
- History & Society
- Science & Tech
- Biographies
- Animals & Nature
- Geography & Travel
- Arts & Culture
- Games & Quizzes
- On This Day
- One Good Fact
- New Articles
- Lifestyles & Social Issues
- Philosophy & Religion
- Politics, Law & Government
- World History
- Health & Medicine
- Browse Biographies
- Birds, Reptiles & Other Vertebrates
- Bugs, Mollusks & Other Invertebrates
- Environment
- Fossils & Geologic Time
- Entertainment & Pop Culture
- Sports & Recreation
- Visual Arts
- Demystified
- Image Galleries
- Infographics
- Top Questions
- Britannica Kids
- Saving Earth
- Space Next 50
- Student Center
- Introduction & Top Questions

Historical overview
- The conservation of matter
- Energy considerations
- Kinetic considerations
- Gas-forming reactions
- Precipitation reactions
- Oxidation-reduction reactions
- The Arrhenius theory
- The Brønsted-Lowry theory
- The Lewis theory
- Decomposition reactions
- Substitution, elimination, and addition reactions
- Polymerization reactions
- Solvolysis and hydrolysis
- Chain reactions
- Photolysis reactions

What are the basics of chemical reactions?
What happens to chemical bonds when a chemical reaction takes place, how are chemical reactions classified.
- What are acids and bases?
- How are acids and bases measured?

chemical reaction
Our editors will review what you’ve submitted and determine whether to revise the article.
- Royal Society of Chemistry - Introducing “chemical reactions”
- Chemistry LibreTexts - Chemical Reactions
- Khan Academy - Chemical reactions
- University of Hawaii pressbooks - Chemical Reactions
- CoolKidFacts.com - Chemical Reactions
- Florida State University - Department of Chemistry and Biochemistry - Chemical Reactions
- BCcampus Open Publishing - Enthalpy and Chemical Reactions
- chemical reaction - Student Encyclopedia (Ages 11 and up)
- Table Of Contents

- A chemical reaction is a process in which one or more substances, also called reactants, are converted to one or more different substances, known as products. Substances are either chemical elements or compounds .
- A chemical reaction rearranges the constituent atoms of the reactants to create different substances as products. The properties of the products are different from those of the reactants.
- Chemical reactions differ from physical changes, which include changes of state, such as ice melting to water and water evaporating to vapor. If a physical change occurs, the physical properties of a substance will change, but its chemical identity will remain the same.
According to the modern view of chemical reactions, bonds between atoms in the reactants must be broken, and the atoms or pieces of molecules are reassembled into products by forming new bonds. Energy is absorbed to break bonds, and energy is evolved as bonds are made. In some reactions the energy required to break bonds is larger than the energy evolved in making new bonds, and the net result is the absorption of energy. Hence, different types of bonds may be formed in a reaction. A Lewis acid-base reaction , for example, involves the formation of a covalent bond between a Lewis base, a species that supplies an electron pair, and a Lewis acid, a species that can accept an electron pair. Ammonia is an example of a Lewis base. A pair of electrons located on a nitrogen atom may be used to form a chemical bond to a Lewis acid.
Chemists classify chemical reactions in a number of ways: by type of product, by types of reactants, by reaction outcome, and by reaction mechanism. Often a given reaction can be placed in two or even three categories, including gas -forming and precipitation reactions. Many reactions produce a gas such as carbon dioxide , hydrogen sulfide , ammonia , or sulfur dioxide . Cake batter rising is caused by a gas-forming reaction between an acid and baking soda (sodium hydrogen carbonate). Classification by types of reactants include acid-base reactions and oxidation-reduction reactions , which involve the transfer of one or more electrons from a reducing agent to an oxidizing agent. Examples of classification by reaction outcome include decomposition, polymerization , substitution , and elimination and addition reactions. Chain reactions and photolysis reactions are examples of classification by reaction mechanism, which provides details on how atoms are shuffled and reassembled in the formation of products.
chemical reaction , a process in which one or more substances, the reactants , are converted to one or more different substances, the products. Substances are either chemical elements or compounds . A chemical reaction rearranges the constituent atoms of the reactants to create different substances as products.
Chemical reactions are an integral part of technology, of culture , and indeed of life itself. Burning fuels, smelting iron , making glass and pottery , brewing beer , and making wine and cheese are among many examples of activities incorporating chemical reactions that have been known and used for thousands of years. Chemical reactions abound in the geology of Earth , in the atmosphere and oceans , and in a vast array of complicated processes that occur in all living systems.

Chemical reactions must be distinguished from physical changes. Physical changes include changes of state, such as ice melting to water and water evaporating to vapour. If a physical change occurs, the physical properties of a substance will change, but its chemical identity will remain the same. No matter what its physical state, water (H 2 O) is the same compound , with each molecule composed of two atoms of hydrogen and one atom of oxygen . However, if water, as ice, liquid, or vapour, encounters sodium metal (Na), the atoms will be redistributed to give the new substances molecular hydrogen (H 2 ) and sodium hydroxide (NaOH). By this, we know that a chemical change or reaction has occurred.
The concept of a chemical reaction dates back about 250 years. It had its origins in early experiments that classified substances as elements and compounds and in theories that explained these processes. Development of the concept of a chemical reaction had a primary role in defining the science of chemistry as it is known today.

The first substantive studies in this area were on gases . The identification of oxygen in the 18th century by Swedish chemist Carl Wilhelm Scheele and English clergyman Joseph Priestley had particular significance. The influence of French chemist Antoine-Laurent Lavoisier was especially notable, in that his insights confirmed the importance of quantitative measurements of chemical processes. In his book Traité élémentaire de chimie (1789; Elementary Treatise on Chemistry ), Lavoisier identified 33 “elements”—substances not broken down into simpler entities. Among his many discoveries, Lavoisier accurately measured the weight gained when elements were oxidized, and he ascribed the result to the combining of the element with oxygen . The concept of chemical reactions involving the combination of elements clearly emerged from his writing, and his approach led others to pursue experimental chemistry as a quantitative science.
The other occurrence of historical significance concerning chemical reactions was the development of atomic theory . For this, much credit goes to English chemist John Dalton , who postulated his atomic theory early in the 19th century. Dalton maintained that matter is composed of small, indivisible particles, that the particles, or atoms , of each element were unique, and that chemical reactions were involved in rearranging atoms to form new substances. This view of chemical reactions accurately defines the current subject. Dalton’s theory provided a basis for understanding the results of earlier experimentalists, including the law of conservation of matter (matter is neither created nor destroyed) and the law of constant composition (all samples of a substance have identical elemental compositions).
Thus, experiment and theory, the two cornerstones of chemical science in the modern world, together defined the concept of chemical reactions. Today experimental chemistry provides innumerable examples, and theoretical chemistry allows an understanding of their meaning.
Basic concepts of chemical reactions
When making a new substance from other substances, chemists say either that they carry out a synthesis or that they synthesize the new material. Reactants are converted to products, and the process is symbolized by a chemical equation . For example, iron (Fe) and sulfur (S) combine to form iron sulfide (FeS). Fe(s) + S(s) → FeS(s) The plus sign indicates that iron reacts with sulfur. The arrow signifies that the reaction “forms” or “yields” iron sulfide, the product. The state of matter of reactants and products is designated with the symbols (s) for solids , (l) for liquids , and (g) for gases .
Examples of Chemical Reactions in Everyday Life
ThoughtCo / Emily Roberts
- Chemistry In Everyday Life
- Chemical Laws
- Periodic Table
- Projects & Experiments
- Scientific Method
- Biochemistry
- Physical Chemistry
- Medical Chemistry
- Famous Chemists
- Activities for Kids
- Abbreviations & Acronyms
- Weather & Climate
- Ph.D., Biomedical Sciences, University of Tennessee at Knoxville
- B.A., Physics and Mathematics, Hastings College
Chemistry happens in the world around you, not just in a lab. Matter interacts to form new products through a process called a chemical reaction or chemical change. Every time you cook or clean, it's chemistry in action. Your body lives and grows thanks to chemical reactions. There are reactions when you take medications, light a match, and draw a breath.
These examples of chemical reactions from everyday life are a small sampling of the hundreds of thousands of reactions you experience as you go about your day.
Key Takeaways: Chemical Reactions in Everyday Life
- Chemical reactions are common in daily life, but you may not recognize them.
- Look for signs of a reaction. Chemical reactions often involve color changes, temperature changes, gas production, or precipitant formation.
- Simple examples of everyday reactions include digestion, combustion, and cooking.
What Is a Chemical Reaction?
A chemical change , often called a chemical reaction , occurs when substances transform into new and distinct substances. Essentially, it involves the rearrangement of atoms. Generally, chemical changes can be identified by temperature changes, light emission, bubble formation, precipitate formation, color changes, and odor release. These effects signify a change in composition, but they may not always be immediately apparent.
Usually, chemical changes are permanent, so they cannot be undone. Conversely, physical changes do not create new substances and can be reversed. Understanding these distinctions is fundamental to the study of chemistry .
Photosynthesis
Frank Krahmer / Getty Images
Plants apply a chemical reaction called photosynthesis to convert carbon dioxide and water into food (glucose) and oxygen. It's one of the most common everyday chemical reactions and also one of the most important because this is how plants produce food for themselves (and animals) and convert carbon dioxide into oxygen. The equation for the reaction is:
6 CO 2 + 6 H 2 O + light → C 6 H 12 O 6 + 6 O 2
Aerobic Cellular Respiration
Kateryna Kon/Science Photo Library / Getty Images
Aerobic cellular respiration is the opposite process of photosynthesis in that energy molecules are combined with the oxygen we breathe to release the energy needed by our cells plus carbon dioxide and water. Energy used by cells is chemical energy in the form of ATP, or adenosine triphosphate .
Here is the overall equation for aerobic cellular respiration:
C 6 H 12 O 6 + 6O 2 → 6CO 2 + 6H 2 O + energy (36 ATPs)
Anaerobic Respiration
Tastyart Ltd Rob White / Getty Images
Anaerobic respiration is a set of chemical reactions that allows cells to gain energy from complex molecules without oxygen. Your muscle cells perform anaerobic respiration whenever you exhaust the oxygen being delivered to them, such as during intense or prolonged exercise. Anaerobic respiration by yeast and bacteria is harnessed for fermentation to produce ethanol, carbon dioxide, and other chemicals that make cheese, wine, beer, yogurt, bread, and many other common products.
The overall chemical equation for one form of anaerobic respiration is:
C 6 H 12 O 6 → 2C 2 H 5 OH + 2CO 2 + energy
Every time you strike a match, burn a candle, build a fire, or light a grill, you see the combustion reaction. Combustion combines energetic molecules with oxygen to produce carbon dioxide and water.
For example, the equation for the combustion reaction of propane, found in gas grills and some fireplaces, is:
C 3 H 8 + 5O 2 → 4H 2 O + 3CO 2 + energy
Alex Dowden/EyeEm / Getty Images
Over time, iron develops a red, flaky coating called rust. This is an example of an oxidation reaction . Other everyday examples include the formation of verdigris on copper and the tarnishing of silver.
Here is the chemical equation for the rusting of iron:
Fe + O 2 + H 2 O → Fe 2 O 3 . XH 2 O
If you combine vinegar and baking soda for a chemical volcano or milk with baking powder in a recipe, you experience a double displacement , or metathesis reaction (plus some others.) The ingredients recombine to produce carbon dioxide gas and water. The carbon dioxide forms bubbles in the volcano and helps baked goods rise .
These reactions seem simple in practice but often consist of multiple steps. Here is the overall chemical equation for the reaction between baking soda and vinegar:
HC 2 H 3 O 2 (aq) + NaHCO 3 (aq) → NaC 2 H 3 O 2 (aq) + H 2 O(l) + CO 2 (g)
Electrochemistry
Batteries use electrochemical or redox reactions to convert chemical energy into electrical energy. Spontaneous redox reactions occur in galvanic cells , while nonspontaneous chemical reactions take place in electrolytic cells .
Peter Dazeley/Photographer's Choice / Getty Images
Thousands of chemical reactions take place during digestion. As soon as you put food in your mouth, an enzyme in your saliva called amylase starts to break down sugars and other carbohydrates into simpler forms your body can absorb. Hydrochloric acid in your stomach reacts with food to further break it down, while enzymes cleave proteins and fats so they can be absorbed into your bloodstream through the walls of the intestines.
Acid-Base Reactions
Lumina Imaging / Getty Images
Whenever you combine an acid (e.g., vinegar, lemon juice, sulfuric acid , or muriatic acid ) with a base (e.g., baking soda , soap, ammonia, or acetone), you are performing an acid-base reaction. These reactions neutralize the acid and base to yield salt and water.
Sodium chloride isn't the only salt that can be formed. For example, here is the chemical equation for an acid-base reaction that produces potassium chloride, a common table salt substitute:
HCl + KOH → KCl + H 2 O
Soap and Detergent Reactions
JGI/Jamie Grill / Getty Images
Soaps and detergents clean by way of chemical reactions . Soap emulsifies grime, which means oily stains bind to the soap so they can be lifted away with water. Detergents act as surfactants, lowering the surface tension of water so it can interact with oils, isolate them, and rinse them away.
Cooking uses heat to cause chemical changes in food. For example, when you hard boil an egg, the hydrogen sulfide produced by heating the egg white can react with iron from the egg yolk to form a grayish-green ring around the yolk . When you brown meat or baked goods, the Maillard reaction between amino acids and sugars produces a brown color and a desirable flavor.
More Examples of Chemistry in Everyday Life
Chemical reactions are everywhere, and in a way, chemistry really makes up everything. From the emotions you feel to peculiar questions such as, "Can bottled water go bad?" Here are some examples of chemistry in everyday life.
- Does the Taurine in Red Bull Really Come From Bull Semen?
- The Tin Man's Toxic Metal Makeup
- Words Made Using Periodic Table Element Symbols
- Chemistry Element Jokes and Puns
- Do You Know If Milk Is an Acid or a Base?
- How to Substitute for Baking Powder and Baking Soda
- What Glows Under Black Light?
- Examples of Organic Chemistry in Everyday Life
- Why Is Chemistry Important in Everyday Life?
- Chemistry in Daily Life
- What Is Mylar?
- Canned Air Isn't Air (Chemical Composition)
- Examples of Endothermic Reactions
- How Glow Stick Colors Work
- What Is an Element in Chemistry? Definition and Examples
- Fun and Interesting Chemistry Facts

- Science Notes Posts
- Contact Science Notes
- Todd Helmenstine Biography
- Anne Helmenstine Biography
- Free Printable Periodic Tables (PDF and PNG)
- Periodic Table Wallpapers
- Interactive Periodic Table
- Periodic Table Posters
- Science Experiments for Kids
- How to Grow Crystals
- Chemistry Projects
- Fire and Flames Projects
- Holiday Science
- Chemistry Problems With Answers
- Physics Problems
- Unit Conversion Example Problems
- Chemistry Worksheets
- Biology Worksheets
- Periodic Table Worksheets
- Physical Science Worksheets
- Science Lab Worksheets
- My Amazon Books
Types of Chemical Reactions

A chemical reaction is a process or chemical change that transforms one set of substances ( the reactants ) into another set of substances (the products ). The process involves breaking chemical bonds between atoms and forming new bonds, so the number and type of atoms are the same for both reactants and products. The chemical change is described by a chemical equation . There are several types of chemical reactions. Here is look at the four main types of chemical reactions, plus additional key reaction types.
4 Main Types of Chemical Reactions
Keep in mind, there are different names for the reaction types. The four main types of chemical reactions are:
- Synthesis or combination reactions
- Decomposition or analysis reactions
- Single replacement, single displacement , or substitution reactions
- Double replacement , double displacement, or metathesis reactions
There are many other types of reactions, though. This table summarizes some key points of the main types of chemical reactions:
| Explanation | ||
| Synthesis or Combination | A + B → AB | Two or more elements or compounds combine to form one compound. |
| Decomposition or Analysis | AB → A + B | A complex molecule breaks into simpler ones. |
| Displacement, Replacement, or Substitution | A + BC → AC + B | One element replaces another in a compound. |
| Double displacement, Double replacement, or Metathesis | AB + CD → AD + CB | exchange partners. |
| A + O → H O + CO | A compound combines with oxygen to form an oxide. Often, carbon dioxide and water form. | |
| Acid-Base or | Acid + Base → Salt + Water | An acid and base react to form a salt and water. |
| A + Soluble salt B → Precipitate + soluble salt C | Two solutions of soluble salts react to form an insoluble salt (a precipitate). |
Let’s take a closer look at the types of reactions and get examples:
Synthesis or Direct Combination Reaction
In a synthesis, direct combination, or composition reaction, two (or more) reactants combine to form a more complex product. The general form of the reaction is: A + B → AB An example of a synthesis reaction is the combination of iron and sulfur to form iron(II) sulfide: 8 Fe + S 8 → 8 FeS
Here are other examples of synthesis reactions:
- 2 Na(s) + Cl 2 (g) → 2 NaCl(s)
- C(s) + O 2 (g) → CO 2 (g)
- S(s) + O 2 (g) → SO 2 (g)
- 2 Fe(s) + O 2 (g) → 2 FeO(s)
- 2 SO 2 + O 2 → 2 SO 3
- 6 C + 3 H 2 → C 6 H 6
- 4 Na + 2 C + 3 O 2 → 2 Na 2 CO 3
Decomposition or Analysis Reaction
A compound breaks or decomposes into smaller pieces in a chemical decomposition or analysis reaction. The general form of the reaction is: AB → A + B An example of a decomposition reaction is the electrolysis of water to form oxygen and hydrogen: 2 H 2 O → 2 H 2 + O 2
Here are additional examples of decomposition reactions:
- CaCO 3 → CaO + CO 2
- 2 KClO 3 → 2 KCl + 3 O 2
- Na 2 CO 3 → Na 2 O + CO 2
Single Replacement, Single Displacement, or Substitution Reaction
A single replacement, single displacement, or substitution reaction is when one element is displaced from a reactant to form a compound with another element. The reaction has the general form: A + BC → AC + B An example of a single replacement reaction is when zinc combines with hydrochloric acid to form zinc chloride and hydrogen. The zinc replaces or displaces the hydrogen in hydrochloric acid: Zn + 2 HCl → ZnCl 2 + H 2
Double Replacement, Double Displacement, or Metathesis Reaction
When the reactant cations and anions “swap partners” the reaction is called a double replacement, double displacement, or metathesis reaction. The general form for a double replacement reaction is: AB + CD → AD + CB An example of a double replacement reaction is the reaction between sodium chloride and silver nitrate to yield sodium nitrate and silver chloride: NaCl(aq) + AgNO 3 (aq) → NaNO 3 (aq) + AgCl(s)
Combustion Reaction
A combustion reaction is the reaction between a fuel and oxygen to form one or more oxides. Because many fuels are carbon-based, carbon dioxide (CO 2 ) is often a product. Sometimes water (H 2 O) is a product.
Here are examples of combustion reactions and their balanced equations :
- C 10 H 8 + 12 O 2 → 10 CO 2 + 4 H 2 O
- H 2 + O 2 → 2 H 2 O
- C 6 H 12 O 6 + 6 O 2 → 6 CO 2 + 6 H 2 O
- 2 Fe 2 S 3 + 9 O 2 → 2 Fe 2 O 3 + 6 SO 2
- 2 Al 2 S 3 + 9 O 2 → 2 Al 2 O 3 + 6 SO 2
- P 4 + 5 O 2 → 2 P 2 O 5
Acid-Base or Neutralization Reaction
An acid-base reaction is a type of double replacement reaction that occurs between an acid and a base. The H + ion in the acid reacts with the OH – ion in the base to form water and an ionic salt: HA + BOH → H 2 O + BA The reaction between hydrobromic acid (HBr) and sodium hydroxide is an example of an acid-base reaction: HBr + NaOH → NaBr + H 2 O
Oxidation-Reduction or Redox Reaction
Redox stands for reduction and oxidation . The two processes occur together. This type of reaction involves electron transfer between reactants and a change in oxidation number. An example is the reduction of I 2 to form I – and oxidation of S 2 O 3 2- (thiosulfate anion) to form S 4 O 6 2- :
2 S 2 O 3 2− (aq) + I 2 (aq) → S 4 O 6 2− (aq) + 2 I − (aq)
Isomerization Reaction
In an isomerization reaction, the structural arrangement of a compound is changed but its net atomic composition remains the same.
For example:
CH 3 CH 2 CH 2 CH 3 (n-butane) → CH 3 CH(CH 3 )CH 3 (i-butane)
Hydrolysis Reaction
A hydrolysis reaction is a reaction in which one or more water molecules is added to a substance. In some cases, this causes both the substance and water molecule to split. The general form of a hydrolysis reaction is: X – (aq) + H 2 O(l) ↔ HX(aq) + OH – (aq)
The reverse reaction is a condensation reaction. In a condensation reaction, water is removed from a substance.
How Many Types of Chemical Reactions Are There?

Technically, there are hundreds or even thousands of different types of chemical reactions. However, chemistry students usually learn to classify them as 4 main types, 5 main types, or 6 main types. The four main types of chemical reactions are synthesis, decomposition, single displacement, and double displacement. But, remember, some people use different names for these reactions. Other important types of reactions are combustion, acid-base, redox reactions, and condensation reactions . It gets even more complicated in organic chemistry, where many reactions have special names. However, these other types of reactions also fit into one of the four main categories!
Types of Chemical Reactions Worksheet
Practice identifying the four main types of chemical reactions with this worksheet. Download and print the PDF worksheet and answer key.
[ Types of Chemical Reactions Worksheet ] [ PDF Answer Key ]
- Atkins, Peter W.; Julio de Paula (2006). Physical Chemistry (4th ed.). Weinheim: Wiley-VCH. ISBN 978-3-527-31546-8.
- IUPAC (1997). “Chemical Reaction”. Compendium of Chemical Terminology (2nd ed.) (the “Gold Book”). doi: 10.1351/goldbook.C01033
- Myers, Richard (2009). The Basics of Chemistry . Greenwood Publishing Group. ISBN 978-0-313-31664-7.
- Wiberg, Egon; Wiberg, Nils; Holleman, Arnold Frederick (2001). Inorganic Chemistry . Academic Press. ISBN 978-0-12-352651-9.
Related Posts
Your browser is not supported
Sorry but it looks as if your browser is out of date. To get the best experience using our site we recommend that you upgrade or switch browsers.
Find a solution
- Skip to main content
- Skip to navigation

- Back to parent navigation item
- Primary teacher
- Secondary/FE teacher
- Early career or student teacher
- Higher education
- Curriculum support
- Literacy in science teaching
- Periodic table
- Interactive periodic table
- Climate change and sustainability
- Resources shop
- Collections
- Remote teaching support
- Starters for ten
- Screen experiments
- Assessment for learning
- Microscale chemistry
- Faces of chemistry
- Classic chemistry experiments
- Nuffield practical collection
- Anecdotes for chemistry teachers
- On this day in chemistry
- Global experiments
- PhET interactive simulations
- Chemistry vignettes
- Context and problem based learning
- Journal of the month
- Chemistry and art
- Art analysis
- Pigments and colours
- Ancient art: today's technology
- Psychology and art theory
- Art and archaeology
- Artists as chemists
- The physics of restoration and conservation
- Ancient Egyptian art
- Ancient Greek art
- Ancient Roman art
- Classic chemistry demonstrations
- In search of solutions
- In search of more solutions
- Creative problem-solving in chemistry
- Solar spark
- Chemistry for non-specialists
- Health and safety in higher education
- Analytical chemistry introductions
- Exhibition chemistry
- Introductory maths for higher education
- Commercial skills for chemists
- Kitchen chemistry
- Journals how to guides
- Chemistry in health
- Chemistry in sport
- Chemistry in your cupboard
- Chocolate chemistry
- Adnoddau addysgu cemeg Cymraeg
- The chemistry of fireworks
- Festive chemistry
- Education in Chemistry
- Teach Chemistry
- On-demand online
- Live online
- Selected PD articles
- PD for primary teachers
- PD for secondary teachers
- What we offer
- Chartered Science Teacher (CSciTeach)
- Teacher mentoring
- UK Chemistry Olympiad
- Who can enter?
- How does it work?
- Resources and past papers
- Top of the Bench
- Schools' Analyst
- Regional support
- Education coordinators
- RSC Yusuf Hamied Inspirational Science Programme
- RSC Education News
- Supporting teacher training
- Interest groups

- More navigation items
Chemical misconceptions
- 2 Alternative conceptions in chemistry teaching
- 3 Concepts in chemistry
- 4 The structure of chemical knowledge
- 5 Overcoming learning impediments
- 6 Scaffolding learning in chemistry
- 7 Chemical axioms
- 8 Chemical structure
- 9 Chemical bonding
- 10 Chemical reactions
- 11 Constructing chemical conceptions
- 12 Elements, compounds and mixtures
- 13 Mass and dissolving
- 14 Changes in chemistry
- 15 Revising acids
- 16 Word equations
- 17 Definitions in chemistry
- 18 Types of chemical reaction
- 19 Revising the periodic table
- 20 Predicting the melting temperature of carbon
- 21 Iron - a metal
- 22 Ionic bonding
- 23 Precipitation
- 24 Spot the bonding
- 25 Hydrogen fluoride
- 26 An analogy for the atom
- 27 Ionisation energy
- 28 Chemical stability
- 29 Stability and reactivity
- 30 Interactions
- 31 Acid strength
- 32 Reaction mechanisms
- 33 Scaffolding explanations
- 34 Chemical comparisons
- 35 Learning impediment diary
Chemical reactions

- No comments
The study of reactions is at the core of chemistry as a subject
The main defining characteristic of a chemical reaction is that it is a process where one or more new substances are formed.
Describing chemical reactions
Reactants → Products
Here, the reactants and products are different chemical substances. The basic way of describing chemical reactions, then, is to write ’chemical equations’ showing the reactants and products in a particular chemical change. Chemical equations have been described as ’an essential part of the common language of scientist.
Chemical ‘equations’ are commonly written as word equations and formulae equations. There is a sense in which formulae equations are easier, as they provide a ready means of checking that no transmutation has been implied (by seeing that the same elements are represented on both sides of the equation). However, formulae equations are more abstract, and so word equations are often introduced first.
Although word equations use the (often) less abstract names of substances, rather than formulae, they can make it more difficult for students to check that the same elements are represented before and after a reaction. Students need to know, for example, that the ending ’-ate’ implies the presence of oxygen, and which elements are present in common substances such as water or ammonia.
Student difficulties with word equations
Word equations are commonly introduced and used in lower secondary science, but national testing shows that many students find it difficult to write or complete word equations for chemical reactions.
This should not be surprising if it is remembered that although chemical names seem familiar to teachers, they may seem somewhat arbitrary to students. The more systematic names, such as tetraoxosulfate(V1) for the SO 4 2– ion, may be especially difficult for students, if the names do not seem to fit an accessible pattern n, then students may well be concerned about the very large number of substances they could hear and read about.
For the teacher, this classification system acts as a set of familiar ’mental pigeon-holes’ into which reactions may be slotted.
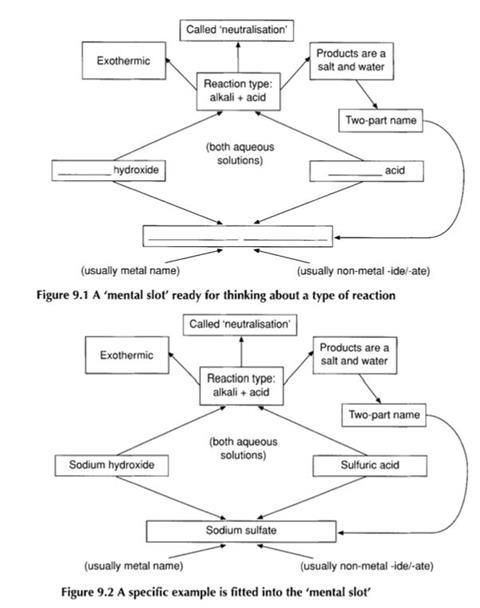
Students, lacking this framework for organising the information, see the equations at a much finer resolution so that a chemical equation that the teacher perceives as an integral unit seems to the students to have many components.
One of the resources included in the companion volume, Word equations , is an exercise on completing word equations. This exercise is not designed around teaching students a set of rules, but rather with giving them an opportunity to see that, although there are many possible chemical reactions, those met in school science may often be fitted into a limited number of common types.
This approach is designed to help students start constructing the reaction-type mental ’slots’ into which they can ‘drop’ reactions
Why do reactions occur?
Explaining ‘why’ reactions occur is a much more difficult task than either completing equations or classifying reactions. Indeed, this topic is not usually dealt with in any meaningful sense until at post- 16 level. In one sense this is understandable, as valid explanations require detailed consideration of bond enthalpies, or - at least - a sophisticated application of notions of electrode potentials and Gibbs free energy. In some cases, a qualitative treatment of entropy can be used.
Yet, research has suggested that many students believe that they do know why reactions occur by the time they complete their secondary science courses. However, the reasons they commonly give are invalid, and may be in contradiction to ideas that they would be expected to learn if they continue their study of chemistry to post-1 6 level.
The most common alternative conception is to suggest that chemical reactions occur ’so that atoms can acquire a full outer electron shell’ or ’an octet of outer electrons’. Students at the end of secondary schooling, or during post-1 6 courses, will commonly give a response along these lines, even if the information given in the question clearly shows this cannot be a valid explanation.
For the full version of this chapter, see downloads below
Buy Chemical Reactions book
Additional information.
These PDFs have been taken from the popular book, Chemical Misconceptions : Prevention, diagnosis and care: Theoretical background, Volume 1, by Keith Taber.

More Keith S Taber

Chemical misconceptions II: Chemical comparisons

Chemical misconceptions I: Concepts in chemistry

Chemical misconceptions II: Scaffolding explanations

Alternative conceptions in chemistry teaching

Concepts in chemistry

The structure of chemical knowledge

Overcoming learning impediments

Scaffolding learning in chemistry

Chemical axioms

Chemical structure

Chemical bonding

Constructing chemical conceptions

Elements, compounds and mixtures

Mass and dissolving

Changes in chemistry

Revising acids

Word equations

Definitions in chemistry

Types of chemical reaction

Revising the periodic table

Predicting the melting temperature of carbon

Iron - a metal

Ionic bonding

Precipitation

Spot the bonding

Hydrogen fluoride

An analogy for the atom

Ionisation energy

Chemical stability

Stability and reactivity

Interactions

Acid strength

Reaction mechanisms

Scaffolding explanations

Chemical comparisons

Learning impediment diary
- Teacher notes
- Misconceptions
- Organic chemistry
- Reactions and synthesis
- Equations, formulas and nomenclature
- Developing teaching practice
Specification
- Ions are formed when atoms lose or gain electrons to obtain the stable electron arrangement of a noble gas.
- Covalent bonds form between non-metal atoms.
- A covalent bond forms when two positive nuclei are held together by their common attraction for a shared pair of electrons.
- Diagrams can be drawn to show how outer electrons are shared to form the covalent bond(s) in a molecule.
- Working out reaction schemes of up to three conversions, recalling familiar reactions.
- Acids, bases and salts. Neutralisation - formation of a salt from an acid and a base.
- Chemical reactions can result in a change in temperature. Exothermic and endothermic reactions (and changes of state).
Related articles
By Keith S Taber
A person can be said to have acquired a concept once they are able to identify examples, and distinguish them from non-examples, this resource aims to support learners do so.
Chemical misconceptions II: Precipitation
Discover more about ionic bonding, and how dissolving into solutions can effect bonds.
Chemical misconceptions II: Reaction mechanisms
Reaction mechanisms are an important part of understanding chemical interactions. This activity helps learners to identify them.
No comments yet
Only registered users can comment on this article., more resources.

Fractional distillation and hydrocarbons | Review my learning worksheets | 14–16 years
By Lyn Nicholls
Identify learning gaps and misconceptions with this set of worksheets offering three levels of support

Chromatography | Review my learning worksheets | 14–16 years
2024-05-10T13:33:00Z By Lyn Nicholls

Solubility | Review my learning worksheets | 14–16 years
- Contributors
- Email alerts
Site powered by Webvision Cloud
What is a Chemical Reaction?
Instructions, simulations.
Youtube ID: 7UUnRtwQPlc
Lesson Summary Video for teachers
Note: This video is designed to help the teacher better understand the lesson and is NOT intended to be shown to students. It includes observations and conclusions that students are meant to make on their own.
Key Concepts:
- A physical change, such as a state change or dissolving, does not create a new substance, but a chemical change does.
- A chemical change is the result of a chemical reaction.
- In a chemical reaction, the atoms and molecules that interact with each other are called reactants .
- In a chemical reaction, the atoms and molecules produced by the reaction are called products .
- In a chemical reaction, only the atoms present in the reactants can end up in the products. No new atoms are created, and no atoms are destroyed.
- In a chemical reaction, reactants contact each other, bonds between atoms in the reactants are broken, and atoms rearrange and form new bonds to make the products.
The teacher will use a small candle flame to demonstrate a chemical reaction between the candle wax and oxygen in the air. Students will see a molecular animation of the combustion of methane and oxygen as a model of a similar reaction. Students will use atom model cut-outs to model the reaction and see that all the atoms in the reactants show up in the products.
Students will be able to explain that for a chemical reaction to take place, the bonds between atoms in the reactants are broken, the atoms rearrange, and new bonds between the atoms are formed to make the products. Students will also be able to explain that in a chemical reaction, no atoms are created or destroyed.

Be sure you and the students wear properly fitting goggles. Be careful when lighting the candle. Be sure that the match and candle are completely extinguished when you are finished with the demonstration.
Materials for the Demonstration
- Tea light candle or other small stable candle
- Glass jar, large enough to be placed over the candle
Materials for Each Student
- Atom cut-outs from the activity sheet
- Sheet of colored paper or construction paper
- Colored pencils
- Glue or tape
Download All Lesson 6.1 Resources
Get the entire lesson plan and Student Activity Sheet for “Lesson 6.1 - What is a Chemical Reaction?"
Download PDF DOCX | Google Doc
Online Assignments
Supplement in-class learning with interactive, multimedia-rich Google Forms lesson modules, perfect for reinforcing key chemistry concepts and scientific investigation skills.
Explore Online Assignments
Standards Alignment
6.1 Next Generation Science Standards (PDF) 6.1 Common Core State Standards (PDF)
More about Standards Alignment
Step 1 Review what happens during a physical change and introduce the idea of chemical change.
Tell students that in previous chapters they have studied different aspects of physical change. When atoms and molecules speed up or slow down, that is a physical change. When they change state from liquid to solid or from gas to liquid, that is a physical change. When a substance is dissolved by water or some other solvent, a new substance has not really been formed. The ions or molecules can still come back together to form the original substance.
Let students know that in this chapter they will explore what happens during a chemical change. In a chemical change, the atoms in the reactants rearrange themselves and bond together differently to form one or more new products with different characteristics than the reactants. When a new substance is formed, the change is called a chemical change .
Step 2 As a demonstration, light a candle and explain what is happening using the terms reactants , products , and chemical reaction .
Explain that in most chemical reactions, two or more substances, called reactants, interact to create different substances called products . Tell students that burning a candle is an example of a chemical reaction.
- Carefully light a tea light candle or other small candle.
- Keep the candle burning as you ask students the questions below. You will put the candle out in the second part of the demonstration
Expected Results
The wick will catch on fire and the flame will be sustained by the chemical reaction.
The following question is not easy, and students are not expected to know the answer at this point. However, thinking about a candle burning in terms of a chemical reaction is a good place to start developing what it means when substances react chemically.
Ask students:
- What do you think are the reactants in this chemical reaction? Wax and oxygen from the air are the reactants.
Students often say that the string or wick is burning. It is true that the string of the wick does burn but it’s the wax on the string and not so much the string itself that burns and keeps the candle flame burning. Explain that the molecules that make up the wax combine with oxygen from the air to make the products carbon dioxide and water vapor.
Point out to students that this is one of the major characteristics of a chemical reaction:
In a chemical reaction, atoms in the reactants combine in new and different ways to form the molecules of the products.
Students may be surprised that water can be produced from combustion. Since we use water to extinguish a fire, it may seem strange that water is actually produced by combustion. You may want to let students know that when they metabolize or “burn” food in their bodies, they also produce carbon dioxide and water.
Step 3 Place a jar over the candle to help students realize that oxygen is a reactant in the burning of a candle.
Remind students that air is a mixture of gases. Explain that when something burns, it reacts with the oxygen in the air.
Ask students to make a prediction:
- Will the candle still burn if one of the reactants (wax or oxygen) is no longer available? Students may guess that the candle will not burn because both reactants are required for the chemical reaction to continue.

- Carefully place a glass jar over the lit candle.
The flame goes out.
- Why do you think the flame goes out when we put a jar over the candle? Placing a jar over the candle limits the amount of oxygen in the air around the candle. Without enough oxygen to react with the wax, the chemical reaction cannot take place and the candle cannot burn.
- When a candle burns for a while, it eventually gets smaller and smaller. Where does the candle wax go? When a candle burns, the candle wax seems to “disappear.” It doesn’t really disappear: It reacts chemically, and the new products go into the air.
Note : Some curious students may ask what the flame is made of. This is a great question and not trivial to answer. The flame is burning wax vapor. The light of the flame is caused by a process called chemiluminescence. Energy released in the chemical reaction makes electrons from different molecules move to a higher energy state. When the electrons come back down, energy is released in the form of light.
Step 4 Introduce the chemical equation for the combustion of methane and explain that atoms rearrange to become different molecules.
Explain to students that wax is made of long molecules called paraffin and that paraffin is made up of only carbon atoms and hydrogen atoms bonded together. Molecules made of only carbon and hydrogen are called hydrocarbons . Tell students that you will use the simplest hydrocarbon (methane) as a model to show how the wax, or any other hydrocarbon, burns.
Project the image Methane and Oxygen React .
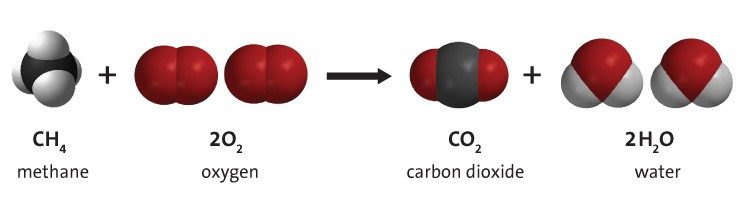
Show students that there is methane and oxygen on the left side of the chemical equation and carbon dioxide and water on the right side. Explain that the molecules on the left side are the reactants and the ones on the right side are the products . When the candle was burning, the paraffin reacted with oxygen in the air to produce carbon dioxide and water, similar to the chemical reaction between methane and oxygen.
Explain to students that the chemical formula for methane is CH 4 . This means that methane is made up of one carbon atom and four hydrogen atoms. Show students that the other reactant is two molecules of oxygen gas. Point out that each molecule of oxygen gas is made up of two oxygen atoms bonded together. It can be confusing for students that oxygen the atom, and oxygen the molecule, are both called oxygen . Let students know that when we talk about the oxygen in the air, it is always the molecule of oxygen, which is two oxygen atoms bonded together, or O 2 .
- Where do the atoms come from that make the carbon dioxide and the water on the right side of the equation? The atoms in the products come from the atoms in the reactants. In a chemical reaction, bonds between atoms in the reactants are broken and the atoms rearrange and form new bonds to make the products.
Note : Leave this equation projected throughout the activity in the Explore section of this lesson. Students will need to refer to it as they model the chemical reaction.
Give each student an activity sheet.
- Lesson 6.1 Student Activity Sheet PDF | DOCX | Google Doc
- Lesson 6.1 Activity Sheet Answers PDF | DOCX | Google Doc
Download the student activity sheet, and distribute one per student.
All Downloads
The activity sheet will serve as the “Evaluate” component of each 5-E lesson plan. The activity sheets are formative assessments of student progress and understanding. A more formal summative assessment is included at the end of each chapter.
Students will record their observations and answer questions about the activity on the activity sheet. The Explain It with Atoms and Molecules and Take It Further sections of the activity sheet will either be completed as a class, in groups, or individually, depending on your instructions. Look at the teacher version of the activity sheet to find the questions and answers.
Step 5 Have students make a model to show that in a chemical reaction the atoms of the reactants rearrange to form the products.
Question to Investigate
Where do the atoms in the products of a chemical reaction come from?
- Atom model cut-outs (carbon, oxygen, and hydrogen)
Prepare the Atoms
- Color the carbon atoms black, the oxygen atoms red, and leave the hydrogen atoms white.
- Use scissors to carefully cut out the atoms.
Build the Reactants
- On a sheet of paper, place the atoms together to make the molecules of the reactants on the left side of the chemical equation for the combustion of methane.
- Write the chemical formula under each molecule of the reactants. Also draw a “+” sign between the reactants.
After you are sure that students have made and written the formula for the reactant molecules, tell students that they will rearrange the atoms in the reactants to form the products.
Build the Products
- Draw an arrow after the second oxygen molecule to show that a chemical reaction is taking place.
- Rearrange the atoms in the reactants to make the molecules in the products on the right side of the arrow.
- Write the chemical formula under each molecule of the products. Also draw a “+” sign between the products.
Tell students that in a chemical reaction, the atoms in the reactants come apart, rearrange, and make new bonds to form the products.
Represent the Chemical Equation
- Have students use their remaining atoms to make the reactants again to represent the chemical reaction as a complete chemical equation.
- Glue or tape the atoms to the paper to make a more permanent chemical equation of the combustion of methane.
Step 6 Help students count the number of atoms on each side of the equation.
Project the animation Combustion of Methane .
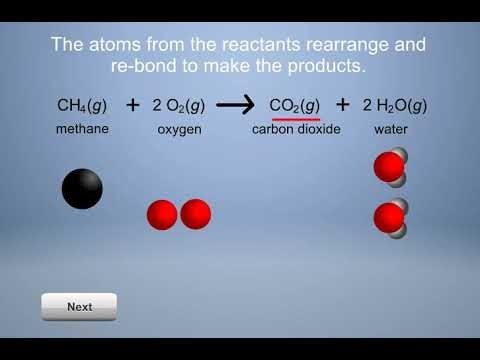
Combustion of Methane
Show students that the atoms in methane and oxygen need to come apart like in their models. Also point out that the atoms arrange themselves differently and rebond to form new products. This is also like their model. Be sure that students realize that the atoms in the products only come from the reactants. There are no other atoms available. No new atoms are created, and no atoms are destroyed.
Note: Explain to students that chemical reactions are more complicated than the simplified model shown in the animation. The animation shows that bonds between atoms in the reactants are broken, and that atoms rearrange and form new bonds to make the products.
In reality, the reactants need to collide and interact with each other in order for their bonds to break and rearrange. Also, the animation shows all of the atoms in the reactants coming apart and rearranging to form the products. But in many chemical reactions, only some bonds are broken, and groups of atoms stay together as the reactants form the products.
Read more about the combustion of methane in Teacher Background.
- Lesson 6.1 Teacher Background PDF
Guide students as you answer the following question together:
- How many carbon, hydrogen, and oxygen atoms are in the reactants compared to the number of carbon, hydrogen, and oxygen atoms in the products? Show students how to use the big number (coefficient) in front of the molecule and the little number after an atom of the molecule (subscript) to count the atoms on both sides of the equation. Explain to students that the subscript tells how many of a certain type of atom are in a molecule. The coefficient tells how many of a particular type of molecule there are. So if there is a coefficient in front of the molecule and a subscript after an atom, you need to multiply the coefficient times the subscript to get the number of atoms. For example, in the products of the chemical reaction there are 2H 2 O. The coefficient means that there are two molecules of water. The subscript means that each water molecule has two hydrogen atoms. Since each water molecule has two hydrogen atoms and there are two water molecules, there must be 2 x 2 = 4 hydrogen atoms.
|
|
|
Carbon | 1 | 1 |
Hydrogen | 4 | 4 |
Oxygen | 4 | 4 |
Note : The coefficients indicate the ratios of the numbers of molecules in a chemical reaction. It is not the actual number as in two molecules of oxygen and one molecule of methane since there are usually billions of trillions of molecules reacting. The coefficient shows that there are twice as many oxygen molecules as methane molecules reacting. It would be correct to say that in this reaction there are two oxygen molecules for every methane molecule.
Step 7 Explain that mass is conserved in a chemical reaction.
- Are atoms created or destroyed in a chemical reaction? No.
- How do you know? There are the same number of each type of atom on both the reactant side and the product side of the chemical equation we explored.
- In a physical change, like changing state from a solid to a liquid, the substance itself doesn’t really change. How is a chemical change different from a physical change? In a chemical change, the molecules in the reactants interact to form new substances. In a physical change, like a state change or dissolving, no new substance is formed.
Explain that another way to say that no atoms are created or destroyed in a chemical reaction is to say, “Mass is conserved.”
Project the image Balanced Equation .
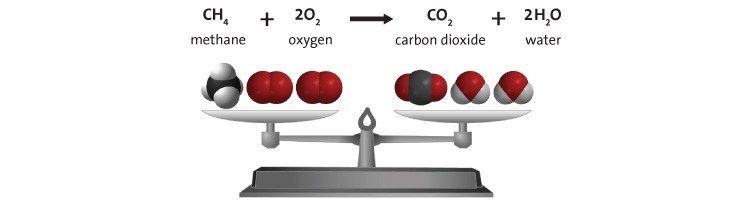
Explain that the balance shows the mass of methane and oxygen on one side exactly equals the mass of carbon dioxide and water on the other. When an equation of a chemical reaction is written, it is “balanced” and shows that the atoms in the reactants end up in the products and that no new atoms are created, and no atoms are destroyed.

Step 8 Introduce two other combustion reactions and have students check to see whether or not they are balanced.
Tell students that, in addition to the wax and methane, some other common hydrocarbons are propane (the fuel in outdoor gas grills), and butane (the fuel in disposable lighters). Have students count the number of carbon, hydrogen, and oxygen atoms in the reactants and products of each equation to see if the equation is balanced. They should record the number of each type of atom in the chart on their activity sheet.
Lighting an outdoor gas grill—Combustion of propane
C 3 H 8 + 5O 2 → 3CO 2 + 4H 2 0
Using a disposable lighter—Combustion of butane
2C 4 H 10 + 13O 2 → 8CO 2 + 10H 2 O
After students have counted each type of atom, review their answers to make sure they know how to interpret subscripts and coefficients
What is the 5-E format?
The 5-E instructional model is an approach to teaching and learning that focuses on active engagement, inquiry-based learning, and collaboration.
Simulations for Lesson 6.1
For Students
For Teachers
- Lesson 6.1 Lesson Plan PDF | DOCX | Google Doc
Resources for the entire Chapter 6
- Chapter 6 Student Reading PDF | DOCX | Google Doc
- Chapter 6 Test Bank PDF | DOCX | Google Doc
More from Chapter 6
Interactive Lesson Modules
- Lesson 6.1 Online Assignments Google Form
Have Questions? Visit Help Center
This lesson is part of: Chapter 6: Chemical Change
Lesson 5.9: Temperature Changes in Dissolving
Lesson 6.2: Controlling the Amount of Products in a Chemical Reaction
Accept & Close The ACS takes your privacy seriously as it relates to cookies. We use cookies to remember users, better understand ways to serve them, improve our value proposition, and optimize their experience. Learn more about managing your cookies at Cookies Policy .
1155 Sixteenth Street, NW, Washington, DC 20036, USA | service@acs.org | 1-800-333-9511 (US and Canada) | 614-447-3776 (outside North America)
- Terms of Use
- Accessibility
Copyright © 2024 American Chemical Society
The Open Reaction Database
Research areas.
Data Management
General Science
Chemical Reactions and Equations
Chemical reactions, what is a chemical reaction.
A chemical reaction is in which the bonds are broken within reactant molecules, and new bonds are formed within product molecules in order to form a new substance.
Chemical reactions are all around us, from the metabolism of food in our body to how the light we get from the sun is the result of chemical reactions. Before beginning with chemical reactions, it is important to know about physical and chemical changes.

A burning candle is the best example of physical and chemical change. Take a candle and light it. As time passes, we can observe that the candle changes to wax. If you cover the candle with a jar, it will extinguish.
In the demonstration, burning of the candle is a chemical change while conversion of the candle to wax is a physical change. In a physical change, there is basically a change of state of the substance but in the case of a chemical change mostly a new substance is formed in which either energy is given off or absorbed. Thus, we can conclude that chemical changes are accompanied by certain physical changes.
Table of Content
Basic concepts of chemical reactions.
- Recommended Videos on Chemical Reactions
- Chemical equations
- Types of Chemical equations
Important Points to Remember
- Frequently Asked Questions – FAQs
- A Chemical Reaction is a process that occurs when two or more molecules interact to form a new product(s).
- Compounds that interact to produce new compounds are called reactants whereas the newly formed compounds are called products.
- Chemical reactions play an integral role in different industries, customs and even in our daily life. They are continuously happening in our general surroundings; for example, rusting of iron, pottery, fermentation of wine and so on.
- In a chemical reaction, a chemical change must occur which is generally observed with physical changes like precipitation, heat production, colour change etc.
- A reaction can take place between two atoms or ions or molecules, and they form a new bond and no atom is destroyed or created but a new product is formed from reactants.
- The rate of reaction depends on and is affected by factors like pressure, temperature, the concentration of reactants.
Recommended Videos
Chemical reactions and equations – all activities in one go.
Chemical Equations
Due to the vast amounts of chemical reactions happening around us, a nomenclature was developed to simplify how we express a chemical reaction in the form of a chemical equation. A chemical equation is nothing but a mathematical statement which symbolizes the product formation from reactants while stating certain condition for which how the reaction has been conducted.
The reactants are on the left-hand side whereas the products formed are on the right-hand side. The reactants and products are connected by a one-headed or two-headed arrows. For example, a reaction
A + B → C + D
Here, A and B are the reactants, which react to form the products C and D. In an actual chemical equation, reactants are denoted by their chemical formula . In order to assure the law of conservation of mass, a chemical equation must be balanced i.e. the number of atoms on both sides must be equal. This is the balancing of the equation.
Let us consider an actual chemical reaction between Methane(CH₄) and Oxygen (O 2 ),
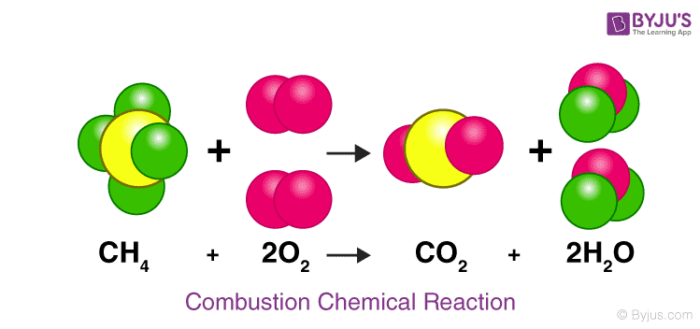
Here we can see how the number of each atom on the left side is balanced on the right side, as stated by the law of conservation of mass.
Types of Chemical Reactions
The basis for different types of reactions is the product formed, the changes that occur, the reactants involved and so on. Different types of reactions are
- Combustion reaction
- Decomposition reaction
- Neutralization reaction
- Redox Reaction
- Precipitation or Double-Displacement Reaction
- Synthesis reaction
1. Combustion Reaction
A combustion reaction is a reaction with a combustible material with an oxidizer to give an oxidized product. An oxidizer is a chemical a fuel requires to burn, generally oxygen. Consider the example of combustion of magnesium metal.
\(\begin{array}{l}2 Mg + O_2 \rightarrow 2 MgO + Heat\end{array} \)
Here, 2 magnesium atoms react with a molecule of oxygen producing 2 molecules of the compound magnesium oxide releasing some heat in the process.
2. Decomposition Reaction
A Decomposition reaction is a reaction in which a single component breaks down into multiple products. Certain changes in energy in the environment have to be made like heat, light or electricity breaking bonds of the compound. Consider the example of the decomposition of calcium carbonate giving out CaO (Quick Lime) which is a major component of cement.
\(\begin{array}{l}Ca C O_3 ( s ) \overset{Heat}{\rightarrow} Ca O ( s ) + CO_2 ( g ) \end{array} \)
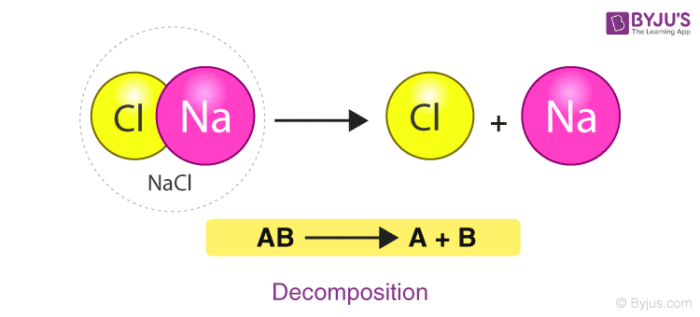
Here, the compound Calcium carbonate when heated breaks down into Calcium Oxide and Carbon Dioxide.
3. Neutralization Reaction
A Neutralization reaction is basically the reaction between an acid and a base giving salt and water as the products. The water molecule formed is by the combination of OH – ions and H + ions. The overall pH of the products when a strong acid and a strong base undergo a neutralization reaction will be 7. Consider the example of the neutralization reaction between Hydrochloric acid and Sodium Hydroxide giving out sodium chloride(Common Salt) and water.
\(\begin{array}{l}H Cl + NaOH \rightarrow NaCl +H_2O\end{array} \)
Here, an acid and a base, Hydrochloric acid and Sodium Hydroxide react in a neutralization reaction to produce Sodium Chloride(Common Salt) and water as the products.
4. Redox Reaction
A RED uction- OX idation reaction is a reaction in which there is a transfer of electrons between chemical species. Let us consider the example of an electrochemical cell-like redox reaction between Zinc and Hydrogen.
\(\begin{array}{l}Zn+2H^{+}\rightarrow Zn^{2+}+H_2\end{array} \)
Here, A Zinc atom reacts with 2 ions of positively charged hydrogen to which electrons get transferred from the zinc atom and hydrogen becomes a stable molecule and Zinc ion is the product.
5. Precipitation or Double-Displacement Reaction
It is a type of displacement reaction in which two compounds react and consequently, their anions and cations switch places forming two new products. Consider the example of the reaction between silver nitrate and sodium chloride. The products will be silver chloride and sodium nitrate after the double-displacement reaction.
\(\begin{array}{l}Ag N O_3 + Na Cl \rightarrow Ag Cl + Na N O_3\end{array} \)
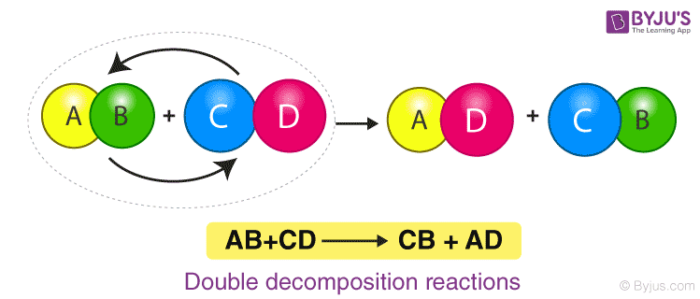
Here, Silver Nitrate and Sodium Chloride undergo a double displacement reaction. Wherein Silver replaces Sodium in Sodium Chloride and Sodium joins with Nitrate becoming Sodium Nitrate along with the Silver Chloride as the product.
6. Synthesis Reaction
A Synthesis reaction is one of the most basic types of reaction wherein multiple simple compounds combine under certain physical conditions giving out a complex product. The product will always be a compound. Let us consider the Synthesis reaction of sodium chloride with reactants solid sodium and chloride gas.
\(\begin{array}{l}2 Na ( s ) + Cl_{2} (g) \rightarrow 2 Na Cl ( s )\end{array} \)
Here, we have 2 Atoms of solid Sodium reacting with Chlorine gas giving out Sodium Chloride viz. Common Salt as the product.
- In a chemical change, a new compound is formed but in a physical change, the substance changes its state of existence.
- Atoms or ions or molecules which react to form a new substance are called reactants; the new atoms or molecules formed are products.
- A chemical reaction follows the law of conservation of mass. That is no atom is destroyed or created but only a new product is formed from reactants.
BYJU’S helps students by delivering chapter wise and detailed solutions to the questions of NCERT books. They can compare their answers with the sample answers given here – NCERT Solutions for class 10 Science Chapter 1 Chemical reactions and equations .
Related Videos

How Will Chemistry Help You Survive Alone on an Island?

Frequently Asked Questions – FAQs
What is meant by a chemical reaction.
A Chemical Reaction is a process that occurs when two or more molecules collide with the right orientation and sufficient force to form a new product. In this process breaking and forming bonds between atoms takes place. Compounds that interact to produce new compounds are called reactants whereas the newly formed compounds are called products.
What is chemical reaction and equation?
A chemical reaction is in which the bonds are broken within reactant molecules, and new bonds are formed within product molecules in order to form a new substance. A chemical equation is nothing but a mathematical statement which symbolizes the product formation from reactants.
What are the chemical reaction types?
On the basis of the product formed, different types of reactions are Combustion reaction, Decomposition reaction, Neutralization reaction, Redox Reaction, Precipitation or Double-Displacement Reaction, Synthesis reaction.
What is a combustion chemical reaction?
A combustion reaction is a reaction in which a substance reacts with oxygen gas, releasing energy in the form of light and heat.
What is a chemical equation?
What is a decomposition reaction, what is a neutralization reaction, what is a redox reaction, what is precipitation or a double displacement reaction, what is a synthesis reaction.

Put your understanding of this concept to test by answering a few MCQs. Click ‘Start Quiz’ to begin!
Select the correct answer and click on the “Finish” button Check your score and answers at the end of the quiz
Visit BYJU’S for all Chemistry related queries and study materials
Your result is as below
Request OTP on Voice Call
| CHEMISTRY Related Links | |
Leave a Comment Cancel reply
Your Mobile number and Email id will not be published. Required fields are marked *
Post My Comment
so easy to understand thank you for giving these resources for free
It nice😊😊😊😊😊 I was satisfied with this information 😊😊😊😊
So interesting easy way of learning
literally it really helped me a lot it is awsm
It is a very good learning platform
It is a very good learning platform for us
Register with BYJU'S & Download Free PDFs
Register with byju's & watch live videos.
- Science Clarified
- Real-Life Chemistry Vol 2
- Chemical Reactions
Chemical Reactions - Real-life applications
Chemical equations.
In every chemical reaction, there are participants known as reactants, which, by chemically reacting to one another, result in the creation of a product or products. As stated earlier, a chemical reaction involves changes in the arrangement of atoms. The atoms in the reactants (or, if the reactant is a compound, the atoms in its molecules) are rearranged. The atomic or molecular structure of the product is different from that of either reactant.
Note, however, that the number of atoms does not change. Atoms themselves are neither created nor destroyed, and in a chemical reaction, they merely change partners, or lose partners altogether as they return to their elemental form. This is a critical principle in chemistry, one that proves that medieval alchemists' dream of turning lead into gold was based on a fallacy. Lead and gold are both elements, meaning that each has different atoms. To imagine a chemical reaction in which one becomes the other is like saying "one plus one equals one."
SYMBOLS IN A CHEMICAL EQUATION.
In a mathematical equation, the sums of the numbers on one side of the equals sign must be the same as the sum of the numbers on the other side. The same is true of a chemical equation, a representation of a chemical reaction in which the chemical symbols on the left stand for the reactants, and those on the right are the product or products. Instead of an equals sign separating them, an arrow, pointing to the right to indicate the direction of the reaction, is used.
- (s) : solid
- (l) : liquid
- (aq) : dissolved in water (an aqueous solution)
The fourth symbol, of course, does not indicate a phase of matter per se (though obviously it appears to be a liquid); but as we shall see, aqueous solutions play a role in so many chemical reactions that these have their own symbol. At any rate, using this notation, we begin to symbolize the reaction of hydrogen and oxygen to form water thus: H (g) + O (g) →H 2 O (l).
This equation as written, however, needs to be modified in several ways. First of all, neither hydrogen nor oxygen is monatomic. In other words, in their elemental form, neither appears as a single atom; rather, these form diatomic (two-atom) molecules. Therefore, the equation must be rewritten as H 2 (g) + O 2 (g) →H 2 O (l). But this is still not correct, as a little rudimentary analysis will show.
Balancing Chemical Equations
When checking a chemical equation, one should always break it down into its constituent elements, to determine whether all the atoms on the left side reappear on the right side; otherwise, the result may be an incorrect equation, along the lines of "1 + 1 = 1." That is exactly what has happened here. On the left side, we have two hydrogen atoms and two oxygen atoms; on the right side, however, there is only one oxygen atom to go with the two hydrogens.
Obviously, this equation needs to be corrected to account for the second oxygen atom, and the best way to do that is to show a second water molecule on the right side. This will be represented by a 2 before the H 2 O, indicating that two water molecules now have been created. The 2, or any other number used for showing more than one of a particular chemical species in a chemical equation, is called a coefficient. Now we have H 2 (g) + O 2 (g) →2H 2 O (l).
Is this right? Once again, it is time to analyze the equation, to see if the number of atoms on the left equals the number on the right. Such analysis can be done in a number of ways: for instance, by symbolizing each chemical species as a circle with chemical symbols for each element in it. Thus a single water molecule would be shown as a circle containing two H's and one O.
Whatever the method used, analysis will reveal that the problem of the oxygen imbalance has been solved: now there are two oxygens on the left, and two on the right. But solving that problem has created another, because now there are four hydrogen atoms on the right, as compared with two on the left. Obviously, another coefficient of 2 is needed, this time in front of the hydrogen molecule on the left. The changed equation is thus written as: 2H 2 (g) + O 2 (g) → 2H 2 O (l). Now, finally, the equation is correct.
THE PROCESS OF BALANCING CHEMICAL EQUATIONS.
What we have done is to balance an unbalanced equation. An unbalanced equation is one in which the numbers of atoms on the left are not the same as the number of atoms on the right. Though an unbalanced equation is incorrect, it is sometimes a necessary step in the process of finding the balanced equation—one in which the number of atoms in the reactants and those in the product are equal.
In writing and balancing a chemical equation, the first step is to ascertain the identities, by formula, of the chemical species involved, as well as their states of matter. After identifying the reactants and product, the next step is to write an unbalanced equation. After that, the unbalanced equation should be subjected to analysis, as demonstrated above.
The example used, of course, involves a fairly simple substance, but often, much more complex molecules will be part of the equation. In performing analysis to balance the equation, it is best to start with the most complex molecule, and determine whether the same numbers and proportions of elements appear in the product or products. After the most complicated molecule has been dealt with, the second-most complex can then be addressed, and so on.
Assuming the numbers of atoms in the reactant and product do not match, it will be necessary to place coefficients before one or more chemical species. After this has been done, the equation should again be checked, because as we have seen, the use of a coefficient to straighten out one discrepancy may create another. Note that only coefficients can be changed; the formulas of the species themselves (assuming they were correct to begin with) should not be changed.
After the equation has been fully balanced, one final step is necessary. The coefficients must be checked to ensure that the smallest integers possible have been used. Suppose, in the above exercise, we had ended up with an equation that looked like this: 12H 2 (g) + 6O 2 (g) →12H 2 O (l). This is correct, but not very "clean." Just as a fraction such as 12/24 needs to be reduced to its simplest form, 1/2, the same is true of a chemical equation. The coefficients should thus always be the smallest number that can be used to yield a correct result.
Types of Chemical Reactions
Note that in chemical equations, one of the symbols used is (aq) , which indicates a chemical species that has been dissolved in water—that is, an aqueous solution. The fact that this has its own special symbol indicates that aqueous solutions are an important part of chemistry. Examples of reactions in aqueous solutions are discussed, for instance, in the essays on Acid-Base Reactions; Chemical Equilibrium; Solutions.
Another extremely important type of reaction is an oxidation-reduction reaction. Sometimes called a redox reaction, an oxidation-reduction reaction occurs during the transfer of electrons. The rusting of iron is an example of an oxidation-reduction reaction; so too is combustion. Indeed, combustion reactions—in which oxygen produces energy so rapidly that a flame or even an explosion results—are an important subset of oxidation-reduction reactions.
REACTIONS THAT FORM WATER, SOLIDS, OR GASES.
Another type of reaction is an acid-base reaction, in which an acid is mixed with a base, resulting in the formation of water along with a salt.
Other reactions form gases, as for instance when water is separated into hydrogen and oxygen. Similarly, heating calcium carbonate (lime-stone) to make calcium oxide or lime for cement also yields gaseous carbon dioxide: CaCO 3 (s) + heat →CaO (s) + CO 2 (g).
There are also reactions that form a solid, such as the one mentioned much earlier, in which solid BaCrO 4 (s) is formed. Such reactions are called precipitation reactions. But this is also a reaction in an aqueous solution, and there is another product: 2KNO 3 (aq) , or potassium nitrate dissolved in water.
SINGLE AND DOUBLE DISPLACEMENT.
The reaction referred to in the preceding paragraph also happens to be an example of another type of reaction, because two anions (negatively charged ions) have been exchanged. Initially K + and CrO 4 2− were together, and these reacted with a compound in which Ba 2+ and NO 3 − were combined. The anions changed places, an instance of a double-displacement reaction, which is symbolized thus: AB + CD →AD + CB.
It is also possible to have a single-displacement reaction, in which an element reacts with a compound, and one of the elements in the compound is released as a free element. This can be represented symbolically as A + BC →B + AC. Single-displacement reactions often occur with metals and with halogens. For instance, a metal(A) reacts with an acid (BC) to produce hydrogen (B) and a salt (AC).
COMBINATION AND DECOMPOSITION.
A synthesis, or combination, reaction is one in which a compound is formed from simpler materials—whether those materials be elements or simple compounds. A basic example of this is the reaction described earlier in relation to chemical equations, when hydrogen and oxygen combine to form water. On the other hand, some extremely complex substances, such as the polymers in plastics and synthetic fabrics such as nylon, also involve synthesis reactions.
When iron rusts (in other words, it oxidizes in the presence of air), this is both an oxidation-reduction and a synthesis reaction. This also represents one of many instances in which the language of science is quite different from everyday language. If a piece of iron—say, a railing on a balcony—rusts due to the fact that the paint has peeled off, it would seem from an unscientific standpoint that the iron has "decomposed." However, rust (or rather, metal oxide) is a more complex substance than the iron, so this is actually a synthesis or combination reaction.
A true decomposition reaction occurs when a compound is broken down into simpler compounds, or even into elements. When water is subjected to electrolysis such that the hydrogen and oxygen are separated, this is a decomposition reaction. The fermentation of grapes to make wine is also a form of decomposition.
And then, of course, there are the processes that normally come to mind when we think of "decomposition": the decay or rotting of a formerly living thing. This could also include the decay of something, such as an item of food, made from a formerly living thing. In such instances, an organic substance is eventually broken down through a number of processes, most notably the activity of bacteria, until it ultimately becomes carbon, nitrogen, oxygen, and other elements that are returned to the environment.
SOME OTHER PARAMETERS.
Obviously, there are numerous ways to classify chemical reactions. Just to complicate things a little more, they can also be identified as to whether they produce heat (exothermic) or absorb heat (endothermic). Combustion is clearly an example of an exothermic reaction, while an endothermic reaction can be exemplified by the process that takes place in a cold pack. Used for instance to prevent swelling on an injured ankle, a cold pack contains an ampule that absorbs heat when broken.
Still another way to identify chemical reactions is in terms of the phases of matter involved. We have already seen that some reactions form gases, some solids, and some yield water as one of the products. If reactants in one phase of matter produce a substance or substances in the same phase (liquid, solid, or gas), this is called a homogeneous reaction. On the other hand, if the reactants are in different phases of matter, or if they produce a substance or substances that are in a different phase, this is called a heterogeneous reaction.
An example of a homogeneous reaction occurs when gaseous nitrogen combines with oxygen, also a gas, to produce nitrous oxide, or "laughing gas." Similarly, nitrogen and hydrogen combine to form ammonia, also a gas. But when hydrogen and oxygen form water, this is a heterogeneous reaction. Likewise, when a metal undergoes an oxidation-reduction reaction, a gas and a solid react, resulting in a changed form of the metal, along with the production of new gases.
Finally, a chemical reaction can be either reversible or irreversible. Much earlier, we described how wood experiences combustion, resulting in the production of ash. This is clearly an example of an irreversible reaction. The atoms in the wood and the air that oxidized it have not been destroyed, but it would be impossible to put the ash back together to make a piece of wood. By contrast, the formation of water by hydrogen and oxygen is reversible by means of electrolysis.
KEEPING IT ALL STRAIGHT.
The different classifications of reactions discussed above are clearly not mutually exclusive; they simply identify specific aspects of the same thing. This is rather like the many physical characteristics that describe a person: gender, height, weight, eye color, hair color, race, and so on. Just because someone is blonde, for instance, does not mean that the person cannot also be brown-eyed; these are two different parameters that are more or less independent.
On the other hand, there is some relation between these parameters in specific instances: for example, females over six feet tall are rare, simply because women tend to be shorter than men. But there are women who are six feet tall, or even considerably taller. In the same way, it is unlikely that a reaction in an aqueous solution will be a combustion reaction—yet it does happen, as for instance when potassium reacts with water.
Studying Chemical Reactions
Several aspects or subdisciplines of chemistry are brought to bear in the study of chemical reactions. One is stoichiometry (stoy-kee-AH-muh-tree), which is concerned with the relationships among the amounts of reactants and products in a chemical reaction. The balancing of the chemical equation for water earlier in this essay is an example of basic stoichiometry.
Chemical thermodynamics is the area of chemistry that addresses the amounts of heat and other forms of energy associated with chemical reactions. Thermodynamics is also a branch of physics, but in that realm, it is concerned purely with physical processes involving heat and energy. Likewise physicists study kinetics, associated with the movement of objects. Chemical kinetics, on the other hand, involves the study of the collisions between molecules that produce a chemical reaction, and is specifically concerned with the rates and mechanisms of reaction.
SPEEDING UP A CHEMICAL REACTION.
Essentially, a chemical reaction is the result of collisions between molecules. According to this collision model, if the collision is strong enough, it can break the chemical bonds in the reactants, resulting in a rearrangement of the atoms to form products. The more the molecules collide, the faster the reaction. Increase in the numbers of collisions can be produced in two ways: either the concentrations of the reactants are increased, or the temperature is increased. In either case, more molecules are colliding.
Increases of concentration and temperature can be applied together to produce an even faster reaction, but rates of reaction can also be increased by use of a catalyst, a substance that speeds up the reaction without participating in it either as a reactant or product. Catalysts are thus not consumed in the reaction. One very important example of a catalyst is an enzyme, which speeds up complex reactions in the human body. At ordinary body temperatures, these reactions are too slow, but the enzyme hastens them along. Thus human life can be said to depend on chemical reactions aided by a wondrous form of catalyst.
WHERE TO LEARN MORE
Bender, Hal. "Chemical Reactions." Clackamas Community College (Web site). <http://dl.clackamas.cc.or.us/ch104-01/chemical.htm> (June 3, 2001).
"Catalysis, Separations, and Reactions." Accelrys (Web site). <http://www.accelrys.com/chemicals/catalysis/> (June 3, 2001).
Goo, Edward. "Chemical Reactions" (Web site). <http://www-classes.usc.edu/engr/ms/125/MDA125/reactions/> (June 3, 2001).
Knapp, Brian J. Oxidation and Reduction. Illustrated by David Woodroffe. Danbury, CT: Grolier Educational, 1998.
Knapp, Brian J. Energy and Chemical Change. Illustrated by David Woodroffe. Danbury, CT: Grolier Educational, 1998.
Newmark, Ann. Chemistry. New York: Dorling Kindersley, 1993.
"Periodic Table: Chemical Reaction Data." WebElements (Web site). <http://www.webelements.com/webelements/elements/text/periodic-table/chem.html> (June 3, 2001).
Richards, Jon. Chemicals and Reactions. Brookfield, CT: Copper Beech Books, 2000.
"Types of Chemical Reactions" (Web site). <http://www.usoe.k12.ut.us/curr/science/sciber00/8th/matter/sciber/chemtype.htm> (June 3, 2001).
Zumdahl, Steven S. Introductory Chemistry: A Foundation, 4th ed. Boston: Houghton Mifflin, 2000.
User Contributions:
Comment about this article, ask questions, or add new information about this topic:.
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Open access
- Published: 06 May 2021
Inferring experimental procedures from text-based representations of chemical reactions
- Alain C. Vaucher ORCID: orcid.org/0000-0001-7554-0288 1 ,
- Philippe Schwaller ORCID: orcid.org/0000-0003-3046-6576 1 ,
- Joppe Geluykens ORCID: orcid.org/0000-0002-3646-6019 1 ,
- Vishnu H. Nair 1 ,
- Anna Iuliano 2 &
- Teodoro Laino 1
Nature Communications volume 12 , Article number: 2573 ( 2021 ) Cite this article
11k Accesses
36 Citations
54 Altmetric
Metrics details
- Cheminformatics
- Computational science
- Synthetic chemistry methodology
- Natural product synthesis
The experimental execution of chemical reactions is a context-dependent and time-consuming process, often solved using the experience collected over multiple decades of laboratory work or searching similar, already executed, experimental protocols. Although data-driven schemes, such as retrosynthetic models, are becoming established technologies in synthetic organic chemistry, the conversion of proposed synthetic routes to experimental procedures remains a burden on the shoulder of domain experts. In this work, we present data-driven models for predicting the entire sequence of synthesis steps starting from a textual representation of a chemical equation, for application in batch organic chemistry. We generated a data set of 693,517 chemical equations and associated action sequences by extracting and processing experimental procedure text from patents, using state-of-the-art natural language models. We used the attained data set to train three different models: a nearest-neighbor model based on recently-introduced reaction fingerprints, and two deep-learning sequence-to-sequence models based on the Transformer and BART architectures. An analysis by a trained chemist revealed that the predicted action sequences are adequate for execution without human intervention in more than 50% of the cases.
Similar content being viewed by others

Automated extraction of chemical synthesis actions from experimental procedures
Large-scale chemical language representations capture molecular structure and properties
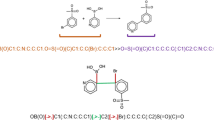
Discovery of novel chemical reactions by deep generative recurrent neural network
Introduction.
In recent years, chemistry has witnessed several successful applications of artificial intelligence (AI) algorithms. Among others, generative models can help design molecules with potentially relevant properties for specific applications 1 , while retrosynthetic models suggest potential routes to synthesize these molecules 2 , 3 .
Reaction prediction algorithms assist chemists in prioritizing the synthetic strategy and in selecting effective routes. However, a synthetic route is insufficient to assemble the experimental procedures required for each synthetic step. The planning of a chemical synthesis requires the knowledge of the precise sequence of operations (addition of chemicals, stirring, filtration, solvent extraction, preparation of intermediate solutions, etc.) and the definition of their optimal parameters (temperature, solvents, atmosphere, etc.). The assembly of these operational tasks is left to happenstance, mostly guided by the chemist’s experience, and characterized by trial and error. It often requires extensive literature search and the use of homology strategies, in which one identifies one or more reported chemical procedures likely to resemble the target chemical transformation, to provide the best initial guess for the experimental protocol. The reasoning behind this approach is that the execution of a chemical reaction should be successful if one follows procedures for known similar reactions. Additional iteration cycles are often required to improve the reaction protocol after inspection of the experimental results.
Hence, in practice, despite the potential benefits provided by AI algorithms, synthesizing the suggested molecules in the laboratory remains an important bottleneck. This is even more relevant if one considers the revived interest in using robotic systems to automate and scale the execution of chemical reactions 4 , 5 , 6 , 7 , 8 , 9 , 10 , 11 , 12 . In fact, while automation is widely present in chemistry 13 , the programming of the robotic systems remains a major impediment towards its wider adoption for general chemistry works due to the need for personnel with both chemical domain expertize and programming skills.
A large-scale adoption of automated synthesis platforms for general purpose chemistry will require virtual assistants to help create specific execution programs for individual chemical reactions. This entails the ability to recommend a precise sequence of operations for the execution of a suggested reaction step, depending on the nature of the substrates, solvents and target products. Concretely, starting from a chemical equation suggested by an AI model, the goal is to determine the series of steps needed to successfully execute that reaction in a laboratory setup.
The use of AI technologies to predict experimental procedures covers, to date, few works focusing on predicting solvents or reaction conditions. For instance, Walker et al. designed a machine learning model to predict adequate solvents for five selected reaction classes 14 . Maser et al. formulated the prediction of reaction conditions as a multiclass prediction problem for four classes of cross-coupling reactions 15 . Their models select categorical values for the categories metal, ligand, base, solvent, additive, temperature, activator, as well as the presence and pressure of a carbon monoxide atmosphere. Nicolaou et al. 8 coupled a retrosynthetic analysis tool to a nearest-neighbor search in a database of previously executed reactions in order to suggest procedures for the automated synthesis of new molecules. Gao et al. 16 trained a neural network to predict reagents, solvents, catalysts, and temperatures for any reaction class. Their model has been combined with retrosynthetic tools to plan syntheses on a robotic platform 5 . However, the chemical procedures had to be revised and complemented manually. The domain complexity and lack of sufficiently curated data hindered further technological developments of AI models predicting entire reaction procedures with limited human intervention.
Here, we present Smiles2Actions, the first AI model to convert chemical equations to fully explicit sequences of experimental actions. We demonstrate it for the realm of batch organic synthesis. The chemical equations, generated by AI algorithms or input by humans, are represented in a text-based format (SMILES). Using a natural language processing model 17 , we generate a data set of 693,517 chemical equations and associated action sequences necessary for training three different data-driven models: a nearest-neighbor model, and two transformer-based sequence-to-sequence models, the original architecture as introduced by Vaswani et al. 18 and the bidirectional and auto-regressive transformer (BART) by Lewis et al. 19 . An overview of our approach is illustrated in Fig. 1 . When comparing the original and predicted chemical procedures as a whole, the best performing model achieves a normalized Levenshtein similarity of 50% for 68.7% of reactions, a 75% match for 24.7% of reactions, and a 100% match for 3.6% of reactions. The models are able to estimate the solubility of products in different solvents (phase separation, extraction) and to anticipate the formation of precipitate (filtration), or when to heat or cool the reaction mixture (endothermic or exothermic reactions), without ever making those concepts explicit. Finally, an academic chemist expert analyzes and assesses 500 predicted action sequences among different chemical reaction classes, finding that the predicted action sequences are adequate for execution without human intervention for more than half of the predicted reactions.
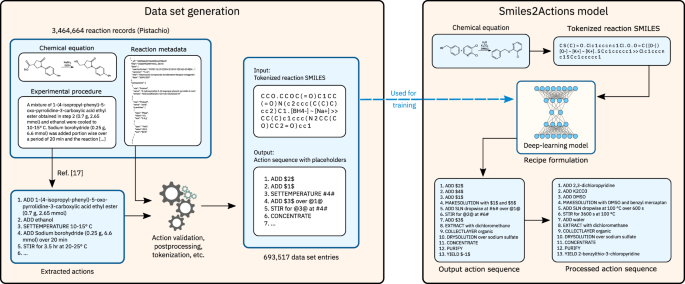
The data set is generated in a sequence of processing and filtering steps, starting from information available in patent reaction records (on the left). The Smiles2Actions model is trained on this data set, after which it can predict the action sequences to execute arbitrary chemical equations (on the right).
Prediction task
We formulate the task of inferring experimental procedure steps as the prediction of an action sequence starting from a chemical equation. The prediction task relates to single reaction steps. For a multi-step synthesis, the experimental procedure steps are predicted separately for each individual reaction.
As in previous works 3 , 20 , 21 , we do not distinguish between reactants and reagents, as the attribution to one class or the other may be subtle or ambiguous 22 . Hence, the chemical equations in the input consist of a set of precursors (reactants + reagents) and a set of product molecules. Figure 2 shows an example for a condensation reaction.
To the left of the arrow, one can identify all the precursor molecules, while the product molecule is shown to its right. On the left-hand side of the reaction, we also include molecules that play a role as reagents or solvents only, such as the first two entities: N,N′-dicyclohexylcarbodiimide and dichloromethane.
We depict chemical equations using a text-based representation of the entire set of molecules involved in the corresponding transformation. Without loss of generality, we use the SMILES 23 , 24 format, which for the example reported in Fig. 2 is equivalent to:
C(=NC1CCCCC1)=NC1CCCCC1.ClCCl.CC1(C)CC(=O)Nc2cc(C(=O)O)ccc21.Nc1ccccc1 >> CC1(C)CC(=O)Nc2cc(C(=O)Nc3ccccc3)ccc21
When processing the SMILES representation of a chemical equation, the generated output is a sequence of synthesis actions. The actions follow the format introduced by Vaucher et al. 17 and each consist of a type with associated properties (specific to the action type). A summary of the action types is given in the Supplementary Note 1 . These actions cover the most common batch operations for the synthesis of organic molecules, and were designed to contain all the required information to reproduce a chemical reaction in a laboratory. A thorough discussion about the action format is available in the original publication 17 .
In order to improve the training performance of the computational models, we restrict the allowed values for two types of properties. The first property relates to the specification of compound names in action sequences. Whenever possible, we use tokens representing the position of the corresponding molecule in the reaction input, allowing the computational models to focus more on relevant instruction patterns instead of trying to learn the naming conventions of molecules. We allow the use of reagents that do not appear in the chemical equation only when they are part of a list of commonly used reagents (reported in the Supplementary Data 1 ). The second property relates to numerical values for temperatures and durations. The success of reactions does not depend on the exact values for these experimental conditions, as long as they lie within adequate ranges. As a consequence, these values are often reported inaccurately. An example is the commonly reported reaction duration overnight. The term has little meaning from a quantitative perspective and only indicates the execution of an unattended reaction outside of working hours. The same applies when reporting reaction temperatures. The specification of wide intervals is often a sign of an uncontrolled process, putting aside the systematic errors connected to the use of different measuring devices. Therefore, we tokenized predefined ranges for temperatures and durations and used these tokens during the training process instead of the exact (noisy) reported values. The predicted procedure steps will contain the optimal tokens corresponding to predefined ranges. At inference time, the tokens can be replaced by actual numerical values in a straightforward manner. These two modifications simplify the design and improve the performance of all computational models, as they remove the necessity to learn the vocabulary and syntax of compound names, durations, or temperatures. Also, they reflect the fact that ranges of durations and temperatures are usually as adequate as precise values.
Another important aspect is the mass scale of the chemical transformation. In fact, compound quantities affect chemical procedures and the format designed by Vaucher et al. 17 is general enough to make this relationship explicit. Hence, it may be possible to introduce a functional dependency with respect to mass quantities by specifying an additional reaction token. However, the information extracted from patents lacks a proper coverage across different mass scales to capture the typical patterns of the operational changes when using production quantities compared to laboratory scale 25 , 26 . Therefore, we removed compound quantities from action sequences, leading to optimal processes that are averaged across the different mass scales.
Taking these points into account, Table 1 shows a possible action sequence for the reaction depicted in Fig. 2 .
The design of models predicting experimental steps requires a data set of chemical equations and associated experimental procedures. Because of the unavailability of open and large-scale ready-to-use options, we created our own data set of chemical reaction procedures from scratch.
The data set was generated in multiple consecutive steps starting from the Pistachio database 27 , which contains 8,377,878 records of reactions published in patents, each including the reaction SMILES string, the experimental procedure, and a mapping of molecular SMILES strings with associated compound names.
Excluding records with no experimental procedure text (2,140,782) and duplicate reaction records (2,772,432), we extracted the action sequences corresponding to the remaining 3,464,664 reactions using a state-of-the-art natural language model (Paragraph2Actions) recently published by our group 17 .
The extracted action sequences underwent a series of postprocessing steps to produce a standardized data set of higher quality. We ignored the reaction records reported in languages other than English, records referring to other procedures, and records containing invalid actions upon processing by the Paragraph2Actions model. We used a few heuristics to normalize the action representation and to add implicit actions. We replaced temperatures, durations, and pH values with tokens corresponding to predefined intervals. We matched compound names to molecules present in the corresponding chemical equation, replacing the chemical names with the respective positional tokens. In cases where we were not able to process the chemical records to meet predefined quality standards the entire record was removed from the data set. For records with identical reaction SMILES we retained only one copy. In the Methods section, we report a more detailed description of all the postprocessing steps. The standardization protocol produced a data set consisting of 693,517 reaction SMILES with associated action sequences, a mere 20% of the intermediate set of 3,464,664 records, or 8% of the initial Pistachio database.
In Table 2 , we list the leading factors responsible for the decrease of the size of the data set compared to the original number of reaction records. More than one fourth of the initial reaction records is connected to unsuccessful mapping of molecules between the chemical equation and the extracted action sequence. This occurs when a molecule is present in the chemical equation but not in the extracted actions, or vice versa. A similar portion of reaction records was ignored because the corresponding experimental procedures do not contain relevant action sequences upon processing by the Paragraph2Actions model, such as records referring to other procedures, very short action sequences, action sequences with invalid actions, and action sequences that are likely to describe multiple reaction steps. Other errors include flaws in the conversion of extracted duration or temperature strings to actual numerical values.
The last processing step described above, involving the identification/removal of duplicate entries, revealed the presence of 326,929 duplicate reaction SMILES in 871,112 reaction records. Out of these, 47,299 contained non-identical action sequences. The inspection of the corresponding action sequences showed that experimental procedures occasionally describe identical operations using different linguistic terms (for instance, a quenching operation described in terms of addition). The analysis also showed that patent records report identical reactions equally successful under different procedure parameters, such as different temperatures, duration, or the order of the added compounds. While this may be a sign of robustness of some chemical processes when exposed to different conditions, there is no possibility to rule out these records as inaccurate, misleading, or even false reporting. In the Supplementary Data 4 , we list all the reaction SMILES and associated action sequences with five or more such sequences. Among the entries with duplicate reaction SMILES, one was picked at random and kept in the data set.
To verify that the data set covers a similar chemical space as the original records, we studied the reaction classes’ coverage in the obtained reaction procedure data set. While it is reasonable to observe changes in relative class frequency due to different statistical samplings, it is essential to ensure the absence of systematic bias in our processing protocol. Figure 3 shows the differences in class frequency between the original reaction data with 3,464,664 entries and the final data set of 693,517 reaction records. Most of the reaction classes have a frequency similar to the initial reaction records: out of 944 reaction classes, in 336 cases, the class prevalence changes by <25%, and in 607 cases by <50%. 66 reaction classes, all with a population of fewer than 100 records in the original set, disappeared in the final data set. More than 88.7% of the reaction classes with a size of 100 entries or more in the original data set did not decrease their prevalence by more than 50%. This analysis comprises the class for unrecognized reactions, the frequency of which changes from 30.7% in the original reaction data to 26.2% in the produced data set. Therefore, we consider the generated data set adequately representative of the original reaction data.
As an example, this figure can be read in the following manner: a total of 60 reaction classes, each occurring between 100 and 999 times in the original reaction data set, are represented between 25% and 50% more frequently in the data set of 693,517 reactions.
In the rest of the work, we use the resulting data set split randomly into 554,813, 69,352, and 69,352 reaction records for training, validation, and testing, respectively. We discuss the class distribution of the different splits in the Supplementary Note 2 .
We used different architectures to design three computational models for inferring action sequences, given a text-based representation of a chemical equation as input. The first model is a nearest-neighbor model using novel reaction fingerprints 21 to identify related reactions in the training set and to provide an action sequence adapted from the closest neighbor with a compatible number of molecules in the chemical equation. The second model has a transformer-based encoder-decoder sequence-to-sequence architecture 18 that formulates the procedure prediction task as a translation from reaction SMILES strings to sequences of actions. This model architecture proved successful in earlier work pertaining to SMILES strings 3 , 20 and synthesis actions 17 . The third model is a bidirectional and auto-regressive transformer model (BART), which builds on the standard transformer architecture and uses recent advances to optimize the pre-training of transformers 19 . The models and their implementation are discussed in the Methods section.
Model evaluation and comparison
We evaluate all of the models on the test set containing 69,352 chemical equations and the associated action sequences. In Table 3 , we show six metrics for the three models studied in this work. The validity measures the syntactical correctness of the predicted action sequences. It is given by the fraction of predictions that can be converted back to actions (as defined in the Supplementary Note 1 ) without error, and that contain a reference to all the molecules present in the chemical equation. The BLEU score 28 is an indicator of the similarity between two strings and is a commonly used metric to evaluate models for machine translation. The 100% accuracy refers to the fraction of sentences for which the full action sequence is identical in the ground truth and in the prediction, including the associated properties. The 90%, 75%, and 50% accuracies are the fractions of sentences that have a normalized Levenshtein similarity of 90%, 75%, 50% or greater, respectively. The Levenshtein similarity is calculated by deducting the Levenshtein distance 29 from one, as implemented in the textdistance library 30 . We also include two baselines that randomly pick action sequences, the first one among all reactions from the training set, and the second one among reactions that have the same number of precursors and products.
All three models perform better than the random baseline, which indicates that all of them are able to learn characteristic reaction patterns. The evaluation of the metrics reported in Table 3 requires extreme care as it assumes that the ground truth is the only correct solution to match. However, this is a weak assumption due to possible ground truth errors as well as the multiple possible ways to achieve the desired chemical transformation in the laboratory or to formulate equivalent action sequences. It is a rigid evaluation scheme that penalizes predicted action sequences that may be chemically equivalent but linguistically different. Still, it is the most reliable scheme to provide a statistically meaningful comparison across the different models.
As an example, we report the actions predicted by the different models for a reductive amination reaction in Table 4 . The deep-learning models predict a sequence identical to the ground truth except for an additional Stir action before the addition of sodium cyanoborohydride. The nearest-neighbor model predicts a longer action sequence including quenching and a more involved work-up. It is noteworthy that all the models predict an identical stirring duration. In the Supplementary Data 2 , we report a selection of 100 reactions and associated predictions, including the one from Table 4 .
Figure 4 a shows the distribution of the number of actions predicted by the different (non-random) models. The sequence lengths of the nearest-neighbor model closely follow the distribution of the ground truth. The transformer model has a pronounced preference for shorter sequences, while the BART model is biased towards middle-length sequences. Both transformer and BART models predict fewer sequences that include 15 or more actions.
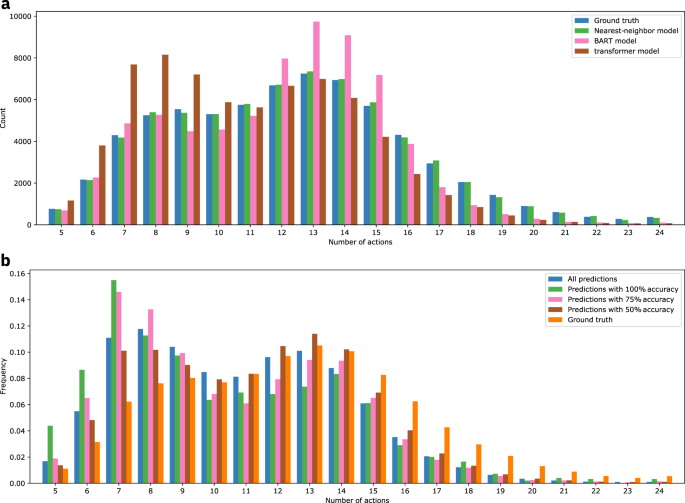
a Comparison of the lengths of predicted action sequences for the different models. b Comparison of the lengths of predicted action sequences for different levels of accuracies.
Figure 4 b shows the distribution of the lengths of action sequences predicted with accuracies of 100%, 75% and 50% using the transformer model. The correct (100%) predictions cover a similar range of sequence lengths as the full data set and are not limited to short sequences. Short sequences are only slightly overrepresented, which is consistent with the higher probability of predicting short sequences correctly.
The fact that the proportion of chemical equations for which the prediction matches the ground truth 100% is under 10% for all models does not mean that their quality is poor. The low percentage of exact matches can be explained, in part, by the presence of sequences with a large number of steps negatively affecting the percentage of entirely correct sequences (multiplicative reduction of the probability if the events were independent), by the noise in the underlying data set (multiple correct ways of doing a reaction, multiple ways of describing the same action sequence) and by errors in the data set (errors in the action sequence extraction from the experimental procedure text). For similar reasons, the differences in the distribution of action sequence length in Fig. 4 a are not a valid argument to judge the quality of the deep-learning models compared to the nearest-neighbor scheme.
In an endeavor to rationalize the model predictions for single actions, one can assume, for illustration purposes, that the prediction of each action is an independent event. We used the average value of the accuracy for the 100% match and the distribution of the sequences’ length to calculate the accuracy of a single action prediction by solving the polynomial equation that sums the probabilities of exactly matching each sequence length to a total value of 3.6%. We found an accuracy of 72.7% for the average single action prediction, which would correspond to the average probability of the model predicting a single action with correct type and associated properties if the action predictions were independent events.
Despite the intrinsic difficulties to assess and compare the absolute performance of the models, it appears that all three schemes are viable options for inferring action sequences from chemical equations. In general, the transformer model performs slightly better than the BART model. The nearest-neighbor model has a better 100% accuracy value (see Table 3 ) than the deep-learning models. The deep-learning models, however, rely on a learned representation rather than on similarities with data points in the training set. As such, they automatically take different aspects of the input into account, such as the type of transformation and presence or absence of functional groups. This makes them more general and more interesting in the context of experimental procedure prediction. For this reason, we will consider only the transformer-based model for further analysis.
In Table 5 , we categorize the differences in the actions predicted by the transformer model compared to the ground truth. In addition to the 3.6% exact predictions, roughly 0.9% represent actions predicted in different orders, with the Add action being the main entity affected by incorrect orderings. In 5.4% of the action sequences, the differences are limited to properties associated with the action types. For 1.4% of reactions, one action is predicted instead of another one - and for 3.9% other cases, some properties of other actions are different in addition to the swap. The swap between similar entities (such as Stir and Reflux ) may indicate the minor importance of the use of a specific action type for a reaction success. In 8.3% of the reactions, an action is missing either in the ground truth or in the predictions. Most often, the extra action is related to work-up or purification. In 18.8% of cases, more than one action is missing in either the ground truth or in the predictions. The remaining 57.0% of the reactions show a combination of multiple types of differences.
For a set of 500 reactions, we did a blind human assessment of the action sequences reported in the ground truth and predicted by the transformer model. For each reaction, we showed a trained organic chemist a chemical reaction diagram together with the ground truth and the predicted action sequences, in a random order. The domain expert, without any possibility to distinguish the predicted sequence from the ground truth one, was asked to judge whether the experimental procedures were adequate or not by selecting among the following options: consider both of them experimentally valid, consider one to be adequate and the other one inadequate, or reject both as experimentally inadequate. Often, the expert labeled sequences as inadequate even if only a single action was considered to be incorrect. We report the collected preferences in Table 6 . While this test cannot be considered statistically significant to assess the quality across different chemistry areas, it is unquestionable that for an academic level expert the predicted procedures are, on average, of equivalent quality to the ground truth. For more than half of the reactions, the chemist considered the predicted action sequence to be adequate. For about two fifths of the reactions, the chemist considered them inadequate. Moreover, the human assessment indicates that the predictions contain slightly fewer inadequate sequences than the ground truth. Therefore, it is reasonable to assume that improving the quality of the ground truth will result in an improvement of the model predictions. We report the human assessment and corresponding analysis of the 500 reaction records in the Supplementary Data 3 .
The Supplementary Information contains additional evaluation information for the transformer model. In the Supplementary Note 3 , we report the effect of shuffling the molecules in the reaction SMILES given as input. We illustrate the performance of the model with respect to the reaction class in the Supplementary Note 4 , and present the class distribution of the reactions assessed by the expert chemist in the Supplementary Note 5 .
In this work, we address the prediction of experimental steps starting from a text-based representation of a chemical equation. We generated a data set beginning with a database of patent reactions to train three predictive models: a nearest-neighbor scheme, and two deep-learning sequence-to-sequence models based on the Transformer and BART architectures. Despite the mathematical differences, the three models exhibit a similar performance.
For most reactions in a random selection of the data set, the predicted action sequences are considered experimentally adequate (313 out of 500) by an expert chemist in a blind assessment. The remaining 187 predictions out of 500 are considered inadequate. The same assessment reveals the presence of 201 inadequate procedures in the ground truth. We demonstrated that it is possible to construct effective deep-learning schemes to learn the characteristic patterns in chemical reaction procedures and, at the same time, we showed that quality issues within the information extracted from patents limit their potential. An inspection of the inadequate procedures in the ground truth highlights some shortcomings in the underlying patent data, such as incorrect chemical equations, but also hint at improvable weaknesses in the generation of the data set. A reduction of the number of inadequate data procedures could for instance be achieved by an improved detection of inexact reaction data, and by further improving the action extraction procedure from experimental reports. Seeing that the transformer model is performing on par with the ground truth, we expect that the model performance will strongly benefit from such quality improvements in training data.
Without loss of generality, we simplified a few aspects of the reaction procedures. We did not include any information about the state or concentration of compounds, as this is not commonly specified in chemical equations. However, the current models could easily be broadened with the availability of higher quality data sets and an extension of the current text-based representation to include the compound state or concentration. The same is true for the relative quantities of precursors and solvents, characterizing the reaction scale, and for the atmosphere under which reactions are executed. These pieces of information are not reported uniformly in the experimental procedures and their use would degrade the overall performance of deep-learning schemes. Therefore, we decided to remove this information during the data standardization process. This decision led to some incomplete action sequences, especially for hydrogenation reactions that take place under hydrogen atmosphere.
We note that action sequence prediction models require chemical equations to include not only reactants and products, but also solvents and reagents. Recently developed retrosynthetic schemes 3 are capable of predicting optimal solvents and catalysts and thus automatically provide the chemical equation in the required format. A recently reported algorithm can be used to infer common reagents and solvents automatically if they are not present in the chemical equation 31 .
Interestingly, a chemical procedure is the equivalent of a computer program for experimental chemists: a series of instructions specified in a human-readable format that unambiguously codifies the operations to execute the chemical experiment 17 , which could either be executed by human operators or by automation hardware. Accordingly, the Smiles2Actions model can be considered to write code for chemical synthesis, and therefore has similarities with machine programming 32 , where the core idea is software creating its own software. The use of artificial intelligence technologies for inferring experimental procedures will reduce the amount of trial and error in a traditional laboratory setup. When coupled with an automation system, this technology will contribute to a wider adoption of automation technologies, laying the foundations for a fully automated synthesis starting from only chemical equations. In fact, we strongly believe that the mathematical architectures presented in this paper will become an essential component to automatize general purpose synthetic chemistry on robotic systems. AI will not replace chemists, and the action sequences predicted by the model introduced in this work should always be verified for safety prior to the actual synthesis. But AI will soon reach a level where the predicted experimental procedures will be production-worthy, without requiring human intervention, and will directly be usable to drive automation hardware in a chemical laboratory or to reduce the amount of trial and error in a traditional laboratory setup.
Action sequence extraction
We used the pristine natural language processing model trained in ref. 17 (Paragraph2Actions) for the extraction of action sequences from experimental paragraphs.
Handling of compound names
The identification of the compounds present in the action sequences extracted from experimental procedures requires the mapping of the compound names to chemical structures. In the next paragraphs, we document the detailed procedure.
Compound name stripping
In Vaucher et al. 17 , the action sequence extraction model was trained to extract explicit compound names. For instance, the sentence “The solution was acidified with 12 mL of a saturated solution of sulfuric acid” contains the extracted compound name “saturated solution of sulfuric acid”. The mapping of extracted names to SMILES strings includes the identification of the root compound name, in this case “sulfuric acid”. Also, in several cases, the extracted compound names refer to multiple chemical compounds, as in “5 mL of a 1.0 M DCM solution of boron tribromide” or “80 mL of 1:1 dioxane-water”.
To obtain the root compound names, we built a series of data-cleaning steps based on heuristics ( https://git.io/JYWDY ) to trim the compound name entities by removing, among others, information about physical state (“solid”, “gaseous”, “(s)”), concentrations (“1.0 M”, “10 wt%”, “saturated”, “concentrated”), temperatures (“cold”, “hot”), or composition ratios (“1:1”, “2/1”, “(80:10:10)”).
Compound name normalization
Chemical compounds often have multiple names. In addition to common synonyms (for instance: “isopropanol”, “2-propanol”, and “propan-2-ol”), the differences among extracted entities are commonly limited to single characters, often as a side-effect of the use of optical character recognition tools in scanned documents. Normalizing compound names with respect to such small differences simplifies the attribution of SMILES strings to compound names.
We implemented a pipeline ( https://git.io/JYWMt ) for the normalization of compound names, in which they undergo several subsequent modifications:
subscript digits are replaced by their ASCII equivalent: H 2 SO 4 → H2SO4.
visually similar characters are unified to avoid common optical character recognition pitfalls: I (uppercase i) → l (lowercase L), 1 (one) → l (lowercase L), 0 (zero) → O (uppercase o).
uppercase characters are converted to lower case.
dash and prime characters are unified or removed.
spelled-out Greek letters are replaced by the Greek letter: alpha-glucose → α-glucose.
spaces are removed: dimethyl sulfoxide → dimethylsulfoxide
a selection of special characters are removed: NiCl2 ⋅ 6H2O → NiCl26H2O, NiCl2 × 6H2O → NiCl26H2O
Note that the resulting names may not be existing compound names. However, the purpose of the compound name normalization is to have a common representation language for names that may differ due to spelling errors.
Name-to-SMILES and SMILES-to-name
In order to match the molecules between the text-based representation of the chemical reaction and the extracted experimental procedure, we implemented a mapping scheme between compound names and SMILES strings.
We built two mappings using pairs of compound names and SMILES strings available in the Pistachio database with hash tables (Python dictionaries). They contain the most common SMILES string for a given normalized compound name, as well as the most common compound name for a given SMILES string. We called the two services name-to-SMILES ( https://git.io/JYWMR ) and SMILES-to-name ( https://git.io/JYWMo ), respectively.
By mapping a compound name to a SMILES string and then back to its compound name, one can obtain the most common synonym for the given compound name. For instance, “dimethyl sulfoxide” is mapped to the SMILES string “ CS(C)=O ”, which maps back to “DMSO”.
Tokenization of temperatures, durations, and pH values
We replaced the extracted temperatures, durations, and pH values with tokens representing predefined intervals ( https://git.io/JYWMS ). We report, in the Supplementary Note 6 , the ranges, tokens, and values used to convert the predicted instructions into numerical values.
Prior to the actual tokenization, we needed to convert the strings of characters representing temperatures, durations, and pH values to actual numbers with associated dimensions. This was achieved with heuristics that account for the multiple ways of representing numbers (“five”, “5”) and units (“C”, “F”, “degrees”, etc.), and that assign predefined values when no actual number is given (“over the weekend”, “overnight”, “RT”, “ambient temperature”, etc.). If the conversion of an extracted value failed, we ignored the associated reaction and did not include it in the data set of experimental procedures.
Data set generation
As a source of chemical reaction data, we selected the Pistachio database, version 3.0 27 . Duplicate reaction records and records with no experimental procedure text were filtered out. This provided a set of 3,464,664 reaction records.
The application of the following standardization steps produced 693,517 reaction SMILES and associated action sequences to train the machine learning models.
Reaction SMILES postprocessing
The Pistachio database provides reaction SMILES strings parsed into reactant, reagent, and product molecules. We merged the reactant and reagent molecules into a list of precursor molecules, and all the SMILES strings were canonicalized with RDKit 33 . For both lists of precursor and product SMILES, we removed the duplicates and reordered the lists alphabetically. The concatenation of the SMILES strings produced the reaction SMILES used for training. Following the reaction SMILES notation, we separated the molecules within the same class using dots (“.”), while the precursor and product lists were separated by “>>”. For fragment bonds, we adopted the convention of using the tilde symbol (“~”) instead of a dot.
For use in language-based models, the reaction SMILES is tokenized by inserting spaces between the SMILES tokens.
Action sequence filtering
Some action sequences are not adequate for training and were removed ( https://git.io/JYWDi ) from the data set. This is the case for reactions with incomplete experimental procedures (such as the ones shortening the description by referring to other procedures), or for unsuccessful or incomplete action sequence extraction.
We ignored experimental procedures when their processing with the Paragraph2Actions model 17 contained any InvalidAction or FollowOtherProcedure action. We filtered out any experimental procedure containing fewer than five actions because such short sequences are not likely to describe a chemical reaction appropriately. We also ignored experimental procedures that likely describe multiple reaction steps. Therefore, any experimental paragraph whose action sequence contains multiple Yield actions interlaid with actions other than purification or work-up were rejected.
Action sequence postprocessing
The action sequence extraction model operates sentence by sentence. When combining the actions for a full experimental procedure, it is crucial to provide a consistent record, guaranteeing correct relative dependencies between instructions. To do so, the action sequence extracted from the experimental procedure text underwent a series of postprocessing steps ( https://git.io/JYWyd ).
We removed all NoAction actions, because these instructions usually arise from sentences that are not relevant to the actual synthesis and can safely be ignored.
Whenever possible, we merged Wait actions with previous actions. This provides a more concise representation when a sentence specifies the duration of an action only in the next sentence. For instance, “The mixture was brought to reflux. After 2 hours, ...” is equivalent to a Reflux action with a time parameter of 2 h.
Often, experimental procedures do not explicitly state whether the precipitate or the filtrate should be kept upon filtering. We therefore inferred this information from the previous or subsequent actions.
The use of preceding actions is also important to complete instructions containing information related to previous actions. For instance, an action specifying the temperature parameter with the “same temperature” value, requires the inspection of the preceding actions to identify the latest set temperature.
Finally, we replaced MakeSolution actions appearing as first instruction in a procedure by a series of Add actions. This reduces the differences between equivalent ways of formulating action sequences while keeping an identical logic in terms of experimental operations.
Updates to individual actions
To further remove unnecessary pieces of information and harmonize differences in action sequences, we applied a series of changes to the individual actions ( https://git.io/JYWyd ). First, we removed all compound quantities. The inclusion of mass information would only decrease the overall accuracy of the model. Better and more evenly distributed data sets will make it possible to skip this simplification step, thereby enriching future predictive capabilities. Second, we tokenized all the values for temperature, duration, and pH values as described above. Third, because of the uneven reporting of the number of repetitions for Extract and Wash actions, we decided to ignore these values and always assume a single repetition. Finally, again because of poor reporting consistency of experimental details, we ignored all indications related to the use of specific atmospheres except when the reported value was “vacuum” for the DrySolid action.
Compound names substitutions
As explained in the Results section, compound names are only admissible in action sequences if the corresponding tokens refer to molecules in the chemical equation or to molecules in a list of common reagents. Therefore, it is necessary to map the molecules present in the chemical equation to the extracted compound names. If the mapping is unsuccessful, we verify if their most common synonyms match any item on the list of common reagents. We report the list of common reagents in the Supplementary Data 1 . The generation of this list is explained in the Supplementary Note 7 .
In addition to the name-to-SMILES and SMILES-to-name mappings introduced above, the Pistachio records provide reaction-specific mappings between compound names and SMILES strings. These mappings are particularly useful when compounds are specified with a general name such as “the compound from Example A”, “title compound”, etc. The mappings from Pistachio have precedence over name-to-SMILES and SMILES-to-name.
We trimmed each compound name in an extracted action sequence and mapped its normalized name to a SMILES string, if possible. We separated extracted compound names containing more than one compound by a slash (“/”) symbol. If the SMILES string corresponded to a molecule present in the chemical equation, we used a placeholder indicating the position in the chemical equation instead of the extracted compound name. If the SMILES string did not correspond to any molecule in the chemical equation, we converted it to its most common synonym; for instance, “dimethyl sulfoxide” is replaced by “DMSO”.
We only kept compound names that did not match any molecule in the chemical equation if they were present in the list of common reagents.
Upon application of these guidelines, if exactly one molecule from the chemical equation and exactly one extracted compound name had not been mapped yet, we considered them to be matching entities. We ignored the entire experimental procedure if, at the end of this protocol, there were still unmapped molecules or extracted compound names.
Textual representation of action sequences
As in previous work 17 , we converted the actions (types and associated properties) to a semi-structured textual representation. This format is concise, human-readable, easily understandable and it enables the use of natural language machine-learning models. This representation can be converted to and from the action type and associated properties without loss ( https://git.io/JYW9e ). The actions listed in Tables 1 and 4 use this textual format.
Nearest-neighbor model
The nearest-neighbor model relies on the computation of reaction fingerprints ( rxnfp ft ), as published in our previous work 21 , for the training and test set. For any test set reaction fingerprint query, we search the nearest neighbors in the training set with faiss 34 . The nearest-neighbor search is constrained to the training reactions that have the same number of precursors as the test reactions.
The data-driven reaction fingerprints from Schwaller et al. 21 were preferred to the connectivity-based reaction fingerprints from Schneider et al. 35 . The latter require a distinction between reactants and reagents, which may be subtle or ambiguous 22 , and have a lower accuracy than the data-driven fingerprints on the reaction classification task 21 .
Transformer model
The transformer model uses a transformer encoder-decoder architecture with 8 attention heads and is trained by minimizing the categorical cross-entropy loss for the output words. The model was implemented with the OpenNMT-py library 36 , 37 . We adopted the hyperparameters suggested by the library with a few changes. First, we reduced the model size by decreasing the number of layers from 6 to 4, the size of the hidden states from 512 to 256, and the size of the word vectors from 512 to 256. Second, we changed the values of the parameters max_generator_batches to 32, accum_count to 4 and label_smoothing to 0.
The BART model was implemented using the reference implementation in the fairseq framework by Facebook AI Research 38 .
Data availability
The generated data set of 693,517 chemical equations and associated action sequences is a derivative work of the Pistachio data set 27 . It is available from the authors upon request.
Code availability
A GitHub repository, available at https://github.com/rxn4chemistry/smiles2actions 39 , contains code for processing compound names and action sequences, as well as instructions to train and use the transformer model presented in this manuscript.
Elton, D. C., Boukouvalas, Z., Fuge, M. D. & Chung, P. W. Deep learning for molecular design — a review of the state of the art. Mol. Syst. Des. Eng. 4 , 828–849 (2019).
Article CAS Google Scholar
Segler, M. H. S., Preuss, M. & Waller, M. P. Planning chemical syntheses with deep neural networks and symbolic AI. Nature 555 , 604–610 (2018).
Article ADS CAS Google Scholar
Schwaller, P. et al. Predicting retrosynthetic pathways using transformer-based models and a hyper-graph exploration strategy. Chem. Sci. 11 , 3316–3325 (2020).
Godfrey, A. G., Masquelin, T. & Hemmerle, H. A remote-controlled adaptive medchem lab: an innovative approach to enable drug discovery in the 21st Century. Drug Discov. Today 18 , 795–802 (2013).
Coley, C. W. et al. A robotic platform for flow synthesis of organic compounds informed by AI planning. Science 365 , eaax1566 (2019).
Steiner, S. et al. Organic synthesis in a modular robotic system driven by a chemical programming language. Science 363 , eaav2211 (2019).
Nicolaou, C. A. et al. Idea2data: toward a new paradigm for drug discovery. ACS Med. Chem. Lett. 10 , 278–286 (2019).
Nicolaou, C. A., Watson, I. A., LeMasters, M., Masquelin, T. & Wang, J. Context aware data-driven retrosynthetic analysis. J. Chem. Inf. Model. 60 , 2728–2738 (2020).
Coley, C. W., Eyke, N. S. & Jensen, K. F. Autonomous discovery in the chemical sciences part i: progress. Angew. Chem. Int. Ed. 59 , 22858–22893 (2020).
Coley, C. W., Eyke, N. S. & Jensen, K. F. Autonomous discovery in the chemical sciences part II: Outlook. Angew. Chem. Int. Ed. 59 , 23414–23436 (2020).
Mehr, S. H. M., Craven, M., Leonov, A. I., Keenan, G. & Cronin, L. A universal system for digitization and automatic execution of the chemical synthesis literature. Science 370 , 101–108 (2020).
Thakkar, A. et al. Artificial intelligence and automation in computer aided synthesis planning. React. Chem. Eng. 6 , 27–51 (2021).
Farrant, E. Automation of synthesis in medicinal chemistry: Progress and challenges. ACS Med. Chem. Lett. 11 , 1506–1513 (2020).
Walker, E. et al. Learning to predict reaction conditions: relationships between solvent, molecular structure, and catalyst. J. Chem. Inf. Model. 59 , 3645–3654 (2019).
Maser, M. R. et al. Multilabel classification models for the prediction of cross-coupling reaction conditions. J. Chem. Inf. Model. 61 , 156–166 (2021).
Gao, H. et al. Using machine learning to predict suitable conditions for organic reactions. ACS Cent. Sci. 4 , 1465–1476 (2018).
Vaucher, A. C. et al. Automated extraction of chemical synthesis actions from experimental procedures. Nat. Commun. 11 , 3601 (2020).
Article ADS Google Scholar
Vaswani, A. et al. Attention is all you need. In Proceedings of the 31st International Conference on Neural Information Processing Systems , NIPS’17, 6000–6010 (Curran Associates Inc., 2017).
Lewis, M. et al. BART: Denoising Sequence-to-sequence Pre-training For Natural Language Generation, Translation, And Comprehension (Association for Computational Linguistics, 2019).
Schwaller, P. et al. Molecular transformer: a model for uncertainty-calibrated chemical reaction prediction. ACS Cent. Sci. 5 , 1572–1583 (2019).
Schwaller, P. et al. Mapping the space of chemical reactions using attention-based neural networks. Nat. Mach. Intell . 3 , 144–152 (2021).
Schneider, N., Stiefl, N. & Landrum, G. A. What’s what: the (nearly) definitive guide to reaction role assignment. J. Chem. Inf. Model. 56 , 2336–2346 (2016).
Weininger, D. SMILES, a chemical language and information system. 1. Introduction to methodology and encoding rules. J. Chem. Inf. Comput. Sci. 28 , 31–36 (1988).
Weininger, D., Weininger, A. & Weininger, J. L. SMILES. 2. Algorithm for generation of unique SMILES notation. J. Chem. Inf. Comput. Sci. 29 , 97–101 (1989).
Hendershot, D. C. & Sarafinas, A. Safe chemical reaction scale up. Chem. Health Saf. 12 , 29–35 (2005).
Brown, D. G. & Boström, J. Analysis of past and present synthetic methodologies on medicinal chemistry: where have all the new reactions gone? J. Med. Chem. 59 , 4443–4458 (2016).
Pistachio. Nextmove Software Pistachio https://www.nextmovesoftware.com/pistachio.html (Accessed 19 Nov 2019).
Papineni, K., Roukos, S., Ward, T. & Zhu, W.-J. Bleu: a method for automatic evaluation of machine translation. In Proc. 40th Annual Meeting of the Association for Computational Linguistics , 311–318 (Association for Computational Linguistics, 2002).
Levenshtein, V. I. Binary codes capable of correcting deletions, insertions, and reversals. Sov. Phys. Dokl. 10 , 707–710 (1966).
ADS MathSciNet Google Scholar
TextDistance library, version 4.1.5. (Accessed 19 Nov 2019).
Vaucher, A. C., Schwaller, P. & Laino, T. Completion of partial reaction equations. Chemrxiv.13273310 . Preprint at https://doi.org/10.26434/chemrxiv.13273310 (2020).
Gottschlich, J. et al. The three pillars of machine programming. In Proc. 2nd ACM SIGPLAN International Workshop on Machine Learning and Programming Languages , MAPL 2018, 69–80 (Association for Computing Machinery, 2018).
Landrum, G. et al. rdkit/rdkit: 2019_03_4 (q1 2019) release (2019).
Johnson, J., Douze, M. & Jégou, H. Billion-scale Similarity Search With gpus http://arxiv.org/abs/1702.08734 (IEEE, 2017).
Schneider, N., Lowe, D. M., Sayle, R. A. & Landrum, G. A. Development of a novel fingerprint for chemical reactions and its application to large-scale reaction classification and similarity. J. Chem. Inf. Model. 55 , 39–53 (2015).
Klein, G., Kim, Y., Deng, Y., Senellart, J. & Rush, A. OpenNMT: open-source toolkit for neural machine translation. In Proc. ACL 2017, System Demonstrations , 67–72 (Association for Computational Linguistics, 2017).
OpenNMT-py library, version 0.9.2. (Accessed 19 Nov 2019).
Ott, M. et al. fairseq: a fast, extensible toolkit for sequence modeling. In Proc. 2019 Conference of the North American Chapter of the Association for Computational Linguistics (Demonstrations) , 48–53 (Association for Computational Linguistics, 2019).
Vaucher, A. C. et al. Inferring Experimental Procedures From Text-based Representations Of Chemical Reactions , rxn4chemistry/smiles2actions, https://doi.org/10.5281/zenodo.4643379 (2021).
Download references
Acknowledgements
We thank the anonymous reviewers for their careful reading of our manuscript and their many insightful comments and suggestions.
Author information
Authors and affiliations.
IBM Research Europe, Rüschlikon, Switzerland
Alain C. Vaucher, Philippe Schwaller, Joppe Geluykens, Vishnu H. Nair & Teodoro Laino
Dipartimento di Chimica e Chimica Industriale, Università di Pisa, Pisa, Italy
Anna Iuliano
You can also search for this author in PubMed Google Scholar
Contributions
The project was conceived and planned by T.L. and A.C.V. and supervised by T.L. A.C.V. generated the dataset and analyzed the results. A.C.V. and V.H.N. trained the transformer model. P.S. and J.G. implemented and trained the nearest-neighbor and BART models, respectively. A.I. assessed the subset of 500 reactions and action sequences. All the authors were involved in discussions about the project. A.C.V. wrote the manuscript with input from all authors.
Corresponding author
Correspondence to Alain C. Vaucher .
Ethics declarations
Competing interests.
The authors declare no competing interests.
Additional information
Peer review information Nature Communications thanks the anonymous reviewer(s) for their contribution to the peer review of this work. Peer reviewer reports are available.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary information, peer review file, description of additional supplementary files, supplementary data 1, supplementary data 2, supplementary data 3, supplementary data 4, rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Cite this article.
Vaucher, A.C., Schwaller, P., Geluykens, J. et al. Inferring experimental procedures from text-based representations of chemical reactions. Nat Commun 12 , 2573 (2021). https://doi.org/10.1038/s41467-021-22951-1
Download citation
Received : 15 November 2020
Accepted : 07 April 2021
Published : 06 May 2021
DOI : https://doi.org/10.1038/s41467-021-22951-1
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
This article is cited by
Gemtelligence: accelerating gemstone classification with deep learning.
- Tommaso Bendinelli
- Luca Biggio
Communications Engineering (2024)
Augmenting large language models with chemistry tools
- Andres M. Bran
- Philippe Schwaller
Nature Machine Intelligence (2024)
A robotic platform for the synthesis of colloidal nanocrystals
- Haitao Zhao
- Xue-Feng Yu
Nature Synthesis (2023)
Language models and protocol standardization guidelines for accelerating synthesis planning in heterogeneous catalysis
- Manu Suvarna
- Alain Claude Vaucher
- Javier Pérez-Ramírez
Nature Communications (2023)
Artificial intelligence driven design of catalysts and materials for ring opening polymerization using a domain-specific language
- Nathaniel H. Park
- Matteo Manica
- Pedro L. Arrechea
By submitting a comment you agree to abide by our Terms and Community Guidelines . If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing: AI and Robotics newsletter — what matters in AI and robotics research, free to your inbox weekly.

References provide the information necessary for readers to identify and retrieve each work cited in the text .
Check each reference carefully against the original publication to ensure information is accurate and complete. Accurately prepared references help establish your credibility as a careful researcher and writer.
Consistency in reference formatting allows readers to focus on the content of your reference list, discerning both the types of works you consulted and the important reference elements (who, when, what, and where) with ease. When you present each reference in a consistent fashion, readers do not need to spend time determining how you organized the information. And when searching the literature yourself, you also save time and effort when reading reference lists in the works of others that are written in APA Style.

Academic Writer ®
Master academic writing with APA’s essential teaching and learning resource

Course Adoption
Teaching APA Style? Become a course adopter of the 7th edition Publication Manual

Instructional Aids
Guides, checklists, webinars, tutorials, and sample papers for anyone looking to improve their knowledge of APA Style
Maintenance work is planned from 21:00 BST on Tuesday 20th August 2024 to 21:00 BST on Wednesday 21st August 2024, and on Thursday 29th August 2024 from 11:00 to 12:00 BST.
During this time the performance of our website may be affected - searches may run slowly, some pages may be temporarily unavailable, and you may be unable to log in or to access content. If this happens, please try refreshing your web browser or try waiting two to three minutes before trying again.
We apologise for any inconvenience this might cause and thank you for your patience.

Physical Chemistry Chemical Physics
Carbon-sulfur bond elongation is the promoting reaction coordinate in the efficient sub-nanosecond intersystem crossing in thianaphthene derivatives.
Thiophene derivatives have become an integral moiety in OLED, photovoltaics, and photodynamic therapy research. A deeper understanding of their excited state dynamics and electronic relaxation mechanisms is expected to provide important physical insights of direct relevance for these applications. In this study, thianaphthene (TN), 2-methylbenzothiophene (2MBT), and 3-methylbenzothiophene (3MBT) are investigated using femtosecond broadband transient absorption and steady-state spectroscopy techniques along with time-dependent density functional calculations in cyclohexane and acetonitrile. The photophysical properties and electronic relaxation mechanisms of these derivatives are elucidated. Small fluorescence quantum yields ranging from 0.4 to 1.1% are measured. It is demonstrated that excitation of TN at 290 nm leads primarily to intersystem crossing to the triplet manifold with a lifetime of 400 ± 15 ps in either solvent, whereas four- to twofold shorter intersystem crossing lifetimes are measured for 2MBT and 3MBT depending on whether cyclohexane or acetonitrile is used. Linear interpolation of internal coordinates evidence that elongation of the S-C bonds enables ultrafast intersystem crossing in these thiophene derivatives involving singlet and triplet states with ππ* and πσ* characters. Excitation at 266 nm results in the observation of an additional 5 ± 1 ps lifetime, which is assigned to intramolecular vibrational relaxation dynamics occurring in the excited singlet state.
Supplementary files
- Supplementary information PDF (9229K)
Article information
Download Citation
Permissions.
C. Griffith, E. Mao, S. J. Hoehn, S. E. Krul and C. E. Crespo-Hernández, Phys. Chem. Chem. Phys. , 2024, Accepted Manuscript , DOI: 10.1039/D4CP02849J
This article is licensed under a Creative Commons Attribution 3.0 Unported Licence . You can use material from this article in other publications without requesting further permissions from the RSC, provided that the correct acknowledgement is given.
Read more about how to correctly acknowledge RSC content .
Social activity
Search articles by author.
This article has not yet been cited.
Advertisements
A Study on the Impact of Chemical Structure on the Evolution of Aggregate Structure in Fiber-shaped High Density Polyethylene Vitrimer
- Research Article
- Special Issue: Dynamic Polymers
- Published: 21 August 2024
Cite this article

- Bing Wang 1 ,
- Yuan-Chu Gao 1 ,
- Hai Wang 1 &
- Hui Niu 1
Vitrimers have emerged as a prominent research area in the field of polymer materials. Most of the studies have focused on synthesizing polymers with versatile dynamic crosslinking structures, while the impact of chemical structure on aggregate structure of vitrimers, particularly during polymer processing, remains insufficiently investigated. The present study employed commercial maleic anhydride-grafted-high density polyethylene (M- g -HDPE) as the matrix and hexanediol as the crosslinker to facilely obtain fiber-shaped HDPE vitrimers through a reaction extrusion and post-drawing process. Through chemical structure characterization, morphology observation, thermal and mechanical properties investigation, as well as aggregate structure analysis, this work revealed the influence of dynamic bonds on the formation of aggregate structures during fiber-shaped vitrimers processing. A small amount of dynamic bonds in HDPE restricts the motion of PE chain during melt-extruding and post-drawing, resulting in a lower orientation of the PE chains. However, lamellar growth and fibril formation during post-drawing at high temperature are enhanced to some extent due to the competition between dynamic bond and chain relaxation. The uneven morphology of fiber-shaped HDPE vitrimers can be attributed to the stronger elastic effect brought by dynamic bonding, which plays a more dominant role in determining the mechanical properties of fiber-shaped vitrimers compared to aggregate structure.
This is a preview of subscription content, log in via an institution to check access.
Access this article
Subscribe and save.
- Get 10 units per month
- Download Article/Chapter or eBook
- 1 Unit = 1 Article or 1 Chapter
- Cancel anytime
Price includes VAT (Russian Federation)
Instant access to the full article PDF.
Rent this article via DeepDyve
Institutional subscriptions
Similar content being viewed by others
One-step and Scalable Synthesis of EAA-based Reprocessable Vitrimer with Superior Mechanical Properties
Fibers of ultra-high molecular weight polyethylene obtained by gel spinning with polyalphaolefin oil.

Unlocking the Potential of Poly(butylene succinate) through Incorporation of Vitrimeric Network Based on Dynamic Imine Bonds
Explore related subjects.
Van Zee, N. J.; Nicolaÿ, R. Vitrimers: Permanently crosslinked polymers with dynamic network topology. Prog. Polym. Sci. 2020 , 104 , 101233.
Article CAS Google Scholar
Ahmadi, M.; Hanifpour, A.; Ghiassinejad, S.; Van Ruymbeke, E. Polyolefins vitrimers: design principles and applications. Chem. Mater. 2022 , 34 , 10249–10271.
Zhang, M. Q. Self-healing polymeric materials: on a winding road to success. Chinese J. Polym. Sci. 2022 , 40 , 1315–1316.
Article Google Scholar
You, Y.; Rong, M. Z.; Zhang, M. Q. Reversibly interlocked polymer networks: design, preparation and applications. Acta Polymerica Sinica (in Chinese) 2023 , 54 , 14–36.
CAS Google Scholar
Mao, H. D.; Zhang, T. T.; Guo, Z. Y.; Bai, D. Y.; Wang, J.; Xiu, H.; Fu, Q. A cross-linked polyethylene with recyclability and mechanical robustness enabled by establishment of multiple hydrogen bonds network reactive melt blending. Chinese J. Polym. Sci. 2023 , 41 , 1104–1114.
Kar, G. P.; Saed, M. O.; Terentjev, E. M. Scalable upcycling of thermoplastic polyolefins into vitrimers through transesterification. J. Mater. Chem. A 2020 , 8 , 24137–24147.
Lessard, J. J.; Scheutz, G. M.; Hughes, R. W.; Sumerlin, B. S. Polystyrene-based vitrimers: inexpensive and recyclable thermosets. ACS Appl. Polym. Mater. 2020 , 2 , 3044–3048.
Zhu, L.; Xu, L.; Jie, S.; Li, B. G. Upgrade SBS into vitrimers with excellent mechanical and physical properties. Eur. Polym. J. 2022 , 180 , 111600.
Wang, W.; Zha, X.; Bao, R.; Ke, K.; Liu, Z.; Yang, M.; Yang, W. Vitrimers of polyolefin elastomer with physically cross-linked network. J. Polym. Res. 2021 , 28 , 210.
Xu, Z.; Meng, S.; Wei, D.; Bao, R.; Wang, Y.; Ke, K.; Yang, W. Hierarchical network relaxation of a dynamic cross-linked polyolefin elastomer for advanced reversible shape memory effect. Nanoscale 2023 , 15 , 5458–5468.
Article CAS PubMed Google Scholar
Wang, A.; Niu, H.; He, Z.; Li, Y. Thermoreversible cross-linking of ethylene/propylene copolymer rubbers. Polym. Chem. 2017 , 8 , 4494–4502.
Liu, S.; Liu, X.; He, Z.; Liu, L.; Niu, H. Thermoreversible cross-linking of ethylene/propylene copolymers based on Diels-Alder chemistry: the cross-linking reaction kinetics. Polym. Chem. 2020 , 11 , 5851–5860.
He, Z.; Niu, H.; Liu, L.; Xie, S.; Hua, Z.; Li, Y. Elastomeric polyolefin vitrimer: dynamic imine bond cross-linked ethylene/propylene copolymer. Polymer 2021 , 229 , 124015.
Gao, Y.; Niu, H. Polypropylene-based transesterification covalent adaptable networks with internal catalysis. Polym. Chem. 2024 , 15 , 884–895.
De Roover, B.; Sclavons, M.; Carlier, V.; Devaux, J.; Legras, R.; Montaz, A. Molecular characterization of maleic anhydride-functionalized polypropylene. J. Polym. Sci., Part A: Polym. Chem. 1995 , 33 , 829–842.
Wunderlich, B. in Appendix - ATHAS table of thermal properties of linear macromolecules. In Thermal analysis , Ed. by Wunderlich, Academic Press, 1990 , p. 417.
Google Scholar
Patterson, A. L. The Scherrer formula for X-ray particle size determination. Phys. Rev. 1939 , 56 , 978–982.
Perret, R.; Ruland, W. Single and multiple X-ray small-angle scattering of carbon fibres. J. Appl. Crystallogr. 1969 , 2 , 209–218.
Tang, Y.; Jiang, Z.; Men, Y. Uniaxial deformation of overstretched polyethylene: in-situ synchrotron small angle X-ray scattering study. Polymer 2007 , 48 , 5125–5132.
Delahaye, M.; Winne, J. M.; Du Prez, F. E. Internal catalysis in covalent adaptable networks: phthalate monoester transesterification as a versatile dynamic crosslinking chemistry. J. Am. Chem. Soc. 2019 , 141 , 15277–15287.
Fu, L.; Lu, Y.; Jiang, Z.; Chen, R.; Men, Y. Towards a better understanding of the crystallization and melting behaviors of high-density polyethylene samples prepared from quasi-isothermal and stretching oriented localized melts. Polymer 2021 , 218 , 123485.
Jiang, Z.; Tang, Y.; Rieger, J.; Enderle, H.; Lilge, D.; Roth, S. V.; Gehrke, R.; Heckmann, W.; Men, Y. Two lamellar to fibrillar transitions in the tensile deformation of high-density polyethylene. Macromolecules 2010 , 43 , 4727–4732.
Download references
Acknowledgments
This work was financially supported by the National Natural Science Foundation of China (Nos. 22278065 and 22073015).
Author information
Authors and affiliations.
State Key Laboratory of Fine Chemicals, School of Chemical Engineering, Dalian University of Technology, Dalian, 116024, China
Bing Wang, Yuan-Chu Gao, Hai Wang & Hui Niu
You can also search for this author in PubMed Google Scholar
Corresponding authors
Correspondence to Hai Wang or Hui Niu .
Ethics declarations
The authors declare no interest conflict.
Electronic Supplementary Information
10118_2024_3176_moesm1_esm.pdf, rights and permissions.
Reprints and permissions
About this article
Wang, B., Gao, YC., Wang, H. et al. A Study on the Impact of Chemical Structure on the Evolution of Aggregate Structure in Fiber-shaped High Density Polyethylene Vitrimer. Chin J Polym Sci (2024). https://doi.org/10.1007/s10118-024-3176-7
Download citation
Received : 06 May 2024
Accepted : 24 May 2024
Published : 21 August 2024
DOI : https://doi.org/10.1007/s10118-024-3176-7
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- High density polyethylene vitrimer
- Fiber-shaped
- Aggregate structures
- Transesterification reaction
- Find a journal
- Publish with us
- Track your research

IMAGES
COMMENTS
The state of matter of reactants and products is designated with the symbols (s) for solids, (l) for liquids, and (g) for gases. Chemical reaction, a process in which one or more substances, the reactants, are converted to one or more different substances, the products. Substances are either chemical elements or compounds.
Alex Dowden/EyeEm / Getty Images. Over time, iron develops a red, flaky coating called rust. This is an example of an oxidation reaction. Other everyday examples include the formation of verdigris on copper and the tarnishing of silver. Here is the chemical equation for the rusting of iron: Fe + O 2 + H 2 O → Fe 2 O 3.
Chemistry articles from across Nature Portfolio. Chemistry is a branch of science that involves the study of the composition, structure and properties of matter. Often known as the central science ...
Here are some broad examples of chemical reactions in daily life: Combustion. Photosynthesis. Aerobic cellular respiration. Anaerobic respiration (including fermentation) Oxidation (including rust) Metathesis reactions (such as baking soda and vinegar) Electrochemistry (including chemical batteries) Digestion.
A chemical reaction is a process that leads to the chemical transformation of one set of chemical substances to another. [ 1] When chemical reactions occur, the atoms are rearranged and the reaction is accompanied by an energy change as new products are generated. Classically, chemical reactions encompass changes that only involve the positions ...
A chemical reaction is a chemical change, which means the starting materials are chemically different from the ending materials. In contrast, matter also changes form via physical changes. But, in a physical change, the chemical identity of matter does not change. For example, when you melt an ice cube into liquid water, the chemical identity ...
The four main types of chemical reactions are synthesis, decomposition, single displacement, and double displacement reactions. A chemical reaction is a process or chemical change that transforms one set of substances (the reactants) into another set of substances (the products).The process involves breaking chemical bonds between atoms and forming new bonds, so the number and type of atoms ...
A reaction mechanism is a sequence of elementary reactions that can explain how an overall chemical reaction proceeds. ... Research Open Access 14 Aug 2024 Nature Communications. Volume: 15, P: 6995.
Machine learning (ML) techniques applied to chemical reactions have a long history. The present contribution discusses applications ranging from small molecule reaction dynamics to computational platforms for reaction planning. ML-based techniques can be particularly relevant for problems involving both computation and experiments. For one, Bayesian inference is a powerful approach to develop ...
The basic way of describing chemical reactions, then, is to write 'chemical equations' showing the reactants and products in a particular chemical change. ... Yet, research has suggested that many students believe that they do know why reactions occur by the time they complete their secondary science courses. However, the reasons they ...
This Perspective presents a personal overview of the current status of the theory of chemical kinetics and mechanisms for complex processes. We attempt to assess the status of the field for reactions in the gas phase, at gas-solid interfaces, in liquid solutions, in enzymes, and for protein folding. Some unifying concepts such as potential energy surfaces, free energy, master equations, and ...
A physical change, such as a state change or dissolving, does not create a new substance, but a chemical change does. A chemical change is the result of a chemical reaction. In a chemical reaction, the atoms and molecules that interact with each other are called reactants. In a chemical reaction, the atoms and molecules produced by the reaction ...
Research on Chemical Intermediates publishes current research articles and concise dynamic reviews on the properties, structures and reactivities of intermediate species in all the various domains of chemistry.. The journal also contains articles in related disciplines such as spectroscopy, molecular biology and biochemistry, atmospheric and environmental sciences, catalysis, photochemistry ...
The ORD schema supports conventional and emerging technologies, from benchtop reactions to automated high-throughput experiments and flow chemistry. The data, schema, supporting code, and web-based user interfaces are all publicly available on GitHub. Our vision is that a consistent data representation and infrastructure to support data sharing ...
The scaling up of a chemical reaction is a complex process that extends far beyond the notion of simply running the same reaction in larger quantities and equipment. Special attention needs to be ...
A chemical reaction occurs when two or more substances (reactants) are mixed together and result in one or more new substances (products). When teaching about chemical reactions, educators show chemical reactions in action and, when possible, let students get hands-on exploring reactions. With classroom science experiments, activities, and ...
An introductory guide to deducing the mechanism of chemical reactions is presented. Following a typical workflow for probing reaction mechanism, the guide introduces a wide range of kinetic and mechanistic tools. In addition to serving as a broad introduction to mechanistic analysis for students and researchers, the guide has also been used by instructors to provide the organizational ...
Chemical Reactions -A chemical reaction is in which the bonds are broken within reactant molecules, and new bonds are formed within product molecules in order to form a new substance. Chemical reactions are all around us. Chemical reactions are continually taking place on our planet. To learn Definition, Equations, Types, Examples with FAQs of Chemical Reactions.
Essentially, a chemical reaction is the result of collisions between molecules. According to this collision model, if the collision is strong enough, it can break the chemical bonds in the reactants, resulting in a rearrangement of the atoms to form products. The more the molecules collide, the faster the reaction.
Khanmigo is now free for all US educators! Plan lessons, develop exit tickets, and so much more with our AI teaching assistant.
Passerini three-component reactions (P-3CRs) based on isonitriles are impressive synthetic protocols in which a number of starting materials react with each other to provide poly-functionalized ...
The experimental execution of chemical reactions is a context-dependent and time-consuming process, often solved using the experience collected over multiple decades of laboratory work or ...
One of the fascinating aspects of this research lies in the team's focus on comproportionation reactions. In these particular types of reactions, two compounds that include the same element but in ...
References provide the information necessary for readers to identify and retrieve each work cited in the text. Consistency in reference formatting allows readers to focus on the content of your reference list, discerning both the types of works you consulted and the important reference elements with ease.
The International Journal of Chemical Kinetics publishes original research in gas phase, condensed phase, and polymer reaction kinetics. Skip to Main Content; Search within. Search term. Advanced Search Citation Search. Search term ... International Journal of Chemical Kinetics: Volume 56, Issue 10. Pages: 569-633. October 2024. Previous Issue.
Electrocatalytic conversion of CO 2 is a promising process to produce renewable chemical feedstocks, such as CO, formic acid, ethylene, and ethanol. To overcome mass transfer limitations imposed by the low solubility of CO 2 in the liquid phase, gas diffusion electrodes (GDEs) are used to ensure an adequate supply of CO 2.The gas-liquid interface within the GDEs is crucial for achieving high ...
Physical Chemistry Chemical Physics. Carbon-Sulfur Bond Elongation is the Promoting Reaction Coordinate in the Efficient Sub-Nanosecond Intersystem Crossing in Thianaphthene Derivatives ... Thiophene derivatives have become an integral moiety in OLED, photovoltaics, and photodynamic therapy research. A deeper understanding of their excited ...
Vitrimers have emerged as a prominent research area in the field of polymer materials. Most of the studies have focused on synthesizing polymers with versatile dynamic crosslinking structures, while the impact of chemical structure on aggregate structure of vitrimers, particularly during polymer processing, remains insufficiently investigated. The present study employed commercial maleic ...