystrophy. N. Engl. J. Med 377 , 1630–1638 (2017).
Article CAS PubMed PubMed Central Google Scholar
Sessa, M. et al. Lentiviral haemopoietic stem-cell gene therapy in early-onset metachromatic leukodystrophy: an ad-hoc analysis of a non-randomised, open-label, phase 1/2 trial. Lancet 388 , 476–487 (2016).
Ungari, S. et al. Design of a regulated lentiviral vector for hematopoietic stem cell gene therapy of globoid cell leukodystrophy. Mol. Ther. Methods Clin. Dev. 2 , 15038 (2015).
Biffi, A. et al. Lentiviral hematopoietic stem cell gene therapy benefits metachromatic leukodystrophy. Science 341 , 1233158 (2013).
Gentner, B. et al. Identification of hematopoietic stem cell-specific miRNAs enables gene therapy of globoid cell leukodystrophy. Sci. Transl. Med 2 , 58ra84 (2010).
Capotondo, A. et al. Safety of arylsulfatase A overexpression for gene therapy of metachromatic leukodystrophy. Hum. Gene Ther. 18 , 821–836 (2007).
Cartier, N. et al. Hematopoietic stem cell gene therapy with a lentiviral vector in X-linked adrenoleukodystrophy. Science 326 , 818–823 (2009).
Biffi, A. et al. Gene therapy of metachromatic leukodystrophy reverses neurological damage and deficits in mice. J. Clin. Invest 116 , 3070–3082 (2006).
Rocca, C. J. et al. CRISPR-Cas9 Gene Editing of Hematopoietic Stem Cells from Patients with Friedreich’s Ataxia. Mol. Ther. Methods Clin. Dev. 17 , 1026–1036 (2020).
Ferrari, G., Thrasher, A. J. & Aiuti, A. Gene therapy using haematopoietic stem and progenitor cells. Nat. Rev. Genet 22 , 216–234 (2021).
Morgan, R. A., Gray, D., Lomova, A. & Kohn, D. B. Hematopoietic Stem Cell Gene Therapy: Progress and Lessons Learned. Cell Stem Cell 21 , 574–590 (2017).
Ferrua, F. et al. Lentiviral haemopoietic stem/progenitor cell gene therapy for treatment of Wiskott-Aldrich syndrome: interim results of a non-randomised, open-label, phase 1/2 clinical study. Lancet Haematol. 6 , e239–e253 (2019).
Taylor, M. et al. Hematopoietic Stem Cell Transplantation for Mucopolysaccharidoses: Past, Present, and Future. Biol. Blood Marrow Transpl. 25 , e226–e246 (2019).
Stok, M. et al. Lentiviral Hematopoietic Stem Cell Gene Therapy Corrects Murine Pompe Disease. Mol. Ther. Methods Clin. Dev. 17 , 1014–1025 (2020).
Chen, C. et al. Non-toxic HSC Transplantation-Based Macrophage/Microglia-Mediated GDNF Delivery for Parkinson’s Disease. Mol. Ther. Methods Clin. Dev. 17 , 83–98 (2020).
Bosch, M. E. & Kielian, T. Neuroinflammatory paradigms in lysosomal storage diseases. Front Neurosci. 9 , 417 (2015).
Cavalca, E. et al. Metallothioneins are neuroprotective agents in lysosomal storage disorders. Ann. Neurol. 83 , 418–432 (2018).
Holley, R. J. et al. Macrophage enzyme and reduced inflammation drive brain correction of mucopolysaccharidosis IIIB by stem cell gene therapy. Brain 141 , 99–116 (2018).
Sailor, K. A. et al. Hematopoietic stem cell transplantation chemotherapy causes microglia senescence and peripheral macrophage engraftment in the brain. Nat. Med 28 , 517–527 (2022).
Wilkinson, F. L. et al. Busulfan conditioning enhances engraftment of hematopoietic donor-derived cells in the brain compared with irradiation. Mol. Ther. 21 , 868–876 (2013).
Plasschaert, R. N. et al. High-throughput analysis of hematopoietic stem cell engraftment after intravenous and intracerebroventricular dosing. Mol. Ther . https://doi.org/10.1016/j.ymthe.2022.05.022 (2022).
Verdonk, F. et al. Phenotypic clustering: a novel method for microglial morphology analysis. J. Neuroinflammation 13 , 153 (2016).
Langhammer, C. G. et al. Automated Sholl analysis of digitized neuronal morphology at multiple scales: Whole cell Sholl analysis versus Sholl analysis of arbor subregions. Cytom. A 77 , 1160–1168 (2010).
Bennett, M. L. et al. New tools for studying microglia in the mouse and human CNS. Proc. Natl Acad. Sci. USA 113 , E1738–E1746 (2016).
Butovsky, O. et al. Identification of a unique TGF-beta-dependent molecular and functional signature in microglia. Nat. Neurosci. 17 , 131–143 (2014).
Chiu, I. M. et al. A neurodegeneration-specific gene-expression signature of acutely isolated microglia from an amyotrophic lateral sclerosis mouse model. Cell Rep. 4 , 385–401 (2013).
Grommes, C. et al. Regulation of microglial phagocytosis and inflammatory gene expression by Gas6 acting on the Axl/Mer family of tyrosine kinases. J. Neuroimmune Pharm. 3 , 130–140 (2008).
Hickman, S. E. et al. The microglial sensome revealed by direct RNA sequencing. Nat. Neurosci. 16 , 1896–1905 (2013).
Matcovitch-Natan, O. et al. Microglia development follows a stepwise program to regulate brain homeostasis. Science 353 , aad8670 (2016).
Lund, H. et al. Competitive repopulation of an empty microglial niche yields functionally distinct subsets of microglia-like cells. Nat. Commun. 9 , 4845 (2018).
Cronk, J. C. et al. Peripherally derived macrophages can engraft the brain independent of irradiation and maintain an identity distinct from microglia. J. Exp. Med. 215 , 1627–1647 (2018).
Bennett, F. C. et al. A Combination of Ontogeny and CNS Environment Establishes Microglial Identity. Neuron 98 , 1170–1183 e1178 (2018).
Bruttger, J. et al. Genetic Cell Ablation Reveals Clusters of Local Self-Renewing Microglia in the Mammalian Central Nervous System. Immunity 43 , 92–106 (2015).
Ulland, T. K. & Colonna, M. TREM2 - a key player in microglial biology and Alzheimer disease. Nat. Rev. Neurol. 14 , 667–675 (2018).
Lee, C. Y. D. et al. Elevated TREM2 Gene Dosage Reprograms Microglia Responsivity and Ameliorates Pathological Phenotypes in Alzheimer’s Disease Models. Neuron 97 , 1032–1048 e1035 (2018).
Benitez, B. A. et al. Missense variant in TREML2 protects against Alzheimer’s disease. Neurobiol. Aging 35 , 1510 e1519–1510 e1526 (2014).
Oakley, H. et al. Intraneuronal beta-amyloid aggregates, neurodegeneration, and neuron loss in transgenic mice with five familial Alzheimer’s disease mutations: potential factors in amyloid plaque formation. J. Neurosci. 26 , 10129–10140 (2006).
Jawhar, S., Trawicka, A., Jenneckens, C., Bayer, T. A. & Wirths, O. Motor deficits, neuron loss, and reduced anxiety coinciding with axonal degeneration and intraneuronal Abeta aggregation in the 5XFAD mouse model of Alzheimer’s disease. Neurobiol. Aging 33 , 196 e129–140 (2012).
Hogg, S. A review of the validity and variability of the elevated plus-maze as an animal model of anxiety. Pharm. Biochem Behav. 54 , 21–30 (1996).
Article CAS Google Scholar
Schambach, A. et al. Woodchuck hepatitis virus post-transcriptional regulatory element deleted from X protein and promoter sequences enhances retroviral vector titer and expression. Gene Ther. 13 , 641–645 (2006).
Tiesjema, B. et al. Viral mediated neuropeptide Y expression in the rat paraventricular nucleus results in obesity. Obes. (Silver Spring) 15 , 2424–2435 (2007).
Sagoo, P. & Gaspar, H. B. The transformative potential of HSC gene therapy as a genetic medicine. Gene Ther . https://doi.org/10.1038/s41434-021-00261-x (2021).
Cartier, N., Lewis, C. A., Zhang, R. & Rossi, F. M. The role of microglia in human disease: therapeutic tool or target? Acta Neuropathol. 128 , 363–380 (2014).
Xu, Z. et al. Efficient Strategies for Microglia Replacement in the Central Nervous System. Cell Rep. 32 , 108041 (2020).
Bohlen, C. J. et al. Diverse Requirements for Microglial Survival, Specification, and Function Revealed by Defined-Medium Cultures. Neuron 94 , 759–773 e758 (2017).
Xu, R. et al. Human iPSC-derived mature microglia retain their identity and functionally integrate in the chimeric mouse brain. Nat. Commun. 11 , 1577 (2020).
Parajuli, B. et al. Transnasal transplantation of human induced pluripotent stem cell-derived microglia to the brain of immunocompetent mice. Glia 69 , 2332–2348 (2021).
Cohen-Pfeffer, J. L. et al. Intracerebroventricular Delivery as a Safe, Long-Term Route of Drug Administration. Pediatr. Neurol. 67 , 23–35 (2017).
Slavc, I. et al. Best practices for the use of intracerebroventricular drug delivery devices. Mol. Genet Metab. 124 , 184–188 (2018).
Duma, C. et al. Human intracerebroventricular (ICV) injection of autologous, non-engineered, adipose-derived stromal vascular fraction (ADSVF) for neurodegenerative disorders: results of a 3-year phase 1 study of 113 injections in 31 patients. Mol. Biol. Rep. 46 , 5257–5272 (2019).
Villabona-Rueda, A., Erice, C., Pardo, C. A. & Stins, M. F. The Evolving Concept of the Blood Brain Barrier (BBB): From a Single Static Barrier to a Heterogeneous and Dynamic Relay Center. Front. Cell Neurosci. 13 , 405 (2019).
Ge, S. & Pachter, J. S. Isolation and culture of microvascular endothelial cells from murine spinal cord. J. Neuroimmunol. 177 , 209–214 (2006).
Bifari, F. et al. Neurogenic Radial Glia-like Cells in Meninges Migrate and Differentiate into Functionally Integrated Neurons in the Neonatal Cortex. Cell Stem Cell 20 , 360–373 e367 (2017).
Benakis, C., Llovera, G. & Liesz, A. The meningeal and choroidal infiltration routes for leukocytes in stroke. Ther. Adv. Neurol. Disord. 11 , 1756286418783708 (2018).
Schlager, C. et al. Effector T-cell trafficking between the leptomeninges and the cerebrospinal fluid. Nature 530 , 349–353 (2016).
Article ADS PubMed Google Scholar
Decimo, I. et al. Meninges: A Widespread Niche of Neural Progenitors for the Brain. Neuroscientist 27 , 506–528 (2021).
Banisadr, G. et al. Highly regionalized neuronal expression of monocyte chemoattractant protein-1 (MCP-1/CCL2) in rat brain: evidence for its colocalization with neurotransmitters and neuropeptides. J. Comp. Neurol. 489 , 275–292 (2005).
Luskin, M. B. Restricted proliferation and migration of postnatally generated neurons derived from the forebrain subventricular zone. Neuron 11 , 173–189 (1993).
Carlen, M. et al. Functional integration of adult-born neurons. Curr. Biol. 12 , 606–608 (2002).
Meeker, R. B., Williams, K., Killebrew, D. A. & Hudson, L. C. Cell trafficking through the choroid plexus. Cell Adh Migr. 6 , 390–396 (2012).
Kunis, G. et al. IFN-gamma-dependent activation of the brain’s choroid plexus for CNS immune surveillance and repair. Brain 136 , 3427–3440 (2013).
Gosselin, D. et al. An environment-dependent transcriptional network specifies human microglia identity. Science 356 , https://doi.org/10.1126/science.aal3222 (2017).
Shemer, A. et al. Engrafted parenchymal brain macrophages differ from microglia in transcriptome, chromatin landscape and response to challenge. Nat. Commun. 9 , 5206 (2018).
Hohsfield, L. A. et al. Effects of long-term and brain-wide colonization of peripheral bone marrow-derived myeloid cells in the CNS. J. Neuroinflammation 17 , 279 (2020).
Cuadros, M. A., Sepulveda, M. R., Martin-Oliva, D., Marin-Teva, J. L. & Neubrand, V. E. Microglia and Microglia-Like Cells: Similar but Different. Front Cell Neurosci. 16 , 816439 (2022).
Li, C., Chen, Y. H. & Zhang, K. Neuroprotective Properties and Therapeutic Potential of Bone Marrow-Derived Microglia in Alzheimer’s Disease. Am. J. Alzheimers Dis. Other Demen 35 , 1533317520927169 (2020).
Mishra, P. et al. Rescue of Alzheimer’s disease phenotype in a mouse model by transplantation of wild-type hematopoietic stem and progenitor cells. Cell Rep. 42 , 112956 (2023).
Simard, A. R., Soulet, D., Gowing, G., Julien, J. P. & Rivest, S. Bone marrow-derived microglia play a critical role in restricting senile plaque formation in Alzheimer’s disease. Neuron 49 , 489–502 (2006).
Simard, A. R. & Rivest, S. Neuroprotective properties of the innate immune system and bone marrow stem cells in Alzheimer’s disease. Mol. Psychiatry 11 , 327–335 (2006).
Visigalli, I. et al. Gene therapy augments the efficacy of hematopoietic cell transplantation and fully corrects mucopolysaccharidosis type I phenotype in the mouse model. Blood 116 , 5130–5139 (2010).
Peviani, M. et al. Lipophilic dye-compatible brain clearing technique allowing correlative magnetic resonance/high-resolution fluorescence imaging in rat models of glioblastoma. Sci. Rep. 10 , 17974 (2020).
Dobin, A. et al. STAR: ultrafast universal RNA-seq aligner. Bioinformatics 29 , 15–21 (2013).
Team, R. C. R: A language and environment for statistical computing . R Foundation for Statistical Computing , Vienna, Austria , https://www.R-project.org/ (2013).
Love, M. I., Huber, W. & Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 15 , 550 (2014).
Bailey, K. R. & Crawley, J. N. In Methods of Behavior Analysis in Neuroscience Frontiers in Neuroscience (eds nd & J. J. Buccafusco) (2009).
Silverman, J. L. et al. Sociability and motor functions in Shank1 mutant mice. Brain Res 1380 , 120–137 (2011).
Download references
Acknowledgements
We would like to thank Hind Bouzid, Guan Wang, Francisco J. Molina Estevez, Valentina Cipolla and Valeria Bortolotto for experimental support on the Alzheimer’s disease project and for supporting the optimization of RNAscope protocol and related analysis. We wish to thank Mauro Giuseppe Spatafora (University of Pavia) for the help with confocal microscope acquisitions and Claudia Balducci (“Mario Negri” Institute for Pharmacological Research) for kindly providing the anti-Amyloid beta antibody. This work was funded by ERC Consolidator Grant #617162, ERC Proof of Concept 2022 Grant TREM2MICROENGINES and sponsored research agreement funds to AB.
Author information
Authors and affiliations.
Division of Hematology, Oncology and Stem Cell Transplantation, Department of Women and Child’s Health, University of Padua, Padua, Italy
Rita Milazzo, Pietro Rigoni, Yuri Ciervo & Alessandra Biffi
San Raffaele Telethon Institute for Gene Therapy, Division of Regenerative Medicine, Stem Cell and Gene Therapy, San Raffaele Scientific Institute, Milan, Italy
Rita Milazzo, Francesca Ferro, Eleonora Cavalca, Alessia Capotondo & Alessandra Biffi
Gene Therapy Program, Dana-Farber/Boston Children’s Cancer and Blood Disorders Center, Boston, MA, USA
Annita Montepeloso, Rajesh Kumar, Francesca Ferro, Eleonora Cavalca, Sabyasachi Das, Danilo Pellin, Marco Peviani & Alessandra Biffi
Department of Biology and Biotechnology “L. Spallanzani”, Cellular and Molecular Neuropharmacology lab, University of Pavia, Pavia, Italy
Paolo Cabras & Marco Peviani
You can also search for this author in PubMed Google Scholar
Contributions
R.M. undertook conceptualization, investigation, methodology and writing of the original draft, review and editing. A.M. carried out conceptualization, investigation, methodology, writing of the original draft, review and editing. R.K., F.F. and E.C. carried out conceptualization, investigation and methodology. S.D., P.R., P.C., Y.C. and D.P. performed methodology. A.C. carried out investigation and methodology. M.P. undertook conceptualization, investigation, methodology, review and editing. A.B. undertook supervision, conceptualization, investigation, review and editing.
Corresponding author
Correspondence to Alessandra Biffi .
Ethics declarations
Competing interests.
A.B., R.M., A.C. and M.P. are authors on a patent application related to this work (intracerebral transplantation of engineered HSCs) filed by Boston Children’s Hospital, Dana Farber Cancer Institute, the San Raffaele Hospital and Politecnico of Milano (application no. PCT/US17/56774); A.B. and M.P. are authors of a provisional patent application related to this work (TREM2 HSC gene therapy for AD) filed by Boston Children’s Hospital and Dana Farber Cancer Institute application no. PCT/US2020/053824). All other authors declare that they have no competing interests.
Peer review
Peer review information.
Nature Communications thanks the anonymous reviewers for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary information, peer review file, reporting summary, source data, source data, rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/ .
Reprints and permissions
About this article
Cite this article.
Milazzo, R., Montepeloso, A., Kumar, R. et al. Therapeutic efficacy of intracerebral hematopoietic stem cell gene therapy in an Alzheimer’s disease mouse model. Nat Commun 15 , 8024 (2024). https://doi.org/10.1038/s41467-024-52301-w
Download citation
Received : 03 October 2022
Accepted : 03 September 2024
Published : 13 September 2024
DOI : https://doi.org/10.1038/s41467-024-52301-w
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
By submitting a comment you agree to abide by our Terms and Community Guidelines . If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily.

IMAGES
VIDEO
COMMENTS
In research, the control group is the one not exposed to the variable of interest (the independent variable) and provides a baseline for comparison. The experimental group, on the other hand, is exposed to the independent variable. Comparing results between these groups helps determine if the independent variable has a significant effect on the outcome (the dependent variable).
A true experiment (a.k.a. a controlled experiment) always includes at least one control group that doesn't receive the experimental treatment.. However, some experiments use a within-subjects design to test treatments without a control group. In these designs, you usually compare one group's outcomes before and after a treatment (instead of comparing outcomes between different groups).
The control group and experimental group are compared against each other in an experiment. The only difference between the two groups is that the independent variable is changed in the experimental group. The independent variable is "controlled", or held constant, in the control group. A single experiment may include multiple experimental ...
Experimental and control groups are the two main groups found in an experiment, each serving a slightly different purpose. Experimental groups are being manipulated to try and change the out come ...
In an experiment, the control is a standard or baseline group not exposed to the experimental treatment or manipulation.It serves as a comparison group to the experimental group, which does receive the treatment or manipulation. The control group helps to account for other variables that might influence the outcome, allowing researchers to attribute differences in results more confidently to ...
You use a computer program to randomly place each number into either a control group or an experimental group. Because of random assignment, the two groups have comparable participant characteristics of age, gender, socioeconomic status, etc. ... Experimental design is the process of planning an experiment to test a hypothesis. The choices you ...
A control group is not the same thing as a control variable. A control variable or controlled variable is any factor that is held constant during an experiment. Examples of common control variables include temperature, duration, and sample size. The control variables are the same for both the control and experimental groups.
Controlled experiments (article) | Khan Academy. If you're seeing this message, it means we're having trouble loading external resources on our website. If you're behind a web filter, please make sure that the domains *.kastatic.org and *.kasandbox.org are unblocked. Khanmigo is now free for all US educators! Plan lessons, develop exit tickets ...
To test its effectiveness, you run an experiment with a treatment and two control groups. The treatment group gets the new pill. Control group 1 gets an identical-looking sugar pill (a placebo). Control group 2 gets a pill already approved to treat high blood pressure. Since the only variable that differs between the three groups is the type of ...
The experimental method involves the manipulation of variables to establish cause-and-effect relationships. The key features are controlled methods and the random allocation of participants into controlled and experimental groups. What is an Experiment? An experiment is an investigation in which a hypothesis is scientifically tested. An ...
A control group in an experiment does not receive the treatment. Instead, it serves as a comparison group for the treatments. Researchers compare the results of a treatment group to the control group to determine the effect size, also known as the treatment effect.. A control group is important because it is a benchmark that allows scientists to draw conclusions about the treatment's ...
In this experiment, the group of participants listening to no music while working out is the control group. They serve as a baseline with which to compare the performance of the other two groups. The other two groups in the experiment are the experimental groups. They each receive some level of the independent variable, which in this case is ...
Table of contents. Step 1: Define your variables. Step 2: Write your hypothesis. Step 3: Design your experimental treatments. Step 4: Assign your subjects to treatment groups. Step 5: Measure your dependent variable. Other interesting articles. Frequently asked questions about experiments.
control group, the standard to which comparisons are made in an experiment. Many experiments are designed to include a control group and one or more experimental groups; in fact, some scholars reserve the term experiment for study designs that include a control group. Ideally, the control group and the experimental groups are identical in every ...
Positive control groups: In this case, researchers already know that a treatment is effective but want to learn more about the impact of variations of the treatment.In this case, the control group receives the treatment that is known to work, while the experimental group receives the variation so that researchers can learn more about how it performs and compares to the control.
Updated on September 07, 2024. A control group in a scientific experiment is a group separated from the rest of the experiment, where the independent variable being tested cannot influence the results. This isolates the independent variable's effects on the experiment and can help rule out alternative explanations of the experimental results.
A control group is an experimental condition that does not receive the actual treatment and may serve as a baseline. A control group may receive a placebo or they may receive no treatment at all. ... Hypothesis Testing, Part 1. 5.1 - Introduction to Hypothesis Testing; 5.2 - Writing Hypotheses. 5.2.1 - Examples; 5.3 - Randomization Procedures.
P < 0.05 tacitly acknowledges the explicate order. Another example of the "subjectivity" of our perception is the level of accuracy we accept for differences between groups. For example, when we use statistical methods to determine if an observed difference between control and experimental groups is a random occurrence or a specific effect, we conventionally consider a p value of less than ...
In controlled experiments, researchers use random assignment (i.e. participants are randomly assigned to be in the experimental group or the control group) in order to minimize potential confounding variables in the study. For example, imagine a study of a new drug in which all of the female participants were assigned to the experimental group and all of the male participants were assigned to ...
The " variables " are any factor, trait, or condition that can be changed in the experiment and that can have an effect on the outcome of the experiment. An experiment can have three kinds of variables: i ndependent, dependent, and controlled. The independent variable is one single factor that is changed by the scientist followed by ...
A true experiment (a.k.a. a controlled experiment) always includes at least one control group that doesn't receive the experimental treatment. However, some experiments use a within-subjects design to test treatments without a control group. In these designs, you usually compare one group's outcomes before and after a treatment (instead of ...
Treatment and control groups. In the design of experiments, hypotheses are applied to experimental units in a treatment group. [1] In comparative experiments, members of a control group receive a standard treatment, a placebo, or no treatment at all. [2] There may be more than one treatment group, more than one control group, or both.
It is the group that you are not conducting experiment. The researcher is changing the independent variable that he thinks will influence the dependent variable. The researcher is not changing the independent variable or set it as a standard value. A good experimental group is identical to the control group in all way except for the difference ...
Data from n° cells/group comprised between 20 and 80 analyzed in n = 4 mice per group across n = 3 experimental replicates, are summarized as mean ± SEM. Source data ( b , c ) are provided as a ...