Warning: The NCBI web site requires JavaScript to function. more...
An official website of the United States government
The .gov means it's official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you're on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
- Browse Titles
NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan-.


StatPearls [Internet].
Salicylic acid (aspirin).
Hasan Arif ; Sandeep Aggarwal .
Affiliations
Last Update: July 5, 2023 .
- Continuing Education Activity
Salicylates have been available since the early 1900s. This activity outlines the indications, mechanism of action, methods of administration, important adverse effects, contraindications, and monitoring, of salicylic acid, so providers can direct patient therapy in treating indicated conditions as part of the interprofessional team.
- Identify the mechanism of action of salicylic acid.
- Describe the potential adverse effects of salicylic acid.
- Review the numerous conditions where salicylic acid is therapeutically indicated.
- Explain the importance of improving care coordination amongst the interprofessional team to ensure the safe use of salicylic acid.
- Indications
Salicylates have been derived from the willow tree bark. The Sumerians were noted to have used remedies derived from the willow tree for pain management as far back as 4000 years ago. [1] Hippocrates used it for managing pain and fever. He even utilized tea brewed from it for pain management during childbirth.
In a 1763 clinical trial, the first of its kind, Reverend Edward Stone studied the effects of willow bark powder for treating fever. About 100 years later, the effects of the willow bark powder were studied for acute rheumatism.
In 1828, Professor Johann Buchner used salicin, the Latin word for willow. Henri Leroux used it to treat rheumatism after isolating it in a crystalline form in 1829. In the 1800s, the Heyden Chemical Company was the first to mass-produce salicylic acid commercially. It was not until 1899 when a modified version named acetylsalicylic acid was registered and marketed by Bayer under the trade name aspirin.
Even though it has been available since the early 1900s, its real mode of action was not known until the late 1970s.
Some of the indications for aspirin use are as follows: [2] [3]
- Angina pectoris
- Angina pectoris prophylaxis
- Ankylosing spondylitis
- Cardiovascular risk reduction
- Colorectal cancer
- Ischemic stroke
- Ischemic stroke: Prophylaxis
- Myocardial infarction
- Myocardial infarction: prophylaxis
- Osteoarthritis
- Revascularization procedures: prophylaxis
- Rheumatoid arthritis
- Systemic lupus erythematosus
- Mechanism of Action
Aspirin is a cyclooxygenase-1 (COX-1) inhibitor. It is a modifier of the enzymatic activity of cyclooxygenase-2 (COX-2). [4] Unlike other NSAIDs (ibuprofen/naproxen), which bind reversibly to this enzyme, aspirin binding is irreversible. [5] It also blocks thromboxane A2 on platelets in an irreversible fashion preventing platelet aggregation. [6] [7]
Researchers hypothesize that due to the blocking of the COX pathway, the arachidonic acids are shuttled into the lipoxygenase pathway. The production of anti-inflammatory lipoxins results from modifying prostaglandin-endoperoxide synthase (PTGS2), also called COX-2, that results in the production of lipoxins, most of which are anti-inflammatory. These compounds are called aspirin-triggered lipoxins, aspirin-triggered resolvins, and aspirin-triggered maresins.
- Administration
Aspirin can be administered via the oral, rectal, and intravenous (IV) route.
It is available in different doses, the lowest being 81 mg, also called a baby aspirin.
- Tablet: 325 mg, 500 mg
- Delayed-release tablet: 81 mg, 325 mg, 500 mg, 650 mg
- Chewable: 81 mg
- Suppository: 60 mg, 120 mg, 200 mg, 300 mg, 600 mg
- Intravenous: 250 mg, 500 mg
Pharmacokinetics [8]
Aspirin absorption from the gastrointestinal (GI) tract depends on the formulation state. When consumed as a liquid preparation, it is rapidly absorbed as opposed to tablets. Its hydrolysis yields salicylic acid. Salicylic acid has a narrow therapeutic window. If maintained within that narrow range, it provides the appropriate anti-inflammatory effect.
The pKa of aspirin is 3.5. Most aspirin is mostly absorbed in the stomach. Aspirin absorption is pH sensitive at the level of the small intestine.
Salicylate elimination occurs through two pathways via the creation of salicyluric acid and salicyl phenolic glucuronide. Salicylic acid is renally cleared, which can be increased by raising the urinary pH. Medications like antacids can increase renal clearance as they raise urinary pH. It can cross the blood-placental barrier. It is also expressed in breast milk.
Pharmacodynamics
Almost 90% of COX inhibition can be achieved with the administration of 160 to 325 mg of aspirin. These effects last for about 7 to 10 days which usually corresponds with the lifespan of a platelet. Prostacyclin inhibition can be achieved with the use of higher doses. This inhibition occurs in the endothelial cells of blood vessels.
- Adverse Effects
Aspirin has had multiple metanalyses, which suggest that aspirin reduces the risk of major adverse cardiovascular events in patients who have diabetes without cardiovascular disease while also causing a trend toward higher rates of bleeding and gastrointestinal complications. [9] [10] [11] [12]
The most common side effect of aspirin is gastrointestinal upset ranging from gastritis to gastrointestinal bleeding. [13]
Hypersensitivity
Hypersensitivity to NSAIDs is common among the general population. The rate is about 1% to 2%. Symptoms could be as mild as a simple rash to angioedema, and anaphylaxis. In patients with asthma or chronic rhinosinusitis, the prevalence of these allergic symptoms could be as high as 26%. If this is accompanied by nasal polyps and inflammation of the respiratory tract with eosinophils, it is called the aspirin triad. NSAID-exacerbated respiratory disease (NERD) is a new term associated with this syndrome due to upper and lower respiratory mucosal inflammation.
Reye Syndrome
Reye syndrome, named after the Australian pathologist Dr. R.D. Reye was first described in 1963. It is a rare but fatal condition with an estimated mortality rate of between 30% to 45%. It is a form of encephalopathy secondary to fatty changes in an otherwise healthy liver. The clinical vignette of Reye syndrome constitutes a viral upper respiratory tract infection in children and concomitant administration of aspirin for the treatment of fever. It is thought that mitochondrial injury secondary to the preceding viral illness is the first hit to both the liver and the brain. Aspirin or similar compounds provide the second hit completing the syndrome. The incidence has dramatically decreased due to better awareness and the use of acetaminophen to manage fever in children instead of aspirin. [14]
Even though the association between aspirin and Reye syndrome exists, some authors argue that at the time of diagnosis, salicylate levels were not routinely checked, biopsies were not obtained, and genetic/inborn errors of metabolism were not ruled out.
Intracerebral Hemorrhage
Aspirin increases the risk of intracranial bleeding (RR = 1.65; 95% CI, 1.06 to 5.99) versus placebo.
- Contraindications
People who are allergic to ibuprofen should not take aspirin as there is cross-reactivity. Patients who have asthma should be cautious if they have asthma or known bronchospasm associated with NSAIDs.
Aspirin increases the risk of GI bleeding in patients who already suffer from peptic ulcer disease or gastritis. The risk of bleeding is still present even without these conditions if there is concomitant alcohol consumption or if the patient is on warfarin. Patients who have inborn coagulopathies such as hemophilia should avoid all salicylates. Acquired diathesis, as in the setting of dengue or yellow hemorrhagic fever, should avoid the use of aspirin.
Patients who have glucose-6-phosphate dehydrogenase deficiency are at risk of acute intravascular hemolytic anemia. Many factors can precipitate these hemolytic episodes. Aspirin is one such known cause.
Avoid using aspirin in children who are suffering from a viral infection to avoid Reye syndrome. [14]
Therapeutic Index and Toxic Doses
Therapeutic drug levels for aspirin are 150 to 300 mcg/mL (salicylate).
Toxic Levels: Greater than 300 mcg/mL
Timing: 1 to 3 hours after the dose
Time to Steady State: 5 to 7 days
Plasma levels of aspirin can range from 3 to 10 mg/dL for therapeutic doses to as high as 70 to 140 mg/dL for acute toxicity. Due to delayed absorption of certain preparations, levels should be checked 4 hours after consumption and every subsequent 2 hours until maximum levels are reached.
Treatment needs to be individualized based on symptomatology as well as levels.
Aspirin levels do not need to be monitored in most cases. For certain diseases, serum creatinine at baseline, along with serum drug levels, if patients have adult or juvenile rheumatoid arthritis, Kawasaki disease, or arthritis/pleurisy.
Patients who have aspirin toxicity can have a myriad of symptoms. Symptoms of mild toxicity can be but are not limited to tinnitus, dizziness, lethargy, nausea, and vomiting. [15] For more severe toxicity, the signs and symptoms include hyperthermia, tachypnea leading to respiratory alkalosis, high anion gap metabolic acidosis, hypokalemia, hypoglycemia, seizures, coma, and cerebral edema. Death commonly occurs due to cardiopulmonary edema secondary to pulmonary edema. [16] [17]
Treatment for salicylate toxicity is based on salicylate concentration, acid-base status, volume status, electrolytes, GI decontamination, airway protection, and respiratory status, and enhanced elimination. [18]
The acuity of exposure, type of formulations, co-ingestions, comorbidities, and clinical status of the patient can affect salicylate levels in serum. Of all of these, particularly acid-base status can influence how the drug is handled by the body the most. Hence, initial and subsequent levels are recommended to assess trajectory. Different laboratories may report salicylate levels differently. One must pay attention to salicylate concentration units. The conversion is as follows:
- 100 milligrams per deciliter (mg/dL) equals
- 1000 milligrams per liter (mg/L), or
- 7.24 millimoles per liter (mmol/L)
One must draw serial salicylate levels to show that the levels are declining and thus also establishing a reduction in absorption.
Aspirin causes high anion gap metabolic acidosis and respiratory alkalosis. The high anion gap comes from the addition of salicylic acid as well as the generation of lactic acid (due to the uncoupling of oxidative phosphorylation causing anaerobic respiration). Respiratory alkalosis is due to direct stimulation of the respiratory center. Acidemia worsens symptomology. Salicylate exists in the blood in both ionized as well as uncharged forms. Acidemia shifts salicylate from its ionized to unionized forms making it more lipophilic and allowing increased penetration into the central nervous system (CNS). Volume status and electrolyte monitoring are paramount as brain glucose utilization increases in the setting of aspirin toxicity, even when serum glucose levels are normal. Hypokalemia worsens acidemia, and hence, supplementation may be required.
Alkalization of the urine can be achieved via a bicarbonate drip (3 ampules of 50 meq/50 ml for a total of 150 meq in 1000 ml of D5W). However, this may worsen hypokalemia, and hence, special attention to potassium supplementation is required.
Activated charcoal and/or bowel irrigation are recommended in both acute and chronic ingestion because of the extended-release preparations available on the market. In the setting of worsening mental status, one must exercise caution to avoid aspiration pneumonia.
Airway protection might be required in the setting of worsening mental status or acute injury to the lung.
Maintaining an alkaline pH is important to avoid CNS toxicity. This can be achieved by increasing minute ventilation to avoid carbon dioxide (CO2) retention. Bicarbonate drips can be used to achieve a pH of no greater than 7.5 during the intubation process.
Hemodialysis is an efficient treatment of salicylate toxicity. Once the protein-bound fraction is saturated, removal of the free fraction is effective through dialysis. Due to this efficiency, the clearance of salicylate is reduced to hours rather than days.
Peritoneal dialysis does not efficiently remove salicylate.
Indications for hemodialysis are as follows: [19]
- Aspirin levels in acute ingestions of 100 mg/dL with or without symptoms
- Aspirin levels in chronic ingestions 40 mg/dL with or without symptoms
- Any neurotoxicity (tinnitus, coma, seizures) with any level
- Renal failure (as the drug needs to be cleared by the kidney)
- Acute pulmonary edema
- Cardiovascular compromise, including volume overload
Hemodialysis does not only clear the drug from circulation but also restores the internal acid-base and electrolyte balance.
- Enhancing Healthcare Team Outcomes
Aspirin is available over the counter and is commonly involved in pediatric overdoses. The medication should be kept in a closed cabinet away from the reach of children. Given its wide availability, potential adverse effects, therapeutic uses, and potential interactions, all interprofessional healthcare team members should be aware of when patients are using aspirin and monitor and counsel the patient to optimize therapeutic outcomes and prevent any potential adverse effects of salicylic acid. This interprofessional team includes all clinicians (MDs, DOs, NPs, PAs), nurses, and pharmacists, all of whom need to have access to the same patient information and coordinate their activities, and openly share information so the patient receives the best possible healthcare, including how aspirin is used. [Level 5]
- Review Questions
- Access free multiple choice questions on this topic.
- Comment on this article.
Disclosure: Hasan Arif declares no relevant financial relationships with ineligible companies.
Disclosure: Sandeep Aggarwal declares no relevant financial relationships with ineligible companies.
This book is distributed under the terms of the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0) ( http://creativecommons.org/licenses/by-nc-nd/4.0/ ), which permits others to distribute the work, provided that the article is not altered or used commercially. You are not required to obtain permission to distribute this article, provided that you credit the author and journal.
- Cite this Page Arif H, Aggarwal S. Salicylic Acid (Aspirin) [Updated 2023 Jul 5]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan-.
In this Page
Bulk download.
- Bulk download StatPearls data from FTP
Related information
- PMC PubMed Central citations
- PubMed Links to PubMed
Similar articles in PubMed
- Prochlorperazine. [StatPearls. 2024] Prochlorperazine. Din L, Preuss CV. StatPearls. 2024 Jan
- Protriptyline. [StatPearls. 2024] Protriptyline. Saef MA, Yilanli M, Saadabadi A. StatPearls. 2024 Jan
- Paroxetine. [StatPearls. 2024] Paroxetine. Shrestha P, Fariba KA, Abdijadid S. StatPearls. 2024 Jan
- Review Safety assessment of Salicylic Acid, Butyloctyl Salicylate, Calcium Salicylate, C12-15 Alkyl Salicylate, Capryloyl Salicylic Acid, Hexyldodecyl Salicylate, Isocetyl Salicylate, Isodecyl Salicylate, Magnesium Salicylate, MEA-Salicylate, Ethylhexyl Salicylate, Potassium Salicylate, Methyl Salicylate, Myristyl Salicylate, Sodium Salicylate, TEA-Salicylate, and Tridecyl Salicylate. [Int J Toxicol. 2003] Review Safety assessment of Salicylic Acid, Butyloctyl Salicylate, Calcium Salicylate, C12-15 Alkyl Salicylate, Capryloyl Salicylic Acid, Hexyldodecyl Salicylate, Isocetyl Salicylate, Isodecyl Salicylate, Magnesium Salicylate, MEA-Salicylate, Ethylhexyl Salicylate, Potassium Salicylate, Methyl Salicylate, Myristyl Salicylate, Sodium Salicylate, TEA-Salicylate, and Tridecyl Salicylate. Cosmetic Ingredient Review Expert Panel. Int J Toxicol. 2003; 22 Suppl 3:1-108.
- Review Clinical pharmacokinetics of the salicylates. [Clin Pharmacokinet. 1985] Review Clinical pharmacokinetics of the salicylates. Needs CJ, Brooks PM. Clin Pharmacokinet. 1985 Mar-Apr; 10(2):164-77.
Recent Activity
- Salicylic Acid (Aspirin) - StatPearls Salicylic Acid (Aspirin) - StatPearls
Your browsing activity is empty.
Activity recording is turned off.
Turn recording back on
Connect with NLM
National Library of Medicine 8600 Rockville Pike Bethesda, MD 20894
Web Policies FOIA HHS Vulnerability Disclosure
Help Accessibility Careers

The History and chemistry behind aspirin
Jul 25, 2014
120 likes | 350 Views
The History and chemistry behind aspirin. By Angela Pacheco. Properties of Aspirin. Antipyretic: reduces fever Analgesic: reduces pain Anti-inflammatory: reduces swelling. Historical pain relief. Some of the earliest pain relievers were parts of plants. For example:
Share Presentation
- salicylic acid
- acetylsalicylic acid
- angela pacheco
- same reaction
- felix hoffman jr

Presentation Transcript
The History and chemistry behind aspirin By Angela Pacheco
Properties of Aspirin • Antipyretic: reduces fever • Analgesic: reduces pain • Anti-inflammatory: reduces swelling
Historical pain relief • Some of the earliest pain relievers were parts of plants. • For example: • The ancient Greeks used bark from willow and poplar trees. Records show Hippocrates prescribing it around 400 BC. • Native Americans made teas from the bark of the willow. • In the mid-1700s, these teas were introduced to Europe by Reverend Edward Stone, who dried willow bark and ground it into a powder. • In the 1800s, the active ingredient, salicylic acid, was identified. It began to be produced in large quantities and not just for pain relief. It was also used as a food preservative.
Problems with salicylic acid • However, salicylic acid was hard on the body. Being highly acidic, it caused irritation to the mouth and digestive system. • Various scientists began to experiment, trying to come up with a suitable alternative.
Felix Hoffman, jr. • A 25 year old chemist working in Bayer Laboratories in Germany in 1893, Felix Hoffman Jr had a personal interest in finding a replacement for salicylic acid. His father relied on the medicine to treat his arthritis, but suffered from the stomach discomfort it caused. • Working after hours, Hoffman Jr synthesized acetylsalicylic acid. The Bayer company would patent the drug and call it Aspirin (a- for the acetyl group and the “sprin” for the group of plants that produce salicylic acid. • In some countries, such as Canada, the name “aspirin” can only be used for the product sold by Bayer.
Aspirin = acetylsalicylic acid • Aspirin is formed by reacting salicylic acid with acetic anhydride. • C7H6O3 + C4H6O3 C9H8O4 + HC2H3O2 • Salicylic acid and acetic anhydride react to form acetylsalicylic acid and acetic acid.
Problems with aspirin • Though not as badly as its salicylic acid predecessor, aspiring still causes blood loss from the stomach. • It interferes with the clotting of blood, which can be a good thing for some heart problems. This is one of the reasons why heart patients may take a “baby” aspirin every day. • Because of the clotting problem, aspirin should not be taken prior to or immediately after surgery. • Aspirin has been linked to “Reyes Syndrome” in children and young teens suffering from viral infections. • Aspirin is toxic in large doses.
Our Aspirin Synthesis • The reaction doesn’t occur quickly, so we will use sulfuric acid as a catalyst. • This is the same reaction that aspirin companies use to make aspirin! • Once we have created our aspirin, we will purify it with ice cold water. Aspirin is not soluble in cold water, but the impurities are. • We will test our product with iron (III) chloride. Iron (III) chloride reacts with a phenol group (found in salicylic acid, but not in aspirin) to produce a colorful result. If your aspirin results look like that of the salicylic acid, then your aspirin is impure. • We will also test for starch. Starch is added to aspirin power to help it form a tablet. In this way, aspirin serves as a “binder.” Iodine reacts with starch to form a blue or black color.
- More by User

The History Behind Earth Day
The History Behind Earth Day. In the beginning…. Problems with the environment have been known long before Earth Day was even a thought.
258 views • 9 slides

Regulatory History of Aspirin
Regulatory History of Aspirin. Cardiovascular and Renal Drugs Advisory Committee Meeting Michelle M. Jackson, Ph.D. Division of Over-The-Counter Drug Products. Cardiovascular and Renal Drugs AC Meeting December 8, 2003. Content. The OTC Drug Monograph Process
579 views • 23 slides


The history behind the Kindle eReader
The history behind the Kindle eReader. 2007-Kindle I 2009 Kindle 2, and the Kindle DX 2010 Kindle 3 2011 Kindle with special offers, Kindle 4, and the Kindle Fire 2012 Kindle 5, Kindle Fire HD, Kindle Paperwhite. Comparison to other eReaders and Tablets. Readability Portability
325 views • 4 slides

Aspirin. Temple College EMS Professions ECA. Aspirin. Mechanism of Action Inhibits platelet aggregation NSAID . Aspirin. Effects Prevents further clotting Has no effect on existing clot Reduces Fever Anti- Inflamatory. Aspirin. Indications Ischemic chest pain. Aspirin.
938 views • 7 slides
The History behind Whipping Boys
Look for words beginning or ending with these prefixes or suffixes as you read “ The History behind Whipping Boys.”. The History behind Whipping Boys. actually .
235 views • 2 slides

The History behind Halloween
The History behind Halloween. Halloween Facts: Halloween candy sales average about 2 billion dollars annually in the United States. The Origins Of Halloween. Halloween can be traced back to the ancient Celts of Ireland, England, and northern France and their festival of Samhain ( Sow-in ). .
439 views • 7 slides

The History of Chemistry
The History of Chemistry. Wait a minute…that’s too much! How about… The History of Chemistry, Abridged. What does Chemistry mean?. In the most general terms, chemistry is the study of chemicals. Chemistry is the science that explains how matter changes.
5.13k views • 23 slides

The History Behind the Tooth Brush
The History Behind the Tooth Brush. By: Gus Koza. The Tooth Brush.
162 views • 3 slides

The Biology and Chemistry Behind HABs
The Biology and Chemistry Behind HABs. By: Barbra Harrell, Ashley McCulloch, Colleen Walsh. THE PLAN…. AP Environmental & GMO courses will complete the KILLER BLOOMS lab Read current HABs news article Complete pre-lab questions using prior knowledge
133 views • 4 slides
Aspirin. A medical miracle. Source: http://jakeandkims.blogspot.com/2010/07/medicine-highlight-aspirin.html. Index. Reason for this topic History Chemistry of aspirin Uses Contra-indications Side effects Conclusion Questions, discussion. Why talk about aspirin?.
1.21k views • 26 slides

The History behind Halloween. Halloween Facts: Halloween candy sales average about 2 billion dollars annually in the United States. The Origins Of Halloween. Halloween can be traced back to the ancient Celts of Ireland, England, and northern France and their festival of Samhain ( Sow-in ).
537 views • 7 slides

The Chemistry Idea Behind This
This is the forest, primeval-- , April 24, 1929 Reproduction of original drawing Published in the Chicago Daily News (1). The Chemistry Idea Behind This. The trees provide the environment with healthy air. It processes carbon dioxide and produces oxygen.
143 views • 5 slides

The History behind the book
The History behind the book. A brief history of Nigeria. 1/3 larger than the state of Texas Located on the west coast of Africa – North of the equator and south of the Sahara Desert. 200 ethnic groups live in present-day Nigeria with their own language, beliefs, and culture.
393 views • 18 slides
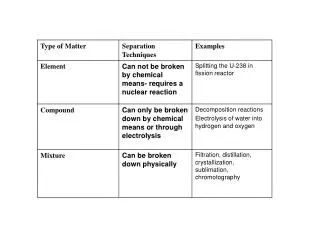
Law of Conservation of Matter: states that mass can neither be created or destroyed during a chemical reaction. The History of Chemistry. About 960 BCE- the Chinese monk Li Tian invented gunpowder (saltpeter- potassium nitrate, sulfur and charcoal)
248 views • 11 slides

The Chemistry Behind the Poison Hemlock
The Chemistry Behind the Poison Hemlock. By Chiara Ramos. What is Poison Hemlock?. Poison Hemlock (conium maculatum) is a deadly poisonous herb of the Apiacea Family, and a close relative to parsley.
726 views • 10 slides

The Secrets and History Behind Disneyland
"Disneyland will never be completed. It will continue to grow as long as there is imagination left in the world.“ -Walt Disney. The Secrets and History Behind Disneyland. By: Kristin Elberling. Thesis:.
850 views • 17 slides
Look for words beginning or ending with these prefixes or suffixes as you read “ The History behind Whipping Boys.”. The History behind Whipping Boys. actually.
244 views • 2 slides

Cell Theory – and the history behind it.
Cell Theory – and the history behind it. Spontaneous Generation. From pre-historic times to about 1850, most people believed that under the right conditions, living things could spontaneously appear from non-living material.
329 views • 32 slides

461 views • 30 slides

Aspirin. Objectives: 1- Acquire the skills of taking focused history and physical examination for aspirin intoxicated patients in ED 2- Acquire the basic approach to the poisoned patient 3- Understand the pahtophysiological and pharmacological effects of aspirin.
614 views • 35 slides

150 views • 10 slides

IMAGES
COMMENTS
Aspirin is synthesized by reacting salicylic acid with acetic anhydride in the presence of sulfuric acid. It has the molecular formula C9H8O4 and is used to treat fever, pain, and inflammatory conditions like arthritis.
The document is a presentation on aspirin submitted by a nursing student. It provides information on aspirin including what it is, its dosages and routes of administration, its actions and indications, contraindications, side effects, and nursing considerations for its use.
This activity outlines the indications, mechanism of action, methods of administration, important adverse effects, contraindications, and monitoring, of salicylic acid, so providers can direct patient therapy in treating indicated conditions as part of the interprofessional team.
This document summarizes key information about aspirin and other nonsteroidal anti-inflammatory drugs (NSAIDs). It discusses aspirin's mechanism of action as an irreversible inhibitor of cyclooxygenase, leading to reduced prostaglandin production and its analgesic, antipyretic and anti-inflammatory effects.
Download this research and presentation activity for groups of 16–18 year old learners including student instructions, a timeline of the development of aspirin, additional supporting information and teacher notes including peer assessment criteria and extra information on alternative analgesics.
The history of aspirin and other medicines dealing with pain, fever or inflammation reveals many interesting points about scientific methodology and the interaction of people and society with technology.
In your presentation, you should include the following points. • The conditions that aspirin helps to relieve or cure, including technical terms such as analgesic, antipyretic, anti-inflammatory and myocardial infarction. • The side effects of aspirin and the precautions or alternative treatments for people affected by them .
Aspirin is a salicylate used to treat pain, fever, inflammation, migraines, and reducing the risk of major adverse cardiovascular events.
Aspirin protects against atherothrombosis while increasing the risk of major bleeding. Although it is widely used to prevent cardiovascular disease (CVD), its benefit does not outweigh its risk for primary CVD prevention in large population settings.
Presentation Transcript. The History and chemistry behind aspirin By Angela Pacheco. Properties of Aspirin • Antipyretic: reduces fever • Analgesic: reduces pain • Anti-inflammatory: reduces swelling. Historical pain relief • Some of the earliest pain relievers were parts of plants.