An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- HHS Author Manuscripts


Protein Separation by Capillary Gel Electrophoresis: A Review
Capillary gel electrophoresis (CGE) has been used for protein separation for more than two decades. Due to the technology advancement, current CGE methods are becoming more and more robust and reliable for protein analysis, and some of the methods have been routinely used for the analysis of protein-based pharmaceuticals and quality controls. In light of this progress, we survey 147 papers related to CGE separations of proteins and present an overview of this technology. We first introduce briefly the early development of CGE. We then review the methodology, in which we specifically describe the matrices, coatings, and detection strategies used in CGE. CGE using microfabricated channels and incorporation of CGE with two-dimensional protein separations are also discussed in this section. We finally present a few representative applications of CGE for separating proteins in real-world samples.
1. Introduction
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE, see Table I for a list of acronyms used in this paper) has been used for size-based separations of proteins for over four decades [ 1 , 2 ], and it is still the workhorse for protein separations and analyses in most biological research laboratories. The basic procedures of this method include: 1) preparing a gel and assembling the gel apparatus, 2) mixing a protein sample with a buffer containing SDS and cooking the mixture at an elevated temperature, 3) loading the protein-SDS mixture into the gel inside the gel apparatus and performing the electrophoresis, and 4) fixing, staining/de-staining, and quantitating the separated proteins. If SDS is allowed to react with sample proteins completely, the reactions should produce SDS-protein complexes having similar charge densities (or mass-to-charge ratios). When these complexes are electrophoretically separated, their mobilities will depend on their sizes, with smaller proteins having greater mobilities. The mobility values decrease linearly with the logarithm of protein molecular masses, which is the basic separation principle of SDS-PAGE. However, the technique is time-consuming and labor-intensive. The many manual operations ( e.g ., gel preparation, sample loading, staining/de-staining, etc.) are believed to be sources of SDS-PAGE irreproducibilities.
List of Acronyms
| Acronym | Representation | Acronym | Representation |
|---|---|---|---|
| 2D | two-dimensional | LIF | laser induced fluorescence |
| Bis | -methylenebis(acrylamide) | LOD | limit of detection |
| CE | capillary electrophoresis | LPA | linear polyacrylamide |
| CGE | capillary gel electrophoresis | MALDI | matrix-assisted laser desorption ionization |
| CIEF | capillary isoelectric focusing | MEKC | micellarelectrokinetic chromatography |
| CPA | cross-linked polyacrylamide | MS | mass spectrometer |
| CSE | capillary sieving electrophoresis | NDA | naphthalene-2,3-dicarboxaldehyde |
| CZE | capillary zone electrophoresis | PA | polyacrylamide |
| EDTA | ethylenediaminetetraacetic acid | PAGE | polyacrylamide gel electrophoresis |
| EOF | electroosmotic flow | PDMA | polydimethylacrylamide |
| FITC | fluoresceinisothiocyanate | PDMS | poly(dimethylsiloxane |
| FQ | 3-(2-furoyl) quinoline-2-carboxaldehyde | PEG | poly-(ethylene glycol) |
| HEC | hydroxyethylcellulose | PEO | poly-(ethylene oxide) |
| HPC | hydroxypropylcellulose | PEOX | poly(2-ethyl-2-oxazoline) |
| HPLC | High performance liquid chromatography | PMMA | poly(methyl methacrylate) |
| HRPN | hydrophilic replaceable polymer network | PVA | poly(vinyl alcohol) |
| HV | High voltage | CPA | replaceable cross-linked polyacrylamide |
| IEF | isoelectric focusing | rMAbs | recombinant monoclonal antibodies |
| IgG | immunoglobulin G | SDS | sodium dodecyl sulfate |
| IPG | immobilized pH gradients | TOF | time-of-flight |
SDS-capillary gel electrophoresis (SDS-CGE), also called capillary sieving electrophoresis (CSE) or capillary gel electrophoresis (CGE), shows many advantages over classical SDS-PAGE. These advantages include on-column detection, automated operation, great resolving power, and capability of accurate protein quantification and molecular weight determination [ 3 – 8 ]. The first papers on CGE were published in the 1980s [ 9 , 10 ]. As in slab-gels, agarose and cross-linked polyacrylamide (CPA) was used as sieving matrices, and these matrices were prepared directly inside the capillary columns. In the early 1990s [ 11 ], linear polyacrylamide (LPA) was introduced to replace CPA, but an in-capillary polymerization procedure was still used for the gel preparation. The lifetimes of these columns were limited (usually less than 10 runs) [ 12 ], and the run-to-run reproducibility was poor. Currently, replaceable and water-soluble linear or slightly branched polymers, such as linear polyacrylamide [ 11 – 13 ], poly(ethylene glycol) [ 11 ], poly(ethylene oxide) [ 14 ], dextran [ 15 – 17 ], pullulan [ 18 , 19 ] and cross-linked polyacrylamide [ 20 – 22 ] are used as sieving matrices for CGE [ 5 , 11 , 23 – 25 ]. Availability of these polymer matrices has led to improved reproducibility and robustness of this methodology.
Recently, CGE has been recognized and established [ 26 ] as an important tool in biopharmaceutical industry to support analytical characterization, process development, and quality control of therapeutic recombinant monoclonal antibodies (rMAbs) [ 26 – 29 ]. In an effort to make CGE-based methods accepted by biotechnology companies, scientists in various pharmaceutical industries and regulatory authorities conducted cross-laboratory research to examine the reliability and robustness of the method [ 30 , 31 ]. It is expected that some CGE methods will soon be used in pharmaceutical and biotechnological industries. In light of this advancement, we write this paper to review briefly the progress of CGE for protein analysis. We focus mainly on the methodology and application aspects of CGE. In the methodology aspect, we review the common sieving matrices, wall coatings, and detection strategies used in CGE. CGE performed in microfabricated channels and CGE as one dimension in two-dimensional (2D) separations are also discussed. In the application aspect, we present a few separations related to or closely related to practical uses. Table II provides a summary of literatures on CGE of proteins based on the sieving matrices used.
Literature Summary Based on Sieving Matrices Used
| Sieving Matrix | Analyte | Reference | Comment |
|---|---|---|---|
| In-capillary/channel prepared polyacrylamide (non-replaceable) | Standard proteins or mixtures | [ , – , , , , , , , ] | Gradient gel was prepared in ref. . Infrared laser desorption/ionization MS was interfaced with gel electrophoresis chip in ref. . 2D gel electrophoresis was performed on chip in ref. , and 115. |
| Human and bovine serum albumin | [ ] | ||
| Interleukin-2 and growth factors | [ ] | ||
| Linear polyacrylamide | Standard proteins or mixtures | [ – ] | |
| Thrombin | [ ] | ||
| Cider proteins | [ , ] | ||
| Beckman SDS gel | Apolipoproteins in human high-density lipoproteins | [ ] | rMabs were labeled with FQ before separation and detection in ref. . 2D separation was performed in ref. and . Performances of Beckman-Coulter ProteomeLab and Agilent 2100 Bioanalyzer were compared in ref. . Bechman SDS-gel goes with their instruments. |
| Recombinant monoclonal antibodies (rMAbs) | [ , , , – ] | ||
| Standard proteins | [ , , , ] | ||
| Protein from E. coli cell | [ ] | ||
| Protein biotoxins | [ ] | ||
| Proteins from soybean | [ , ] | ||
| Erythrocyte membrane proteins | [ ] | ||
| Myofibrillar proteins (actin/myosin) | [ ] | ||
| Rotavirus-like particles | [ ] | ||
| Agilent 2100 kit | Standard proteins | [ ] | Separations on chip were performed in ref. – , , and . Agilent SDS-gel goes with their instrument. |
| Glycoproteins and de-N-glycosylated Human serum glycoproteins | [ , , ] | ||
| Proteins from soybean cultivars | [ ] | ||
| Monoclonal antibody | [ ] | ||
| Bio-Rad CE-SDS run buffer | Polyethylene glycolylated interferon (PEG-IFN) | [ , ] | Bio-Rad CE-SDS run buffer does not require coated capillaries for SDS-CGE. |
| PEG-modified granulocyte-colony stimulating factor | [ ] | ||
| RuBisCo | [ , ] | ||
| Monoclonal antibody | [ ] | ||
| Water-/salt-solubale proteins from bovine and ostrich meat | [ ] | ||
| Slightly cross-linked polyacrylamide (replaceable) | Standard proteins or mixtures | [ , , ] | MALDI-MS was interfaced with CGE in ref. . |
| Proteins in crude cell extract | [ ] | ||
| E. coli AcrA protein | [ ] | ||
| PEG/Dextran | Proteins in MCF-7 breast cancer cell | [ ] | The work in ref. was focused on selection of an internal standard for separation. 2D separation was performed in ref. 98. Performances of PEG, dextran and LPA were compared in ref. . Detection limit of sub-pM were obtained in ref. . |
| Proteins in Barrett’s Esophagus Tissue homogenate | [ ] | ||
| Proteins in rat plasma | [ ] | ||
| Proteins in E. coli cell extract | [ ] | ||
| Standard proteins | [ , , , , , , ] | ||
| Proteins from AtT-20 cellular homogenate | [ , ] | ||
| Proteins from Barretts esophagus homogenate | [ ] | ||
| Tryptic digests | [ ] | ||
| Carbonylated proteins from rat muscle | [ ] | ||
| Pullulan | Standard proteins | [ , , , , ] | 2D separation was performed in ref. , and . |
| Protein homogenate from D. radiodurans | [ ] | ||
| Proteins from breast cancer cell | [ ] | ||
| Polyethylene oxide (PEO) | Proteins in HT29 human colon adenocarcinoma cell extract | [ ] | Proteins were labeled with FQ before separation and LIF detection in ref. . Proteins were labeled with SYPRO Red before separation and LIF detection in ref. . 2D separation was performed in ref. and . |
| Casein in milk | [ ] | ||
| Proteins from E. coli cell | [ ] | ||
| Standard proteins | [ ] | ||
| Hydroxypropylcellulose (HPC) | Standard proteins or mixtures | [ , ] | |
| Proteins in HT29 human colon adenocarcinoma cell extract | [ ] | ||
| Poly-N- hydroxethylacrylamide | Standard proteins | [ ] | An acid-labile surfactant was used to replace SDS in ref. . |
| Hydroxyethylcellulose (HEC) | Lanthanide chelate-labeled proteins | [ ] | A time-resolved fluorescence detector was used in ref. . |
2. Methodology
The basic apparatus for CGE is identical to that of capillary zone electrophoresis (CZE) and consists of a capillary column, an on-column detector, and a high voltage power supply. The major difference between the two techniques is the separation media: a sieving matrix is employed in CGE while a background electrolyte solution is utilized in CZE.
2.1. Sieving Matrices for CGE
Polyacrylamide (PA ) has been widely used in slab gel electrophoresis of proteins, and consequently it is frequently utilized in CGE. Initially, PA gels were synthesized in-situ inside capillaries [ 10 , 32 , 33 ]. Typically, a capillary column was prepared by mixing acrylamide (monomer), N,N’-methylenebis(acrylamide) (Bis, cross-linker), ammonium peroxy-disulfate or ammonium persulfate (radical initiator), N,N,N’,N’-tetramethylethylenediamine (TEMED, catalyst) and other background electrolytes, introducing the mixture into the capillary, and allowing the solution to polymerize inside the capillary. While this worked in general, problems occasionally arose when PA shrank during polymerization, breaking PA gel into segments and/or forming bubbles inside the column. Additionally, a good column could work well for only the first a few runs, as large molecules and particulate materials accumulated at the injection end of the column, which deteriorated and eventually shut down the separation.
To address this issue, a replaceable sieving polymer – a low viscous LPA solution – was prepared. This sieving matrix was successfully used for DNA sequencing [ 34 ], as well as for protein sizing [ 12 ]. Because the sieving polymer inside a separation column could be replaced after each run, the run-to-run reproducibility was improved considerably.
It should be noted that, when LPA was developed, its low viscosity (or replaceability) was emphasized. This might be why CPA was rarely investigated as a replaceable sieving matrix initially, because common sense tells that a cross-linked polymer would have a high viscosity. In 2005, Lu et al. [ 35 ] noticed that, if the degree of cross-linking was carefully controlled, CPA was an excellent replaceable sieving matrix – superior over LPA for protein separations in many aspects. Using this sieving matrix, CGE was capable of resolving proteins ranging from ∼4–250 kD in less than 20 min.
When PA sieving matrices are used to run CGE, capillary walls often need to be coated for achieving high quality separations. Poly(N,N-dimethylacrylamide)-grafted PA , a derivative of PA, was prepared by Zhang et al. [ 36 ] in 2006, and when this polymer was used to sieve proteins, capillary wall coating could be avoided. This is because poly(N,N-dimethylacrylamide)-grafted PA is capable of coating capillary walls dynamically.
Various polysaccharides form another important type of sieving matrices for protein separations. One advantage of polysaccharides is that these polymers do not absorb as much UV light as PA does. Ganzler et al. [ 11 ] separated SDS-protein complexes using dextran and poly(ethylene glycol) (PEG). The separated proteins were detected at 214 nm in which dextran and PEG are transparent. These matrices had moderate viscosities and could be conveniently replenished. Luo et al. [ 17 ] performed high-throughput protein analysis by multiplexed SDS-CGE, and Xu et al. [ 37 ] realized separation and characterization of SDS-protein complexes on a microchip with UV adsorption detection using similar matrices. Hydroxypropyl cellulose (HPC) is another polysaccharide sieving matrix used in CGE. For example, Hu et al. [ 38 ] developed a CGE-laser-induced fluorescence (LIF) method for separating proteins from HT29 cancer cells. Pullulan [ 24 , 39 , 40 ] and hydroxyethyl cellulose [ 41 ] were used for CGE, as well.
Other polymers have also been utilized for protein sieving. Yu et al. [ 42 ] used poly(vinyl alcohol) (PVA) to perform on-line protein concentration and separation. Bernard et al. [ 43 ] used poly(2-ethyl-2-oxazoline) for CGE and achieved separation efficiencies of ∼10 million plates per meter. Hu et al. [ 8 ] used polyethylene oxide (PEO) to analyze the protein contents in a single HT29 cancer cell and obtained protein profiles similar to those determined by other methods.
Using dynamic light-scattering, Sumitomo et al. [ 44 ] evaluated the mesh size and homogeneity of three sieving polymer solutions, PA , PEO and HPC. Based on their experimental results, these authors concluded that an optimal sieving polymer for separating proteins ranging from 14.3 to 97.2 kD is a homogeneous polymer network with a mesh size of less than 10 nm. Sumitomo et al. also stated that PEO in solution can aggregate, degrade into smaller pieces, and form polymer–micelle complexes with SDS. This disturbs protein–SDS complexation and impairs the protein separation efficiency. Recently, the same group surveyed the composition of the separation buffers, and results showed that Tris-CHES buffer was able to suppress SDS adsorption to PEO and achieve separation of six proteins [ 45 ].
Commercial sieving kits are now available to run CGE. These kits are largely from Beckman-Coulter ( www.beckmancoulter.com ), Agilent Technologies ( www.agilent.com ) and Bio-Rad Laboratories ( www.bio-rad.com ) and they are optimized for their CGE instruments. Beckman SDS Gel was demonstrated to be capable of sizing membrane proteins [ 46 ], protein biotoxins [ 47 ], and antibodies [ 48 ], but coated capillaries or channels were usually needed to achieve good separations [ 49 ]. Using Bio-Rad CE-SDS run buffer, Na et al. [ 50 , 51 ] used uncoated fused-silica capillaries for CGE and separated poly(ethylene glycol)-modified proteins. This kit was also employed for quantitative analysis of antibodies [ 52 ], RuBisCo in spinach [ 53 ], and water-/salt-proteins from bovine and ostrich meat [ 54 ]. Agilent commercialized the first microchip capillary electrophoresis system (Agilent 2100 Bioanalyzer), along with its microchips. Agilent 2100 Kit was provided with this instrument and utilized for analysis of half-antibody [ 55 ] and glycoproteins by microchip CGE [ 56 , 57 ]. In these applications, the gel was pipetted into the designated reservoirs on a chip and propelled, by use of a syringe, into the chip channels.
2.2. Capillary Coatings
The interior walls of capillaries used in CGE are often coated for two purposes: reducing protein-wall interactions and suppressing electroosmotic flow (EOF). An uncoated wall can interact with proteins electrostatically (if part of the protein molecule is positively charged) and/or hydrophobically (if a portion of the protein molecule is hydrophobic), and these interactions deteriorate separation efficiencies. In CGE, the strengths of these interactions are greatly reduced because proteins have reacted with SDS forming hydrophilic and negatively charged SDS-protein complexes. Therefore, wall coating in CGE is used primarily for EOF suppressions.
Running CGE at low or zero EOF is important for achieving reproducible results. If one uses an uncoated capillary to run CGE, the EOF will carry the sieving matrix from anode to cathode while SDS-protein complexes migrate in the opposite direction. Some of the proteins will never pass the detector, unless the EOF is so large that it brings all SDS-protein complexes to the detector. Usually, this condition cannot be guaranteed. Another problem associated with EOF is its instability as the wall conditions change. The fluctuation of EOF causes the migration time change, and subsequently the separations become irreproducible. Including an internal standard in samples can mitigate this problem, as long as the standard does not interfere with protein peak detections. Pugsley et al. [ 58 ] developed a dye (fluorescently-labeled aspartic acid) that worked well as an internal standard, because it migrates faster than all fluorescently-labeled SDS-protein complexes.
Numerous approaches have been explored to control/suppress EOF, and the most commonly used approach is to derivatize capillary walls via either dynamic coatings [ 59 – 62 ] or covalent coatings [ 63 – 65 ]. Progress in the field of polymeric coatings can be found in a number of reviews [ 66 – 68 ].
A dynamic coating, due to its simplicity, is a convenient way to modify capillary wall properties. It is normally produced by putting appropriate additives (often polymers) into SDS-SGE run buffers (or sieving matrices) and flushing the capillary columns with these run buffers before separation. Several polymers, including polydimethylacrylamide [ 61 ], epoxy poly(dimethylacrylamide) [ 69 – 71 ], and poly(-hydroxyethylacrylamide) [ 62 ] were used to create a dynamic coating. The exposure of silica surfaces to very dilute solutions of these polymers causes development of dense polymer layers via hydrogen bonding, electrostatic attractions and/or hydrophobic forces [ 72 ]. The molecular weight of the polymer has a strong impact on the stability of the coating since the adhesive forces/energies per chain increase in proportion to the number of monomer units [ 73 ]. Some CGE sieving matrices are excellent dynamic coating additives [ 8 , 51 , 58 , 74 ]. With these matrices, bare capillaries can be used directly for protein separations.
Covalent coatings are generally more stable than dynamic coatings. These coatings are obtained by chemically bonding desired substances to capillary walls. One of the most common coating protocols was introduced by Hjerten in 1985 [ 65 ]. Typically, 3-(trimethoxysilyl)propyl methacrylate is first attached to a capillary wall, leaving acrylic groups exposed on the wall surface. The capillary is then filled with a polymerizing solution containing acrylamide and a polymerization initiator. The free acrylic groups attached to a capillary wall serve as anchors for growing linear polymer chains. A problem of this coating is that linear molecules cannot cover the capillary wall completely. The poorly covered area will adsorb proteins and create EOF. To improve this situation, a CPA coating was developed by Gao and Liu in 2004 [ 75 ] and successfully used for SDS-CGE [ 35 ].
2.3. Microfabricated channels for CGE
Microfabricated (or microchip) devices are developed with a goal to perform and integrate multiple analytical processes (e.g. sample pretreatment, solution distribution/mixing, separation, detection, etc.) on a chip platform [ 76 , 77 ]. Due to the short column length and high separation efficiency, microchip CGE is generally fast, typically from a few seconds to a few minutes. Yao et al. [ 78 ] is recognized as the first who performed SDS-PAGE in a microfabricated channel, and the separations were completed in less than 1 min. By combining an on-chip dye staining with an electrophoretic dilution step (similar to a destaining step), Bousse et al. [ 79 ] obtained excellent resolutions for microchip CGE of proteins. On the basis of this work, the first commercial microchip instrument was constructed by Agilent Technologies. In 2004, Han et al. [ 22 ] and Herr et al. [ 80 ] applied an in-channel photopolymerization approach to prepare CPA gels inside a microchip channel for SDS-PAGE, and a separation speed of <30 s per run was demonstrated. These authors also prepared a gradient CPA gel for on-chip protein sizing [ 20 ] and successfully implemented sample pre-concentration using these photo-patterned gels [ 21 ]. Huang et al. [ 81 ] combined isotachophoresis (ITP) to concentrate proteins for subsequent CGE. Xu et al. [ 82 ] performed on-line electrokinetic supercharging preconcentration on a microchip to improve method sensitivity. Tsai et al. [ 83 ] tested simultaneous separations of both native and SDS-denatured proteins on a single microchip with 36 microchannels. Herr et al. [ 84 ] recently integrated saliva pretreatment (mixing, incubation, and enrichment) with subsequent quantitative immunoassays and measured the concentration of endogenous MMP-8 in saliva. More recently, He and Herr [ 85 ] photopatterned different gels inside a microfluidic chamber for protein immunoblotting. Fig. 1 presents the immunoblotting chip. Gel-separation was first performed in the vertical dimension, and the separated proteins were then transferred to the immunoblotting gel in the horizontal dimension. Electric fields were applied to the chamber via the parallel microchannels, and the microchannel arrays were designed such that uniform electric fields were produced over the chamber area during separation and transfer steps in both the vertical and horizontal dimensions.

(a) Schematic design of the immunoblot chip for analysis of native proteins. The sample (2), sample waste (3), buffer (1, 4, 5, 6) and buffer waste (7, 8) reservoirs are indicated in sketch (not to scale). The middle region of the device (indicated as Chamber) has a length of 1.5 mm, a width of 1 mm and a depth of 20 µm. (b) Three separate zones inside the Chamber to facilitate protein immunoblotting: a large-pore-size protein loading gel on the top, a smaller-pore-size protein separation gel on the bottom-left and an antibody-functionalized blotting gel on the bottom-right. Colored dyes were used to visualize the different gel regions. Reprinted from ref. [ 85 ] with permission.
In 2005, Fruetel et al. [ 47 ] reported a hand-held microchip instrument called µChemLab™ that is capable of performing CZE and CGE in parallel. The instrument consisted of a microfluidics module, a dual channel LIF detection module, an integrated multichannel high-voltage power supply, and a main control board containing the laser diode drivers, user interface, and an embedded microprocessor (see Fig. 2A ). It has an approximate volume of 7×8×4.5 cubic inches (see Fig. 2B ).

(a) The µChemLab instrument with the top off showing the separation platform, the control panel, the back of the LCD display, and the battery pack. The instrument is approximately 7″×8″×4.5″ and weighs 6 lbs. (b) The separation platform houses the microfluidic chip in a compression manifold that connects the chip to eight fluid reservoirs, two sample injection ports, and a LIF detection module. The overall size of the platform is approximately 5″×3″×4″. Reprinted from ref. [ 48 ] with permission.
Microchip devices were originally fabricated on glass substrates [ 86 , 87 ]. Over the past decade, polymeric chips have attracted growing attention, due to the low material and fabrication costs. Polystyrene [ 88 ], polyesters [ 88 ], polycarbonate [ 89 ], poly(dimethylsiloxane) (PDMS) [ 90 ] and poly(methyl methacrylate) (PMMA) [ 91 ] were used to fabricate microchips. Hybrid materials are also used [ 49 ]. All these chips have been tested for CGE separation of proteins. Performance of microchip-based gel electrophoresis has been compared with that of capillary-based gel electrophoresis [ 41 , 56 , 57 , 92 – 94 ]. In general, the performances are comparable, while microchip CGE provides faster separations.
2.4. CGE as One Separation Dimension in Two-Dimensional Protein Analyses
2D separation techniques are powerful tools for protein analysis, because the peak capacity of a 2D analysis is the multiplication product of the peak capacities of the two individual dimensions. To realize this resolving power, Chen et al. [ 90 ] constructed a 2D separation device using reconfigurable PDMS slabs in 2002. Four slabs were used to make channels and reservoirs to perform the first dimensional (1 st -D) separation – isoelectric focusing (IEF). Then, the middle two slabs containing the IEF-resolved proteins were inserted into another two pieces of slabs which contained multiplexed channels for the second dimensional (2 nd -D) separation – CGE. Because of their elastomeric nature, PDMS slabs could be attached and detached reversibly without fluid leaking. Although 2D separations were performed, high resolving power was not demonstrated using this device.
Griebel et al. [ 19 ] fabricated 300 parallel channels (64 mm long × 50 µm wide × 50 µm deep) on a PMMA chip. A 50-µm opening was produced at one end of chip across all these parallel channels. To prepare for the separation, these parallel channels were filled with 15 % (w/v) pullulan. IEF (the 1 st -D separation) was performed first on a separate device – a conventional immobilized pH gradients (IPG) strip. After IEF, the IPG strip was brought to the opening on the chip for parallel CGE (the 2 nd -D) separations. However, 2D separation results were not disclosed in this paper.
IEF and CGE were incorporated in the above devices, but they were coupled in an off-line fashion. To implement on-line integration, Li et al. [ 89 ] integrated IEF with CGE on a polycarbonate microchip using PEO as their sieving matrix. Fig. 3 presents the channel layout of this chip: one horizontal channel intersected by eight parallel vertical channels. The IEF was performed in the horizontal channel, and SDS-PEO gel electrophoresis was performed in the vertical channels. Preferably, an SDS-PEO sieving matrix should be filled in the vertical channels before IEF was performed. However, the device as designed had a limitation in this regard. Because the 1 st -D and the 2 nd -D channels were directly connected, the SDS in the SDS-PEO matrix in the 2 nd -D channels would bleed into the 1 st -D channel due to molecular diffusion and electric field distortion at the channel intersections during IEF. The presence of SDS in the 1 st -D channel would bind to proteins (which would add negative charges on the proteins), and therefore ruin the IEF. To circumvent this problem, the authors filled the 2 nd -D channels with a matrix containing PEO but not SDS. The SDS required for the 2 nd -D separation was electrokinetically introduced to the matrix after IEF was complete. During the SDS introduction, the protein bands focused based on their p I values were diffused/broadened before they were conjugated with SDS and electrokinetically injected to the 2 nd -D channels. Thus, some IEF resolution was lost.

Reprinted from ref. [ 90 ] with permission.
In 2008, Liu et al. [ 95 ] carried out IEF and parallel SDS gel electrophoresis on a similar device. PA gel plugs were patterned via photopolymerization at various locations to stop hydrodynamic flows between reservoirs/channels and thus prevent unwanted bleeding/mixing. These gel plugs may cause problems when channels require frequent washing.
It should be noted that the concept of this 2D separation chip had been discussed earlier [ 96 ], but 2D separation results were never published.
In a separate effort, Yang et al. [ 97 ] combined capillary isoelectric focusing (CIEF) with CGE in a linear format (see Fig. 4A ) via a polyethersulfone dialysis hollow fiber interface. Fig. 4B illustrated the detailed structure of this interface. After hemoglobin variants were focused in the CIEF capillary, the catholyte in the reservoir on the methacrylate plate was replaced by a CGE buffer. The CGE buffer also served as a chemical mobilization solution for the CIEF. As voltages were applied to both capillaries, CIEF-resolved protein bands were chemically mobilized to the hollow fiber. At the same time, negatively charged SDS continuously migrated into the hollow fiber and reacted with the proteins (forming SDS-protein complexes), and the SDS-protein complexes were subsequently injected into the CGE capillary for the 2 nd -D separation. Because the CIEF-resolved proteins were continuously injected into the CGE capillary, some of the resolving power was sacrificed.

(A) Overall Arrangement of experimental setup. (B) Dialysis hollow fiber interface: (1) methacrylate plate, (2) capillaries, (3) Teflon tubes, (4) hollow fiber and (5) buffer reservoir. Reprinted from ref. [ 98 ] with permission.
Additionally, Michels et al. [ 18 ] coupled CGE (the 1 st -D) with MEKC (the 2 nd -D) by connecting the exit-end of a CGE capillary to the sampling-end of an MEKC capillary. A small gap was left between the two capillaries and filled with an MEKC running buffer to facilitate electric field application and sample injection for MEKC. Two high voltage (HV) power supplies were used in this work. HV1 was used to execute sample injection and separation, and HV2 was utilized for MEKC. After a period of initial CGE separation, a fixed length (e.g., 10 s migration) of CGE-resolved protein band(s) was allowed to enter the gap. HV2 was turned on to apply a potential to the gap solution so that there was no electric field across the CGE capillary (to stop the CGE), while an electric field was created across the MEKC capillary to inject the proteins in the gap into the MEKC capillary and execute the MEKC separation. [Note: HV1 was on all the time.] When the MEKC separation was complete, HV2 was turned off for a given period of time ( e.g ., 10 s) so that more CGE-resolved proteins entered the gap. Then, HV2 was turned on again to execute the sample injection and MEKC separation. These operations were repeated until the proteins inside the CGE capillary were exhausted. This separation technique was successfully applied for separations of proteins from bacterium Deinococcus radiodurans [ 18 ] and proteins from single mammalian cells [ 40 ]. The method was later modified, and the separation speed was improved from 3–5 h per run to ∼1 h per run [ 98 ].
In 2006, Shadpour et al. [ 91 ] incorporated CGE with MEKC on a PMMA device. Fig. 5A shows the channel layout of the microchip. By applying a vacuum to reservoir D while reservoirs E and F were sealed, a sieving matrix was aspirated into d 1 channel from reservoir C . As soon as the sieving matrix reached point d 2 (as shown in Fig. 5B ), the vacuum on reservoir D was removed. An MEKC buffer was pressurized into d 3 channel from reservoirs F while reservoirs A - C were sealed. A protein mixture was injected into d 1 for CGE. As the first protein peak approached point d 2 , appropriate voltages were applied to various reservoirs to stop CGE and effect MEKC. After MEKC was complete, voltages on the reservoirs were changed to stop MEKC and resume CGE for a short period of time (e.g., 0.5 second) to allow a fraction of CGE-resolved protein band to migrate toward point d 2 . These operations were repeated until all CGE-resolved proteins were separated by MEKC. Complex proteins samples were analyzed using a similar chip and approach [ 99 ].

(A) Geometrical layout of the microchip used for SDS-µCGE-MEKC. (B) Fluorescence image of the sieving matrix/MEKC interface at the intersection of the SDS-µCGE and MEKC dimensions. Reprinted from ref. [ 92 ] with permission.
2.5. Detection Strategies
UV absorption is the most commonly used detection mode in CE, including CGE [ 15 , 100 , 101 ]. Proteins can be detected easily by a UV absorbance detector, because the peptide bonds between amino acids and aromatic side groups in protein molecules absorb UV light at 200–220 nm and 280 nm, respectively. Owing to the limited optical path length, the concentration sensitivities of UV absorption detection are normally low, especially when narrow capillaries are used.
LIF detectors are commonly used in CGE to improve concentration sensitivities. When an LIF detector is used, proteins need to be fluorescently “labeled”. Proteins have been covalently labeled by fluorescent dyes, such as naphthalene−2,3-dicarboxaldehyde (NDA) [ 102 ], 3-(2-furoyl) quinoline−2- carboxaldehyde (FQ) [ 8 , 103 , 104 ] and fluoresceinisothiocyanate (FITC) [ 105 , 106 ]. In 2007, Michels et al. [ 107 ] reported an improved fluorescent derivatization method for proteins analysis by CGE. In this assay, rMAbs were derivatized with FQ in the presence of cyanide (CN − ). This technique minimized sample preparation artifacts and greatly improved detection sensitivity of FQ-labeled rMAbs.
Covalent labeling method has an intrinsic problem. A protein molecule usually has a number of sites that can react with a fluorescent labeling dye. Because these sites have different reactivities, it is challenging to make all sites to be labeled with the dye. This labeling reaction produces a mixture in which some proteins are un-labeled, some are fully-labeled, while the majority is partially-labeled. This mixture will cause peak-broadening or even multiple peaks in CE separations [ 108 ]. A postcolumn labeling method is often a good approach to address this problem. In 2009, Kaneta et al. [ 16 ] reported a postcolumn derivatization method for CGE separations of proteins. The method used a labeling dye of naphthalene-2, 3-dicarbaldehyde in the presence of 2-mercaptoethanol which played a role of a reducing agent in the derivatization reaction. Recently, these researchers replaced 2-mercaptoethanol with ethanethiol as the reducing agent and improved the method limits of detection by 1.4- to 4.5-fold [ 109 ].
Alternatively, proteins can be dynamically labeled with fluorescent dyes [ 110 ]. In 2001, Jin et al. [ 111 ] showed that SDS-protein complexes could be dynamically labeled with NanoOrange. NanoOrange does not fluoresce much in aqueous solutions, but as it binds to a protein-SDS complex, it fluoresces substantially. Sano et al. [ 112 ] took a similar approach for CGE analysis of collagenase. Chiu et al. [ 74 ] labeled proteins with SYPRO Red and accomplished LIF detection using a low-cost He-Ne laser. In 2007, Wu et al. [ 113 ] developed an elegant approach for protein labeling. First a pseudo SDS dye was synthesized by attaching an alkyl chain to an ionic fluorescent dye (e.g., FITC). Since the long carbon chain is equivalent to the dodecyl group while the negatively charged fluorescent group resembles the sulfate group of SDS, the pseudo-SDS dye has the same function as SDS when binding to proteins. As a mixture of SDS and pseudo-SDS dye reacts with proteins, protein molecules are dynamically labeled with some pseudo-SDS dye. Fig. 6 presents a schematic demonstration of pseudo-SDS dye-protein-SDS complex. Because each protein can be associated with many pseudo-SDS dye molecules, the detection sensitivity can be improved considerably. Using this approach, these authors obtained an LOD of 0.13 ng/mL and a dynamic range of ∼5 orders of magnitude for CGE analysis of BSA.

Reprinted from ref. [ 114 ] with permission.
Fluorescence imagers have also been used as detectors for SDS-CGE [ 19 , 90 , 114 , 115 ]. A fluorescence imager is a great tool for early stage technology development since it allows researchers to see the migration of proteins inside a capillary or a microfabricated channel. The imaging area depends on the field of view of the imager but normally it will be about a few millimeters to a few centimeters in diameter.
Mass spectrometers (MS) are excellent detectors, because they are capable of identifying proteins. Coupling CGE with an MS, however, is challenging, because MS does not normally have access to CGE resolved proteins. In addition, the SDS in the sieving matrix interferes severely with MS detection. To address these issues, Lu et al. [ 116 ] developed an approach to couple SDS-CGE with matrix-assisted laser desorption ionization time-of-flight MS (MALDI-TOF-MS). Fig. 7 presents a schematic diagram of the experimental setup for this work. Basically, a PTFE membrane was used to collect CGE-resolved proteins (so that a MS detector will have access to these proteins). [Note: The collected proteins were actually SDS-protein complexes that could not be analyzed directly by MS.] After the collection, the SDS-protein complexes on the membrane were washed using an optimized solution to remove SDS while proteins were retained on the membrane. After SDS removal, a MALDI matrix was introduced onto the membrane for MALDI-TOF-MS analysis.

(a) SDS-CGE setup with membrane collectror; (b) split view of membrane collector. Reprinted from ref. [ 117 ] with permission.
3. Applications
In the literatures we surveyed, a lot of the papers still dealt with standard (or commercially-purchased) proteins (see Table II ). Here, we discuss only a few representative papers closely related to practical applications.
3.1. Proteins in Biological Fluids
Analysis of proteins in biological fluids is challenging due to the complexity of sample media. CGE offers a powerful tool to analyze these samples. In 2000, Lin et al . [ 46 ] used CGE to analyze erythrocyte membrane proteins in blood samples. The erythrocyte membrane samples were extracted from washed red cells, and spectrin in the samples was removed before CGE run. Erythrocyte membrane proteins in normal red cell indices or from healthy blood donors were utilized as controls. The same samples were analyzed by both CGE and SDS-PAGE, and similar profiles were obtained.
In 2008, Obubuafo et al. [ 117 ] analyzed thrombin, an important marker for various hemostasis-related diseases and conditions, by affinity microchip CGE for human plasma samples and also for rabbit plasma sample. The method employed a PMMA microchip and used LPA as sieving matrix. Two fluorescently labeled aptamer affinity probes, HD1 and HD22, which bind respectively to thrombin exosites I and II were investigated. HD22 affinity assays of thrombin produced baseline-resolved peaks with favorable efficiency due to its higher binding affinity, whereas HD1 assays showed poorer resolution of the free aptamer and complex peaks. Therefore, HD22 was selected in determining the level of thrombin in human plasma.
In 2011, Debaugnies et al. [ 118 , 119 ] evaluated an automated CGE system, the Experion instrument from BioRad, for its ability to separate and quantify the erythrocyte membrane proteins. The major erythrocyte membrane proteins were extracted and purified from membrane ghosts by centrifugation, immunoprecipitation and electroelution. Analyses were performed using SDS-PAGE and SDS-CGE to establish a separation profile of the total ghosts. As the SDS-CGE method was able to achieve the same conclusion as with SDS-PAGE, except for the patient with elliptocytosis, Debaugnies et al. concluded that the new SDS-CGE method could be proposed as an automated alternative method to the labor-intensive SDS-PAGE analysis. Kaneta et al. [ 109 ] applied CGE with postcolumn derivatization/LIF detection to analyses of two biological samples, namely a cell lysate and a milk sample.
3.2. Proteins in Food Products
Monitoring food safety and food quality has become increasingly important in recent years. Sample preparations are essential for these analyses. To examine the quality of seafood products, Sotelo et al. [ 120 ] applied CGE for analysis of myofibrillar proteins in fish and squid muscles. A Beckman-Coulter P/ACE 2000 capillary electrophoresis system was used in this work, and the manufacturer recommended procedure was followed. Myosin and actin contents in fish and squid muscles were measured, and these results were comparable to the results from a slab-gel SDS-PAGE system. While the resolving powers of the two methods were comparable, CGE had two significant advantages – automated operations and short separation times. However, P/ACE 2000 could only analyze one sample per run. When a batch of samples was to be analyzed, a technician could run all of them in a slab gel in one run, and the differences between samples were readily recognized by direct lane-to-lane comparisons. If these samples were analyzed serially by CGE, results comparisons were not as straightforward, especially when the reproducibility was poor.
Meat quality can be indicated by the profile and quantity of water-soluble and salt-soluble proteins. Vallejo-Cordoba et al. [ 54 ] employed CGE and analyzed these proteins in bovine and ostrich meats. Briefly, meats were mixed with water or saline buffer (typically, 0.6 M NaCl/0.01 M phosphate buffer, pH 6.0, 0.5% polyphosphates), blended and centrifuged. The filtered supernatant, sample buffer, benzoic acid (as internal standard) and mercaptoethanol were mixed, boiled and then cooled down. Proteins in this sample were injected for CGE analysis. CGE separations were carried out on a Bio-Rad CE system (BioFocus 3000), and the manufacturer recommended protocols were followed. Profiles and concentrations of water-soluble and salt-soluble proteins were measured successfully in this work.
Gomis et al. [ 121 ] analyzed cider proteins and determined their relative molecular masses. Various methods were hired to isolate cider proteins for CGE [ 122 ]. Chiu et al. [ 74 ] described a segmental filling method for analysis of SYPRO Red labeled SDS-proteins by CE-LIF with electroosmotic counterflow of PEO. This method was capable of determining casein in cow’s milk below 0.5 mM.
3.3. Proteins in Agricultural Products
RuBisCo accounts for more than 50% of the soluble protein in chloroplasts and is a key enzyme in the photosynthetic fixation of carbon dioxide [ 123 ]. An accurate measurement of the quantity of RuBisCo subunits would provide an indication of a plant’s physiological status. Nicolas et al. [ 53 ] established a CGE method for analysis of RuBisCo in Spinach leaves. To prepare samples for this method, spinach leaves were freshly harvested and ground in a chilled mortar with a portion of inert sand and some chilled buffer (100 mM Tris–hydrochloride, 0.1 mM EDTA and 1 mM ascorbic acid at pH 8.0). The homogenate was centrifuged, and the supernatant was desalted. This sample was diluted 1:1 with the CE-SDS protein sample buffer (CE-SDS Protein Kit: Bio-Rad, Hercules, CA, USA), and benzoic acid was added as an internal standard (CE-SDS Protein Kit) to a final concentration of 50 µg/mL. After SDS-protein complexes were formed, the sample was ready for analysis. An HP3D capillary electrophoretic system (Hewlett-Packard, Wilmington, DE, USA) was used in the work.
Chen et al. [ 124 ] also analyzed RuBisCo from leaves of Vigna unguiculata. Leaf tissues were ground to a fine powder in liquid nitrogen. Proteins were extracted from leaf tissue at 0–4 °C in 80 mM Tris buffer containing 0.1 M P-mercaptoethanol, 2% (w/v) SDS, and 15% (v/v) glycerol. The extract was centrifuged and the supernatant was used for protein analysis. CGE was performed with a Bio-Rad 3000 system. Proteins in soybean seeds were also analyzed using CGE by Gerber et al. [ 125 ]. Blazek and Caldwell [ 93 ] compared SDS-CGE with the lab-on-a-chip technology to quantify the relative amount of 7S and 11S fractions in twenty different soybean cultivars.
Marchetti-Deschmann et al. [ 126 ] recently evaluated a one-step single-grain wheat extraction process followed by a CGE-on-a-chip analysis for fast and reliable wheat variety control [ 119 ]. Based on the results of 15 different wheat varieties grown in Austria, Marchetti-Deschmann et al. concluded that the CGE-on-a-chip system was a promising alternative for the time-consuming and labor-intensive SDS-PAGE for high-throughput food analysis.
3.4. Proteins in Clinical and Pharmaceutical Studies
Recombinant immunoglobulin G4 (IgG4), as well as other IgG antibodies, is made up of two light chains and two heavy chains. In a normal human IgG4, disulfide bonds are formed between a light chain (L) and a heavy chain (H), and also between two HL dimmers. In an abnormal IgG4, there are no disulfide bonds between HL dimmers (the dimmers are linked together by only noncovalent interactions). Vasilyeva et al. [ 55 ] used an Agilent 2100 Bioanalyzer to quantitate these HL dimmers of abnormal IgG4 in rMAb samples. The microchip method described in this paper was suitable for analyzing samples containing HL from approximately 0.6% to at least 5.2% (may be extended up to 80%). The LOD and limit of quantitation (LOQ) were determined to be 0.05% and 0.59%, respectively. Good correlations were obtained between this method and conventional SDS-PAGE, and between this method and reversed-phase HPLC.
With the increasing therapeutic use of rMAbs, Analyzing the quality and purity of rMAbs becomes an important and routine task for rMAb manufacturers. Hunt and Nashabeh [ 26 ] developed a CGE method for analysis of rMAbs in biopharmaceutical industry. The method included precolumn protein labeling, CGE separation and LIF detection. 5-carboxytetramethylrhodamine succinimidyl ester was used as a labeling reagent. CGE separations were performed on a Bio-Rad BioFocus 3000 CE system equipped with a LIF detector. This method was validated according to the guidelines of the International Committee on Harmonization and had been used as part of a control system for the release of an rMAb pharmaceutical in Genentech, Inc. This method was optimized recently [ 30 ].
Guo et al . [ 52 ] developed a non-reduced SDS-CGE method and used it to study disulfide heterogeneity in IgG2 antibodies. This method was proved to be a powerful tool to get information on the backbone structure of IgG molecules. Zhang et al. [ 48 ] optimized a similar method to analyze mAb1 drug substance under both reduced and non-reduced conditions. Lancher et al. [ 127 ] established a generic method for monitoring disulfide isomer heterogeneity in IgG2 antibodies, and applied this method for purity analysis of reduced and non-reduced IgG2 mAbs [ 128 ]. Rustandi et al. [ 129 ] reported a wide range of applications of CGE for mAb product development, including purity analyses for product release, product-related impurities during process and cell-culture development, and product stability evaluation. Cherkaoui et al. [ 130 ] used CGE to evaluate the IgG structural integrity under various reduction conditions and track antibody reduction fragments.
Carbonyl-modified proteins are considered markers of oxidative damage in aged tissues and diseases such as Parkinson’s, diabetes, emphysema, and atherosclerosis [ 131 , 132 ]. Feng et al. [ 133 ] developed a carbonyl detection method based on the reaction of Alexa 488 hydrazide with carbonyls and on the separation of the Alexa 488-labeled compounds by CGE with a sheath flow cuvette. Because carbonyls on lipids, carbohydrates, and nucleic acids could also react with Alexa 488 [ 134 ], yielding products that would interfere with the detection of carbonyl-modified proteins, the Alexa 488-labeled proteins were further labeled with another fluorogenic reagent – FQ. FQ only reacted with proteins, and its fluorescence showed little spectral overlap with that of Alexa 488. Therefore, protein peaks with fluorescence characteristics of both Alexa 488 and FQ belonged to carbonylated proteins. The method was adequate for analyzing nanogram protein samples with femtomole levels of carbonyls.
Mellado et al. [ 135 ] described an application of CGE for the analysis of rotavirus virus-like particles. Particle’s apparent molecular masses and quantities were determined, and these results were validated by comparing them with those obtained from traditional SDS-PAGE and MALDI-TOF-MS.
4. Conclusions
In conclusion, CGE is a powerful tool for protein analysis. Automated operation and short separation times are two most significant advantages of CGE over conventional slab gel electrophoresis. Reproducibility is still a shortcoming for CGE, although a lot of progress has been made. Currently, CGE separations are performed usually in series, which makes lane-to-lane comparisons not as convenient as in multilane slab gel electrophoresis [ 120 , 136 ]. Microchip CGE is a promising platform for high speed protein analysis. At the time being, however, most practical applications have been conducted using capillary-based systems. While UV absorption detection is still a popular detection scheme for CGE, LIF detection is gaining a lot of ground. The reason might be that reliable and affordable fluorescence labeling dyes are commercially available, and that multiple labeling is less an issue for CGE. CGE has been used as a separation dimension for 2D separations, but so far the resolving power of these schemes could not compete with that of conventional 2D gels. In terms of practical application, CGE has already been utilized for quality control and purity test of monoclonal antibody products. Other imminent applications include clinical diagnosis, food quality monitoring, etc. We expect CGE to be an important analytical technique in all these areas in the near future.[ 50 , 117 , 137 – 147 ]
Acknowledgement
This work is partially supported by NIH through grant RO1 GM078592, NSF through grant CHE 1011957, Department of Energy (SC0006351), and OCAST.
Biographies
Mr. Zaifang Zhu earned his bachelor’s degree of Science in chemistry from Lanzhou University (Lanzhou, P. R. China). He is currently a Ph.D student in the Department of Chemistry and Biochemistry at the University of Oklahoma. His research is on exploiting capillary-based systems for bioanalysis.
Ms. Joann J. Lu received her Master’s degree from Texas Tech University in 1994. Ms. Lu worked as a research associate and scientist at Bayor in West Heaven, Connecticut, Inhale Therapeutic in San Carlos, California, and Oculex Pharmaceuticals in Sunnyvale, California. She is now a Research Scientist in the Department of Chemistry and Biochemistry at University of Oklahoma. Her research is focused on protein analysis.
Professor Shaorong Liu received his Ph.D. degree from Texas Tech University in 1995. After worked as a postdoctoral fellow at Northeastern University in 1996 and University of California at Berkeley in 1997, he joined Molecular Dynamics in Sunnyvale, California as a Scientist in 1998 and Manager of Technology Development in 2000. Dr. Liu joined Texas Tech University as an Associate Professor in 2002, and Professor in 2007. Since 2008, Dr. Liu is a Professor in the Department of Chemistry and Biochemistry at University of Oklahoma. His research is focused capillary electrophoresis and microfluidic devices for high-speed and high-throughput bioanalysis.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
SDS-PAGE and Western Blotting: Basic Principles and Protocol
- First Online: 10 May 2022
Cite this protocol

- Mukesh Bhatt 5 ,
- Vishal Rai 6 ,
- Ashok Kumar 6 ,
- Ajay Kumar Yadav 6 ,
- Kaushal Kishor Rajak 6 ,
- Vikas Gupta 7 ,
- Vishal Chander 6 &
- R. K. Avasthe 5
Part of the book series: Springer Protocols Handbooks ((SPH))
1855 Accesses
2 Citations
Western blotting is an important analytical technique used in cell and molecular biology for last four decades. It involves separation of proteins in SDS-PAGE and then transfer of proteins to a membrane followed by detection. By using a western blot, one can identify specific protein from a complex mixture of proteins. Along with its use as a diagnostic aid, it can also be used to verify proteins of interest in exploratory proteomic studies to identify different disease mechanisms. The ease of performing the technique, low cost, and accessibility further support the use of western blot in proteomic research. However, a good understanding, initial training, and optimization are of utmost importance because being a multi-step technique, it is prone to false results and incorrect interpretation. This chapter attempts to explain the technique and theory behind western blot along with some ways to troubleshoot.
This is a preview of subscription content, log in via an institution to check access.
Access this chapter
Subscribe and save.
- Get 10 units per month
- Download Article/Chapter or eBook
- 1 Unit = 1 Article or 1 Chapter
- Cancel anytime
- Available as PDF
- Read on any device
- Instant download
- Own it forever
- Available as EPUB and PDF
- Compact, lightweight edition
- Dispatched in 3 to 5 business days
- Free shipping worldwide - see info
- Durable hardcover edition
Tax calculation will be finalised at checkout
Purchases are for personal use only
Institutional subscriptions
Similar content being viewed by others

2D SDS PAGE in Combination with Western Blotting and Mass Spectrometry Is a Robust Method for Protein Analysis with Many Applications

Proteomics: Tools of the Trade

Determination of Protein Molecular Weights on SDS-PAGE
Towbin H, Staehelin T, Gordon J (1979) Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A 76(9):4350
Article CAS Google Scholar
Burnette WN (1981) “Western blotting”: electrophoretic transfer of proteins from sodium dodecyl sulfate—polyacrylamide gels to unmodified nitrocellulose and radiographic detection with antibody and radioiodinated protein A. Anal Biochem 112(2):195–203
Southern EM (1975) Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol 98(3):503–517
Alwine JC, Kemp DJ, Stark GR (1977) Method for detection of specific RNAs in agarose gels by transfer to diazobenzyloxymethyl-paper and hybridization with DNA probes. Proc Natl Acad Sci U S A 74(12):5350–5354
Gwozdz T, Dorey K (2017) Western blot. In: Basic science methods for clinical researchers. Academic, Boca Raton, pp 99–117
Chapter Google Scholar
Counts SE (2010) Western blot. In: Encyclopedia of movement disorders, vol 2010. Academic, pp 323–326 . ISBN 9780123741059. https://doi.org/10.1016/B978-0-12-374105-9.00297-5
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193(1):265–275
Smith PK, Krohn RI, Hermanson GT, Mallia AK, Gartner FH, Provenzano MD et al (1985) Measurement of protein using bicinchoninic acid. Anal Biochem 150(1):76–85
Kurien BT, Scofield RH (2015) Western blotting: an introduction. Methods Mol Biol 1312:17–30. https://doi.org/10.1007/978-1-4939-2694-7_5 . PMID: 26043986; PMCID: PMC7304528
Article CAS PubMed PubMed Central Google Scholar
Wilson K, Walker JM, Hofmann A, Clokie S (2018) Wilson and Walker’s principles and techniques of biochemistry and molecular biology. Cambridge University Press, New York, NY
Google Scholar
Kurien BT, Scofield RH (2009) Protein blotting and detection: methods and protocols. Humana Press, New York
Book Google Scholar
Kattoor JJ, Saurabh S, Malik YS, Sircar S, Dhama K, Ghosh S, Bányai K, Kobayashi N, Singh RK (2017) Unexpected detection of porcine rotavirus C strains carrying human origin VP6 gene. Vet Q 37(1):252–261. https://doi.org/10.1080/01652176.2017.1346849
Article PubMed Google Scholar
Todd D, McNulty MS, Allan GM (1984) The use of polyacrylamide gel electrophoresis of virus RNA in the study of rotavirus infections. In: McNulty MS, McFerran JB (eds) Recent advances in virus diagnosis. Current topics in veterinary medicine and animal science, vol 29. Springer, Dordrecht. https://doi.org/10.1007/978-94-009-6039-8_11
Urzainqui A, Tabarés E, Carrasco L (1987) Proteins synthesized in African swine fever virus-infected cells analyzed by two-dimensional gel electrophoresis. Virology 160(1):286–291. https://doi.org/10.1016/0042-6822(87)90076-6
Article CAS PubMed Google Scholar
Bhatt M, Mohapatra JK, Pandey LK, Mohanty NN, Das B, Prusty BR, Pattnaik B (2018) Mutational analysis of foot and mouth disease virus nonstructural polyprotein 3AB-coding region to design a negative marker virus. Virus Res 243:36–43. https://doi.org/10.1016/j.virusres.2017.10.010
Greenlee JJ, Kunkle RA, Smith JD, Greenlee MHW (2016) Scrapie in swine: a diagnostic challenge. Food Saf 4(4):110–114. https://doi.org/10.14252/foodsafetyfscj.2016019 . PMID: 32231914; PMCID: PMC6989210
Article Google Scholar
Hedman C, Otero A, Douet JY, Lacroux C, Lugan S, Filali H, Corbière F, Aron N, Badiola JJ, Andréoletti O, Bolea R (2018) Detection of PrPres in peripheral tissue in pigs with clinical disease induced by intracerebral challenge with sheep-passaged bovine spongiform encephalopathy agent. PLoS One 13(7):e0199914. https://doi.org/10.1371/journal.pone.0199914
Ameri M, Zhou EM, Hsu WH (2006) Western blot immunoassay as a confirmatory test for the presence of anti-mycoplasma hyopneumoniae antibodies in swine serum. J Vet Diagn Investig 18(2):198–201. https://doi.org/10.1177/104063870601800210
Plotzki E, Keller M, Ivanusic D, Denner J (2016) A new western blot assay for the detection of porcine cytomegalovirus (PCMV). J Immunol Methods 437:37–42. https://doi.org/10.1016/j.jim.2016.08.001
Yang S, Li L, Yin S et al (2018) Single-domain antibodies as promising experimental tools in imaging and isolation of porcine epidemic diarrhea virus. Appl Microbiol Biotechnol 102:8931–8942. https://doi.org/10.1007/s00253-018-9324-7
Download references
Author information
Authors and affiliations.
ICAR Research Complex for NEH Region, Sikkim Centre, Gangtok, Sikkim, India
Mukesh Bhatt & R. K. Avasthe
ICAR-IVRI, Bareilly, UP, India
Vishal Rai, Ashok Kumar, Kiran, Ajay Kumar Yadav, Kaushal Kishor Rajak & Vishal Chander
National Institute of Animal Health, Baghpat, UP, India
Vikas Gupta
You can also search for this author in PubMed Google Scholar
Editor information
Editors and affiliations.
National Research Centre on Pig, Indian Council of Agricultural Research, Guwahati, Assam, India
Ajay Kumar Yadav
Swaraj Rajkhowa
College of Animal Biotechnology, Guru Angad Dev Veterinary and Animal Sciences University, Ludhiana, Punjab, India
Yashpal Singh Malik
Rights and permissions
Reprints and permissions
Copyright information
© 2022 The Author(s), under exclusive license to Springer Science+Business Media, LLC, part of Springer Nature
About this protocol
Bhatt, M. et al. (2022). SDS-PAGE and Western Blotting: Basic Principles and Protocol. In: Deb, R., Yadav, A.K., Rajkhowa, S., Malik, Y.S. (eds) Protocols for the Diagnosis of Pig Viral Diseases. Springer Protocols Handbooks. Humana, New York, NY. https://doi.org/10.1007/978-1-0716-2043-4_23
Download citation
DOI : https://doi.org/10.1007/978-1-0716-2043-4_23
Published : 10 May 2022
Publisher Name : Humana, New York, NY
Print ISBN : 978-1-0716-2042-7
Online ISBN : 978-1-0716-2043-4
eBook Packages : Springer Protocols
- Publish with us
Policies and ethics
- Find a journal
- Track your research
Maintenance work is planned from 21:00 BST on Sunday 18th August 2024 to 21:00 BST on Monday 19th August 2024, and on Thursday 29th August 2024 from 11:00 to 12:00 BST.
During this time the performance of our website may be affected - searches may run slowly, some pages may be temporarily unavailable, and you may be unable to log in or to access content. If this happens, please try refreshing your web browser or try waiting two to three minutes before trying again.
We apologise for any inconvenience this might cause and thank you for your patience.

Metallomics
Native sds-page: high resolution electrophoretic separation of proteins with retention of native properties including bound metal ions.
* Corresponding authors
a Department of Chemistry and Biochemistry, University of Wisconsin-Milwaukee, 3210 N Cramer Street, Milwaukee, WI 53201, USA E-mail: [email protected] Tel: +1 414-229-5853
Sodium dodecyl-sulfate polyacrylamide gel electrophoresis (SDS-PAGE) is commonly used to obtain high resolution separation of complex mixtures of proteins. The method initially denatures the proteins that will undergo electrophoresis. Although covalent structural features of resolved proteins can be determined with SDS-PAGE, functional properties are destroyed, including the presence of non-covalently bound metal ions. To address this shortcoming, blue-native (BN)-PAGE has been introduced. This method retains functional properties but at the cost of protein resolving power. To address the need for a high resolution PAGE method that results in the separation of native proteins, experiments tested the impact of changing the conditions of SDS-PAGE on the quality of protein separation and retention of functional properties. Removal of SDS and EDTA from the sample buffer together with omission of a heating step had no effect on the results of PAGE. Reduction of SDS in the running buffer from 0.1% to 0.0375% together with deletion of EDTA also made little impact on the quality of the electrophoretograms of fractions of pig kidney (LLC-PK 1 ) cell proteome in comparison with that achieved with the SDS-PAGE method. The modified conditions were called native (N)SDS-PAGE. Retention of Zn 2+ bound in proteomic samples increased from 26 to 98% upon shifting from standard to modified conditions. Moreover, seven of nine model enzymes, including four Zn 2+ proteins that were subjected to NSDS-PAGE retained activity. All nine were active in BN-PAGE, whereas all underwent denaturation during SDS-PAGE. Metal retention after electrophoresis was additionally confirmed using laser ablation-inductively coupled plasma-mass spectrometry and in-gel Zn-protein staining using the fluorophore TSQ.

Article information
Download citation, author version available, search articles by author, advertisements.
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Open access
- Published: 09 October 2015
SDS-PAGE analysis of Aβ oligomers is disserving research into Alzheimer´s disease: appealing for ESI-IM-MS
- Rosa Pujol-Pina 1 na1 ,
- Sílvia Vilaprinyó-Pascual 1 na1 ,
- Roberta Mazzucato 1 na1 ,
- Annalisa Arcella 2 na1 ,
- Marta Vilaseca 3 na1 ,
- Modesto Orozco 2 , 4 na1 &
- Natàlia Carulla 1 na1
Scientific Reports volume 5 , Article number: 14809 ( 2015 ) Cite this article
19k Accesses
88 Citations
87 Altmetric
Metrics details
- Alzheimer's disease
- Supramolecular assembly
The characterization of amyloid-beta peptide (Aβ) oligomer forms and structures is crucial to the advancement in the field of Alzheimer´s disease (AD). Here we report a critical evaluation of two methods used for this purpose, namely sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE), extensively used in the field and ion mobility coupled to electrospray ionization mass spectrometry (ESI-IM-MS), an emerging technique with great potential for oligomer characterization. To evaluate their performance, we first obtained pure cross-linked Aβ40 and Aβ42 oligomers of well-defined order. Analysis of these samples by SDS-PAGE revealed that SDS affects the oligomerization state of Aβ42 oligomers, thus providing flawed information on their order and distribution. In contrast, ESI-IM-MS provided accurate information, while also reported on the chemical nature and on the structure of the oligomers. Our findings have important implications as they challenge scientific paradigms in the AD field built upon SDS-PAGE characterization of Aβ oligomer samples.
Similar content being viewed by others

Conformational strains of pathogenic amyloid proteins in neurodegenerative diseases

Limited proteolysis–mass spectrometry reveals aging-associated changes in cerebrospinal fluid protein abundances and structures

Aggregation and Cellular Toxicity of Pathogenic or Non-pathogenic Proteins
Introduction.
Aβ oligomers, species that form early during Aβ aggregation, are considered the pathogenic molecular form of Aβ in AD 1 . Consequently, they have been singled out as a target to treat this disease 2 . However, the characterization of Aβ oligomers is challenging because they are heterogeneous—comprising a range of aggregation states—and because they form transiently—evolving as a function of time 3 , 4 . In spite of these difficulties, different approaches and techniques have been developed to characterize them 5 , 6 , 7 , 8 , 9 , 10 , 11 , 12 . One such approach relies on the production of cross-linked oligomers by means of the photo-induced cross-linking of unmodified proteins (PICUP) reaction and their subsequent analysis by SDS-PAGE ( Fig. 1 ) 5 , 13 . Aβ1-40 (Aβ40) and Aβ1-42 (Aβ42) are the two principal forms of Aβ, differing by only two hydrophobic residues at the C-terminus. Analysis of PICUP cross-linked Aβ40 and Aβ42 samples by SDS-PAGE revealed that Aβ40 oligomerizes through dimers up to tetramers while Aβ42 does so mainly through pentamers and hexamers 5 , 13 . Since Aβ42 has been shown to have a more prominent role in AD 14 , 15 , PICUP/SDS-PAGE analysis led to the conclusion that pentamers and hexamers constituted the basic building blocks for Aβ aggregation 5 , 13 .
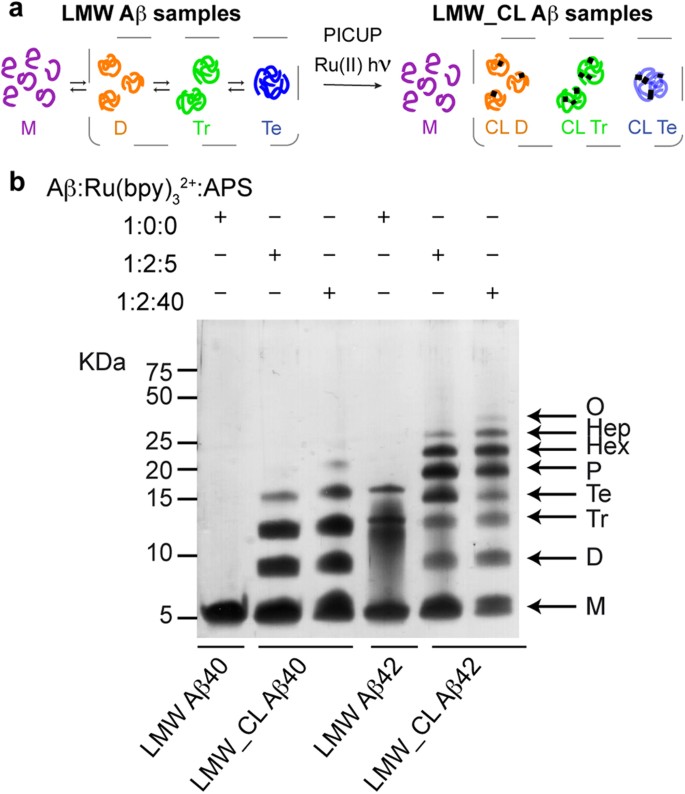
PICUP and SDS-PAGE analysis apparently indicate that Aβ40 and Aβ42 oligomerize through distinct pathways.
( a ) LMW Aβ samples contain monomers in equilibrium with low order Aβ oligomers (left). Synthetic LMW_CL Aβ samples are obtained by subjecting their LMW counterparts to PICUP (right). ( b ) Characterization of LMW and LMW_CL Aβ40 and Aβ42 oligomer distribution by SDS-PAGE analysis. LMW_CL Aβ40 and Aβ42 samples were prepared using two different Aβ/Ru(bpy) 3 2+ /APS ratios, namely 1:2:5 and 1:2:40.
The importance of pentamers and hexamers in Aβ aggregation has become a scientific paradigm in the field. For example, according to the Web of Science, there are six key papers 7 , 8 , 9 , 16 , 17 , 18 , frequently cited together (more than one thousand times), that constitute the foundational core for the research front entitled “Aβ oligomers, fibrils and AD”. Three of these six papers are based directly 16 , 18 or indirectly 7 on the conclusions derived from PICUP/SDS-PAGE analysis. However, SDS-PAGE analysis might be biased since SDS has been suggested to affect the oligomerization state of Aβ, especially that of Aβ42 8 , 19 , 20 . Given the potential artifacts of SDS, it is critical to evaluate the usefulness of SDS-PAGE to characterize Aβ oligomers to have a criteria with which to revise the conclusions derived from its use. For the purpose of this evaluation, it is important to consider new techniques that are not dependent on SDS. ESI-IM-MS is an emerging method with great potential for the characterization of oligomers 7 , 10 , 21 , 22 , 23 , 24 . ESI-IM-MS offers the possibility to resolve heterogeneous oligomer samples on the basis of differences in the order and/or in the structure of the oligomers present in a sample. Moreover, the time scale of IM experiments (tens of ms) is much shorter than that of conventional structural techniques (s to hrs), thus making them ideal for the structural characterization of heterogeneous and dynamic samples such as Aβ oligomers. Furthermore, since ESI-MS allows the preservation of many non-covalent interactions within complexes 25 , even several involving a reduced interaction surface 26 , 27 , 28 , 29 , 30 , ESI-IM-MS emerges as a suitable method for the characterization of not only cross-linked oligomers but also non-covalent ones.
Here we provide a critical evaluation of SDS-PAGE and ESI-IM-MS for the characterization of Aβ oligomers. To this end, we first developed a protocol to obtain pure PICUP cross-linked (CL) Aβ40 and Aβ42 oligomers of defined order. Using these samples, we unequivocally established that SDS-PAGE leads to artifacts in determining the order and distribution of Aβ42 oligomers On the other hand, ESI-IM-MS was further established as a reliable technique through which to characterize the order, distribution, chemical modifications and structure of both covalently and non-covalently linked Aβ oligomers. Our results have important implications as they challenge previous scientific paradigms in the field built upon results obtained through the SDS-PAGE characterization of Aβ oligomers. In this context, we demonstrate that pentamers and hexamers are artifacts of SDS-PAGE analysis. Moreover, we identify Aβ40 and Aβ42 dimers and trimers, adopting a globular structure and lacking defined secondary structure, as the earliest forms to be considered in the design of therapeutic strategies targeting Aβ oligomerization.
PICUP and SDS-PAGE apparently indicate that Aβ40 and Aβ42 oligomerize through distinct pathways
Following previously described protocols 31 , 32 , we obtained Aβ40 and Aβ42 samples in their lowest aggregation state using size exclusion chromatography (SEC). We refer to these samples as low molecular weight (LMW) Aβ40 and Aβ42 ( Fig. 1a ), as various techniques have shown that they comprise monomers in rapid equilibrium with low order oligomers 31 . To freeze this dynamic equilibrium, LMW Aβ samples were cross-linked by means of PICUP 5 , 13 . We refer to these samples as LMW cross-linked (LMW_CL) Aβ40 and Aβ42 ( Fig. 1a ). Initially, we used previously described conditions—an Aβ/tris(bipyridyl) Ru(II) complex (Ru(bpy) 3 2+ )/ammonium persulfate (APS) ratio of 1:2:40—to obtain LMW_CL Aβ40 and Aβ42 5 , 13 , 33 . Analysis of these samples by SDS-PAGE and silver staining reproduced previous results described in the literature ( Fig. 1b ). Uncross-linked LMW Aβ40 ran with a molecular weight consistent with that of monomers. LMW_CL Aβ40 presented an oligomer distribution characterized by monomers through to pentamers—displaying decreasing intensity with increasing oligomer order. Uncross-linked Aβ42 produced predominantly three bands consistent with monomers, trimers and tetramers. LMW_CL Aβ42 ran as monomers through to trimers—displaying decreasing intensity with increasing oligomer order—and as a Gaussian-like distribution comprising tetramers through to octamers, with a maximum at pentamers and hexamers. These results apparently support the hypothesis from Teplow and co-workers that Aβ40 and Aβ42 oligomerize through distinct pathways 5 , 13 .
To determine the homogeneity of the LMW_CL Aβ samples, we analyzed LMW_CL Aβ40 by reversed phase high-pressure liquid chromatography (RP-HPLC) and LC coupled to high resolution MS (LC-HRMS). This analysis revealed that an Aβ/Ru(bpy) 3 2+ /APS ratio of 1:2:40 led to various degrees of oxidized byproducts ( Supplementary Fig. S1a and Table S1 ). We then optimized PICUP conditions and found that an Aβ/Ru(bpy) 3 2+ /APS ratio of 1:2:5 largely overcame the formation of these byproducts ( Supplementary Fig. S1b,c ). Although the optimized conditions led to a lower yield of cross-linked oligomers ( Fig. 1b ), they were used throughout our study since they ensured chemically well-defined CL Aβ oligomers.
Aβ42 pentamers and hexamers are artifacts of SDS-PAGE analysis
Various studies have suggested that SDS affects the oligomerization state of Aβ samples 8 , 19 , 20 . To address this possibility, we sought to determine the oligomer order and distribution of LMW_CL Aβ samples using a new strategy that involved the same principles as those used in SDS-PAGE, that is, denaturation/disaggregation followed by size fractionation, but without using SDS. Denaturation/disaggregation was accomplished by lyophilizing LMW and LMW_CL Aβ40 and Aβ42 samples and later resuspending them in 6.8 M guanidine thiocyanante (GdnHSCN), conditions used to solubilize plaque cores 34 . Therefore, treatment of LMW and LMW_CL Aβ samples with 6.8 M GdnHSCN should break all non-covalent Aβ-Aβ interactions preserving only the covalent ones formed during the cross-linking reaction. Size fractionation of the resulting oligomers was attained by SEC using 10 mM ammonium acetate at pH 8.5 as the elution buffer. This buffer was chosen because it prevented aggregation of the samples during SEC fractionation. After this treatment, referred to as GdnHSCN-SEC analysis, LMW Aβ40 and Aβ42 eluted as a single peak ( Fig. 2a,b ), confirming that GdnHSCN broke all the non-covalent interactions and that aggregation was prevented during SEC fractionation. In contrast, LMW_CL Aβ40 and Aβ42 samples eluted as four main peaks ( Fig. 2c,d ). ESI-MS analysis confirmed that the peaks eluting at 39.5, 34.7 and 32.6 mL in LMW_CL Aβ40 and at 38.4, 33.5 and 31.6 mL in LMW_CL Aβ42 samples corresponded to “monomers”, CL dimers and CL trimers of Aβ40 ( Fig. 2e ) and Aβ42 ( Fig. 2f ), respectively. Although peaks eluting at 31.3 mL in LMW_CL Aβ40 ( Fig. 2c ) and at 30.5 mL in LMW_CL Aβ42 ( Fig. 2d ) samples were low in abundance, ESI-MS analysis revealed the presence of +8 and +7 charge states corresponding to tetramers. Of note, ESI-MS analysis of peaks corresponding to “monomers” in LMW and LMW_CL Aβ samples also showed that they contained charge states corresponding to non-covalent dimers and trimers ( Fig. 2e,f ). These observations are consistent with previous results indicating that Aβ monomers exist in rapid equilibrium with low order Aβ oligomers ( Fig. 1a ) 31 . Altogether, GdnHSCN-SEC analyses indicated that the oligomer distribution for LMW_CL Aβ40 and Aβ42 samples was the same, comprising dimers, trimers and tetramers ( Fig. 2c,d ). This result is inconsistent with that obtained when the same samples were analyzed by SDS-PAGE ( Fig. 1b ), thus suggesting that SDS affects Aβ oligomerization, particularly that of Aβ42.
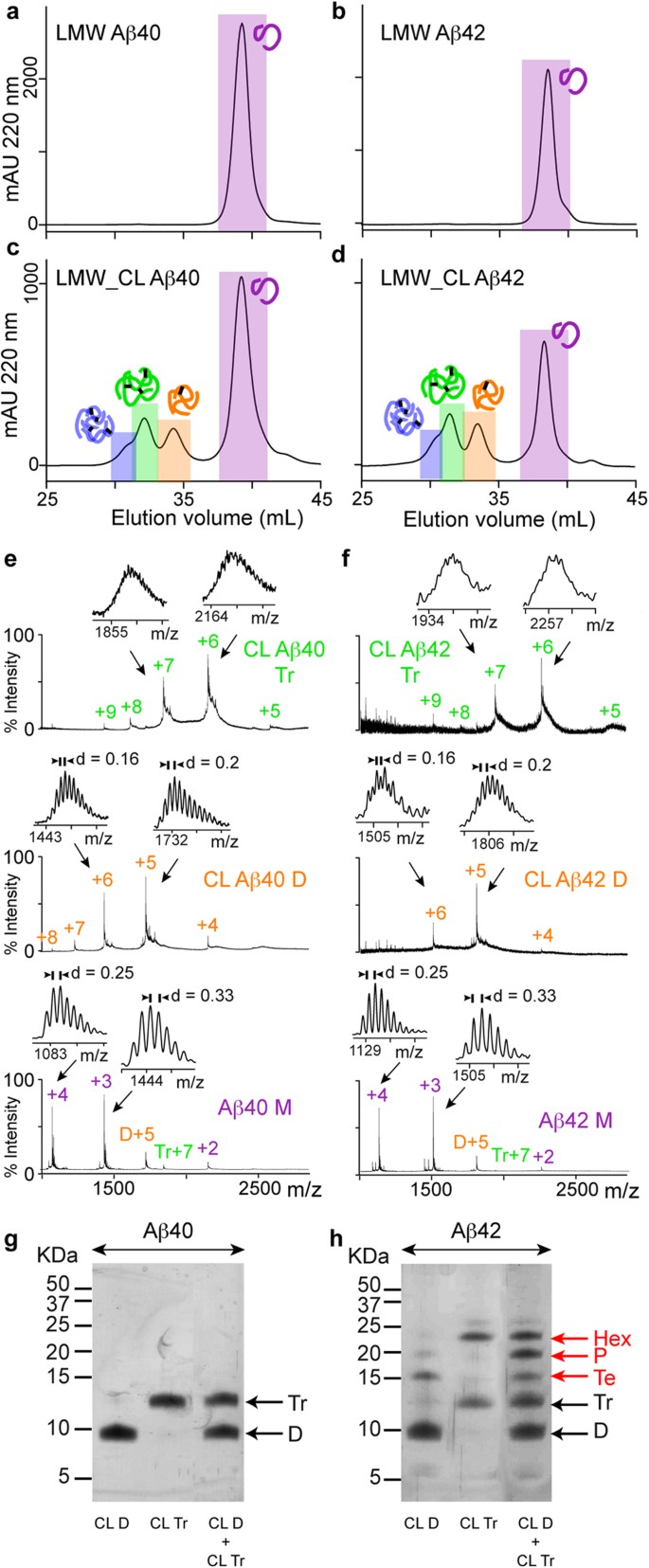
Aβ42 pentamers and hexamers are artifacts of SDS-PAGE analysis.
GdnHSCN-SEC analysis of ( a ) LMW Aβ40, ( b ) LMW Aβ42, ( c ) LMW_CL Aβ40 and ( d ) LMW_CL Aβ42. ESI-MS spectra corresponding to peaks detected after GdnHSCN-SEC analysis of ( e ) LMW_CL Aβ40 and ( f ) LMW_CL Aβ42 samples. SDS-PAGE analysis of isolated CL dimers and trimers, as well as mixtures of them for ( g ) Aβ40 and ( h ) Aβ42 obtained after GdnHSCN-SEC fractionation. M = monomers, D = dimers, Tr = trimers, Te = tetramers, P = pentamers and Hx = hexamers. The red arrows indicate oligomers formed artifactually in the presence of SDS.
Having access to pure synthetic CL Aβ oligomer samples of well-defined order offered us a unique opportunity to study the effect of SDS on Aβ oligomerization. We analyzed isolated CL Aβ40 and Aβ42 dimers and trimers as well as mixtures of them, obtained after GdnHSCN-SEC, by SDS-PAGE. CL Aβ40 dimers and trimers ran as expected ( Fig. 2g ). However, CL Aβ42 dimers run also as tetramers, CL Aβ42 trimers also as hexamers and mixtures of CL Aβ42 dimers and trimers also as tetramers, pentamers and hexamers ( Fig. 2h ). These findings have two important implications: i) SDS is responsible for affecting the oligomerization state of Aβ42 oligomers. In fact, the temperature and incubation time prior to SDS-PAGE analysis did not have an effect in the oligomerization state of the samples ( Supplementary Fig. S2 ). Consequently, SDS-PAGE is not a reliable technique to characterize the order and distribution of Aβ oligomers present in a sample; ii) they challenge the widely accepted view that pentamers and hexamers are the basic building blocks for Aβ aggregation.
ESI-IM-MS analysis reveals that Aβ40 and Aβ42 predominantly oligomerize through dimers and trimers
Given that SDS-PAGE analysis leads to artifacts in the characterization of Aβ oligomers, we consider it critical to establish techniques that can provide reliable results. As detailed in the introduction, ESI-IM-MS is an emerging technique with great potential to characterize the oligomers present in a sample 7 , 10 , 22 , circumventing the problems associated with the use of SDS 8 , 19 , 20 . To evaluate the feasibility of ESI-IM-MS for the characterization of Aβ oligomers, we next studied LMW and LMW_CL Aβ40 and Aβ42 samples in 10 mM ammonium acetate at pH 8.5 ( Fig. 3 and Supplementary Figs S3–S5 ). We began ESI-IM-MS analysis by first assigning all the peaks in each spectrum to specific Aβ oligomers (see Supplementary Text ). Briefly, assignments were performed on the basis of the 13 C isotope distribution ( Fig. 3b,c and Supplementary Figs S3b,c–S5b,c ), as proposed in the literature 10 . Whenever resolution was not enough, assignment was carried out by the detection of at least two consecutive charge states for a specific Aβ oligomer ( Fig. 3a and Supplementary Figs S3a–S5a ). The LMW and LMW_CL Aβ40 and Aβ42 samples ranged predominantly from monomers to trimers, although low intensity charge states for tetramers, pentamers and hexamers were also detected (M = monomers, D = dimers, Tr = trimers, Te = tetramers, P = pentamers and Hx = hexamers) ( Fig. 3a and Supplementary Figs S3a–S5a ). Next, we determined the oligomer distribution in each sample by using all charge state ions across the whole spectrum ( Fig. 3d ). There were no significant differences between the oligomer distribution of Aβ40 and Aβ42 when compared in the LMW or LMW_CL samples. Hence, we concluded that the oligomer distribution for Aβ40 and Aβ42 was the same, comprising mainly dimers and trimers and that PICUP provided an accurate snapshot of this distribution. Noticeably, the ESI-IM-MS oligomer distribution was consistent with the GdnHSCN-SEC analysis ( Supplementary Fig. S6 ), thereby supporting the reliability of ESI-IM-MS for the characterization of Aβ oligomers. Furthermore, in contrast to GdnHSCN-SEC analysis, ESI-IM-MS allowed detection of low abundant oligomers such as pentamers and hexamers and characterization of the oligomer order and distribution of non-covalently linked oligomers.
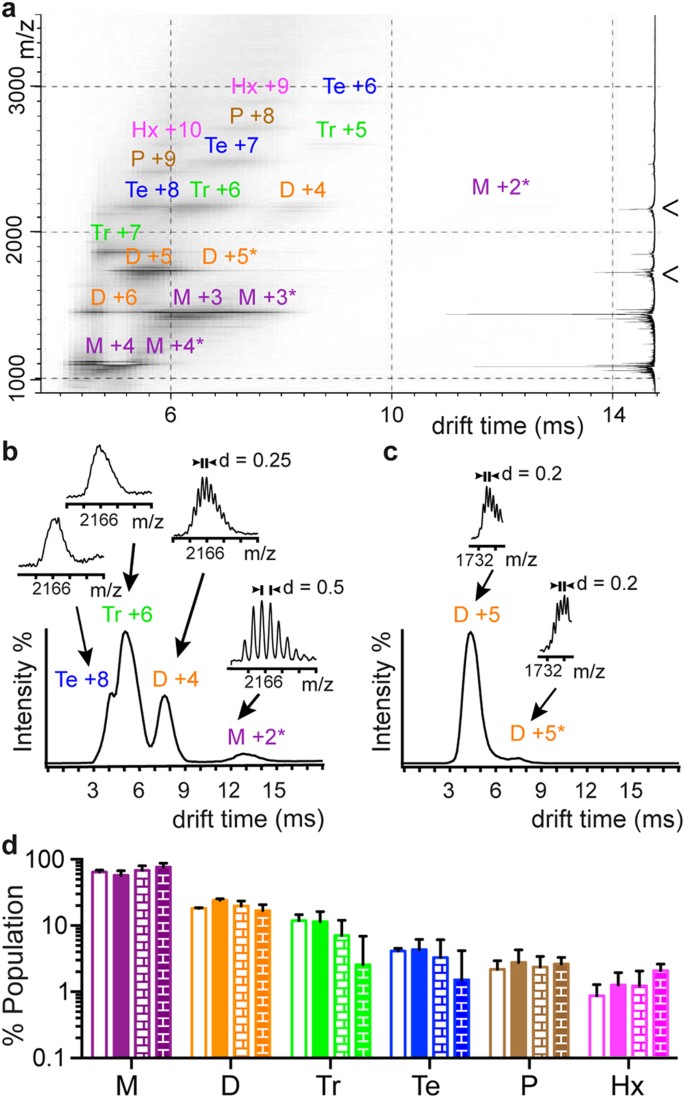
ESI-IM-MS analysis reveals that Aβ40 and Aβ42 oligomerize predominantly through dimers and trimers.
( a ) ESI-IM-MS spectra for LMW Aβ40. M = monomers, D = dimers, Tr = trimers, Te = tetramers, P = pentamers and Hx = hexamers. The number adjacent to each aggregation state refers to the charge state of the ion. The summed m/z spectrum is shown on the right. Projections of the ESI-IM-MS spectra on the drift time axis for ( b ) m/z 2166 (M +2, D +4, Tr +6 and Te +8) and ( c ) m/z 1732 (D +5), both indicated by an arrowhead in ( a ). For each of the mobility peaks detected, their associated m/z spectrum is also shown. Peaks were assigned following the considerations described in the Supplementary Text . ESI-IM-MS analysis of LMW_CL Aβ40, LMW Aβ42 and LMW_CL Aβ42 samples is shown in Supplementary Figs S3–S5 , respectively. Relative population of M (purple), D (orange), Tr (green), Te (blue), P (brown) and Hx (pink) in LMW Aβ40 (empty bars), LMW Aβ42 (filled bars), LMW_CL Aβ40 (empty brick pattern bars) and LMW_CL Aβ42 (filled bricked pattern bars) samples obtained from ( d ) the intensity counts of all charge states detected in ESI-IM-MS spectra.
Aβ40 and Aβ42 dimers and trimers adopt a globular shape devoid of defined secondary structure
In addition to characterizing the Aβ oligomer order and distribution, ESI-IM-MS has the potential to identify chemical modifications, as well as to provide dynamic and structural information on the oligomers present in the sample. As per the characterization of chemical modifications, ESI-IM-MS analysis revealed that the mass of LMW_CL Aβ oligomers is consistent with the expected loss of two hydrogens for each covalent bond formed in the PICUP reaction ( Supplementary Fig. S7 ) 35 .
To establish the effect of cross-linking on Aβ oligomer dynamic, we measured the line width of the mobility peaks associated with specific Aβ oligomers. The line width corresponding to non-covalent Aβ oligomers in LMW samples was significantly larger than that of CL ones in LMW_CL samples ( Supplementary Fig. S8 ). This finding indicated that during the mobility experiment each of the detected non-covalent Aβ oligomer sampled more conformations than their corresponding CL counterparts. A result consistent with the general expectation that cross-linking, which entails the formation of covalent bonds, reduces the number of accessible conformations.
Regarding structural information, ESI-IM-MS experiments can provide collisional cross-section (Ω) values 8 , 11 , 22 , 23 , 24 , which are key structural constraints related to oligomer size and shape. We determined Ω for the most abundant Aβ oligomers detected in the LMW and LMW_CL Aβ40 and Aβ42 samples ( Fig. 4a and Supplementary Table 2 ) and compared the same type of oligomer in the four samples. First, we compared the same type of oligomer in the LMW Aβ40 and Aβ42 samples and no significant differences in drift time ( Supplementary Fig. S8a ) or Ω values ( Fig. 4a ) were found, thereby indicating that they were similar. Next, we carried out, the same comparison for the LMW_CL Aβ40 and Aβ42 samples and found significant differences in drift time ( Supplementary Fig. S8a ) and Ω values ( Fig. 4a ). This result was not surprising since cross-linking reduces the conformational dynamics of Aβ oligomers ( Supplementary Fig. S8 ) so we expected that differences in shape would be easier to distinguish among the CL oligomers than among the non-covalent ones. However, although this finding might indicate that LMW_CL Aβ42 oligomers have a more extended structure than their Aβ40 counterparts, the difference observed was consistent with the fact that Aβ42 has two more amino acids than Aβ40 ( Supplementary Fig. S9 ). Accordingly, we concluded that Aβ40 and Aβ42 oligomers of the same order adopt the same shape, regardless of whether they are cross-linked.
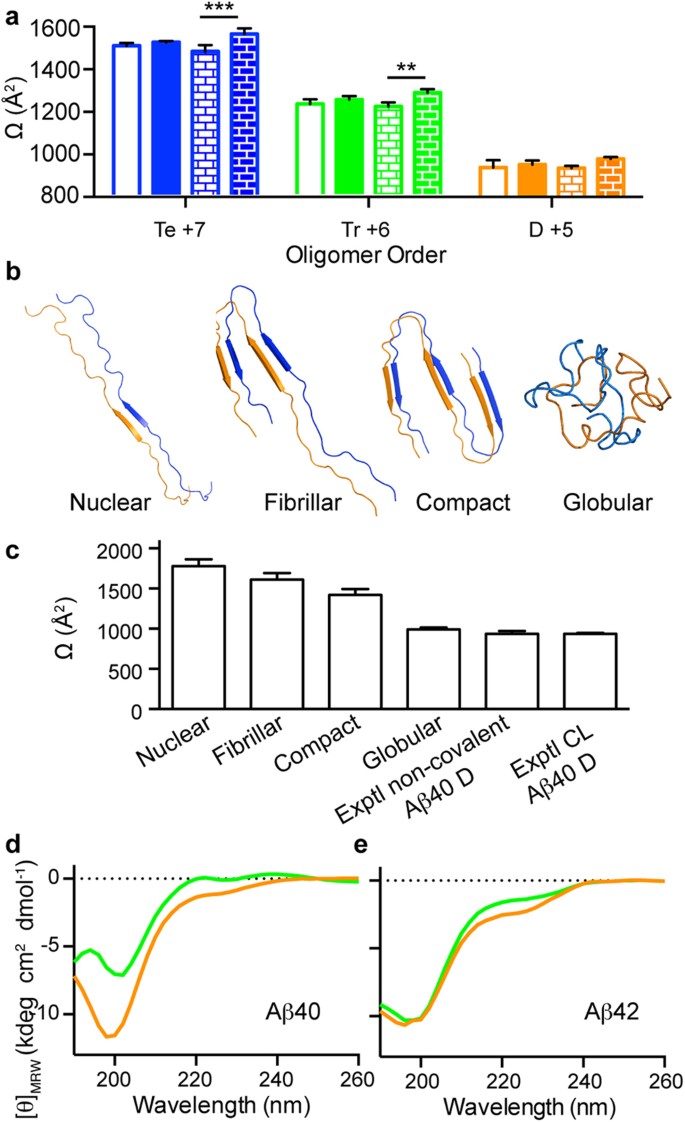
Aβ40 and Aβ42 dimers and trimers adopt a globular shape devoid of defined secondary structure.
( a ) Ω values obtained from the mobility peaks associated with the most abundant charge states for the most abundant Aβ oligomers detected (Te = tetramers, Tr = trimers and D = dimers) Te +7 (blue), Tr +6 (green) and D +5 (orange) for LMW Aβ40 (empty bars), LMW Aβ42 (filled bars), LMW_CL Aβ40 (empty brick-pattern bars) and LMW_CL Aβ42 (filled brick-pattern bars). Data are the mean ± s.d. of three independent experiments. p-values are calculated using unpaired two-tailed Student’s t-test (**p < 0.01 and ***p < 0.001). ( b ) Theoretical Aβ40 dimer models constructed using reported structural restraints for Aβ aggregates (nuclear 36 , fibrillar 37 and compact 9 , 38 ), as well as those obtained from REMD simulations (globular). ( c ) Comparison of Ω values obtained from theoretical models of the dimer structures described in (b) with the experimental measures for the non-covalent and CL Aβ40 dimers reported in (a). CD analysis of CL dimers (orange) and CL trimers (green) for ( d ) Aβ40 and ( e ) Aβ42 obtained after GdnHSCN-SEC fractionation.
To rationalize the experimentally obtained Ω values, we constructed three structural models of Aβ dimers for Aβ40 ( Fig. 4b ) and Aβ42 ( Supplementary Fig. S10a ). These models were built and named nuclear 36 , fibrillar 37 , or compact 9 , 38 , on the basis of various structural restraints reported for Aβ aggregates. All the predicted Ω values ( Fig. 4c and Supplementary Fig. S10b ) were markedly larger than the experimental ones, thereby indicating that none of the proposed models fitted the experimental data. Thus, to build more realistic structural models and achieve better sampling, we ran atomistic molecular dynamics (MD) simulations using the replica exchange method (REMD) and simulation conditions similar to those in a ESI-MS experiment 39 , 40 . In the first part of the simulations, all the models, irrespective of their original conformation, collapsed to adopt globular structures ( Supplementary Fig. S11 ). Of the three structural models, the ensemble of globular structures for the nuclear form had the lowest energy ( Supplementary Fig. S12 ). Thus, we took this low-energy ensemble to represent the structure of a globular dimer model ( Fig. 4b and Supplementary Fig. S10a ). The average Ω value of this ensemble was very similar to the experimental values obtained for the non-covalent and CL dimers ( Fig. 4c and Supplementary Fig. S10b ), thereby indicating that Aβ dimers have a globular shape. Next, to validate the lack of defined secondary structure adopted by the Aβ oligomers, we measured a CD spectrum of Aβ40 and Aβ42 CL dimers and trimers obtained immediately after SEC ( Fig. 4d,e ). The spectra for all four samples showed a minimum at around 200 nm, indicative of a lack of defined secondary structure. Additionally, an arm around 230 nm was also observed, which may reflect the existence of dynamic structural nuclei. In summary, the results from ESI-IM-MS, MD simulations and CD spectroscopy were consistent with Aβ dimers and trimers adopting a globular shape devoid of defined secondary structure ( Fig. 4 and Supplementary Fig. S10 ).
Our results establish that SDS-PAGE leads to artifacts in the determination of the order and distribution of Aβ42 oligomer samples and present ESI-IM-MS as a powerful technique to characterize the order, distribution, chemical modifications and structure of both covalent and non-covalently linked Aβ oligomers ( Fig. 5 ). Moreover, our findings indicate that pentamers and hexamers are artifacts of SDS-PAGE analysis and reveal Aβ40 and Aβ42 dimers and trimers, adopting a globular structure and lacking defined secondary structure, as the earliest oligomers formed during Aβ aggregation.
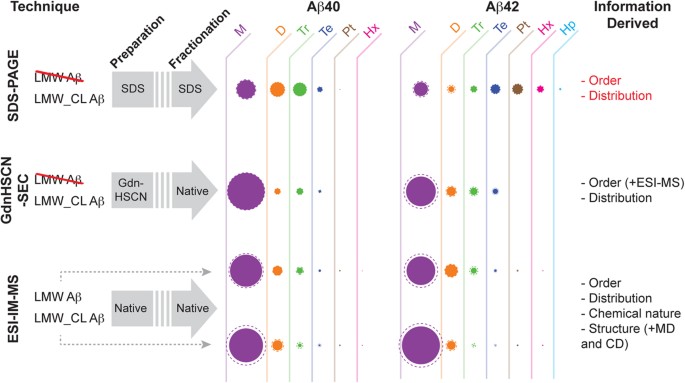
Schematics describing the conditions and the information derived from the different techniques used in this study to characterize LMW and LMW_CL Aβ samples.
Techniques involving denaturing conditions in the preparation and/or fractionation of the samples are not suitable for the characterization of LMW Aβ samples. Filled and dashed circles indicate, respectively, the average and the standard deviation of the M = monomers (purple), D = dimers (orange), Tr = trimers (green), Te = tetramers (blue), P = pentamers (brown), Hx = hexamers (pink) and Hp = heptamers (cyan) relative population in each of the samples studied. The information obtained from each of the techniques is shown in the last column. Additional methods used to further support and/or validate specific information derived from either GdnHSCN-SEC or ESI-IM-MS are indicated in parentheses. The results derived from SDS-PAGE analysis are indicated in red because we have shown that this technique provides flawed information regarding the order and distribution of oligomers present in a sample ( Fig. 2 ).
Although no other laboratory has studied LMW_CL Aβ40 and Aβ42 samples by ESI-IM-MS, they have addressed LMW Aβ40 and Aβ42 samples 7 , 10 . In this regard, it is important to note that our results are consistent only with studies that used the 13 C isotopic distribution to assign peaks separated in the mobility dimension. This is the case for findings on LMW Aβ40 samples reported by Dadlez and co-workers 10 . They reported the presence of various oligomers, ranging from dimers to hexadecamers, adopting two conformations, namely compact and extended 13 . The Ω values for the oligomers detected in our study are in agreement with those reported by Dadlez´s group. Differences in the number of oligomers detected in the two studies can be reconciled by taking into account that Dadlez´s work was performed using a higher Aβ40 concentration (200 μM versus 30 μM). On the contrary, our results are not consistent with those of Bowers and co-workers 7 , who detected a single charge state (D -5, they work on negative mode) specific for an Aβ oligomer, which included various mobility peaks, assigned by the authors based upon injection energy studies and PICUP/SDS-PAGE experiments 7 , 21 , to dimers and tetramers for Aβ40 and to dimers, tetramers, hexamers and dodecamers for Aβ42. These same assignments were further repeated in subsequent papers from the group 41 , 42 , 43 , 44 , 45 . Since their instrumentation did not allow measuring the 13 C isotope distributions, they could not distinguish whether a mobility peak originated from a structural variant of an oligomer of the same order ( e.g. , compact and extended forms) or from oligomers of distinct order. We indeed observed two mobility peaks associated with the D+5 charge state for Aβ40 and Aβ42 ( Supplementary Fig. S13 ). However, on the basis of 13 C isotopic distribution, we assigned these peaks to compact and extended conformations of the dimer rather than to distinct oligomer forms. Therefore, an important conclusion for the application of ESI-IM-MS to the study of Aβ oligomers is that assignments should be based on the 13 C isotopic distribution of the peaks separated in the mobility dimension, as initially proposed by Dadlez and co-workers 10 . Whenever there is not enough resolution to determine the charge state, we propose that the detection of at least two consecutive charge states for a particular oligomer should be required to assign a mobility peak to a specific oligomer.
A key aspect of the present study is that the GdnHSCN-SEC strategy allowed us access to synthetic Aβ oligomers of well-defined order. This protocol greatly improves previous efforts to fractionate CL Aβ oligomers from SDS-PAGE bands. Indeed, a protocol for the isolation of CL Aβ oligomers of defined order from SDS-PAGE has been described for Aβ40 16 . Attempts to apply the same protocol to CL Aβ42 samples were unsuccessful 16 . According to the authors of that study, isolated CL Aβ42 oligomers were not stable upon re-electrophoreses. This result reinforces our findings that Aβ42 pentamers and hexamers are not bona fide cross-linked oligomers but are dimers and trimers that evolve into pentamers and hexamers in the presence of SDS. Having developed a fractionation protocol that permitted obtaining Aβ oligomers of well-defined order also allowed us to characterize them by CD spectroscopy. By combining CD analysis with structural information derived from Ω values obtained from ESI-IM-MS studies and atomistic MD simulations, we show that dimers and trimers have a globular fold lacking defined secondary structure ( Fig. 4 and Supplementary Fig. S10 ). This observation contrasts with previous publications where SDS-PAGE-purified CL Aβ40 dimers, trimers and tetramers were suggested to adopt a β-sheet structure 16 . In the aforementioned work, the authors used PICUP conditions that, in our hands, afforded a heterogeneous mixture of oxidized products when analyzed by RP-HPLC and LC-HRMS ( Supplementary Fig. S1 and Table S1 ). Moreover, the authors fractionated the oligomers using SDS-PAGE and although several purification steps were applied, there is the possibility that SDS contaminated the samples, thus affecting the structure of the purified oligomers.
In summary, our results reveal that SDS-PAGE leads to artifactual results in determining the order and distribution of Aβ42 oligomers, while ESI-IM-MS is established as a powerful technique to characterize the order, distribution, chemical modifications and structure of both covalent and non-covalently linked Aβ40 and Aβ42 oligomers. These findings challenge previous scientific paradigms in the AD field built upon the use of SDS-PAGE to characterize Aβ oligomers samples. Among these paradigms, pentamers and hexamers have been established as the building blocks for Aβ aggregation. However, here we demonstrate that pentamers and hexamers form artifactually from dimers and trimers in the presence of SDS. Importantly, we identify Aβ40 and Aβ42 dimers and trimers, adopting a globular structure and lacking defined secondary structure, as the earliest oligomers formed during Aβ aggregation. This result is in agreement with an elegant study addressing homo- and hetero-molecular Aβ40/Aβ42 aggregation 46 . This study reveals that from all possible stages of aggregation, Aβ40 and Aβ42 interact significantly only at the level of primary nucleation. The authors explain this result by suggesting that the structure of primary nuclei is less organized than that of the fibrils. This lower level of organization would allow for better accommodation of the two-residue C-terminal mismatch. Our findings indicating that Aβ40 and Aβ42 dimers and trimers adopt very similar shapes lacking defined secondary structure would be in complete agreement with the proposed explanation. Finally, since structural models for other higher order Aβ oligomers have been reported to adopt a β-sheet structure 9 , 38 , 47 , our findings that Aβ dimers and trimers are globular and lack defined secondary structure indicate that major structural rearrangements occur during fibril formation. These observations highlight the importance of defining the structures and properties of the various species involved in Aβ aggregation. Indeed, it is only through this level of molecular detail that we will be able to interfere with Aβ aggregation in a rational manner.
Preparation of LMW Aβ40 and Aβ42
Aβ peptides of 40 and 42 residues, Aβ40 and Aβ42, were synthesized and purified by Dr. James I. Elliott (New Haven). LMW Aβ40 and Aβ42 preparations were obtained using Size-Exclusion Chromatography (SEC). Aβ peptide was dissolved in 6.8 M GdnHSCN (Life Technologies) at 8.5 mg/mL, sonicated for 5 min and diluted to 5 mg/mL of peptide and 4 M GdnHSCN with H 2 O. It was then centrifuged at 10,000 g for 6 min at 4 °C and passed through a 0.45-μm Millex filter (Millipore). The resulting Aβ solution was injected into a HiLoad Superdex 75 prep grade column (GE Healthcare). The column was equilibrated using 10 mM sodium phosphate pH 7.4 and eluted at 4 °C at a flow rate of 1 mL/min. The peak attributed to LMW Aβ was collected and its protein concentration determined (see section Quantifying Aβ40 and Aβ42 protein concentration). The peptide solution was then diluted to 150 μM, frozen and kept at −20 °C until used.
Quantifying Aβ40 and Aβ42 protein concentration
LMW Aβ concentration obtained from SEC fractions was determined by reversed phase high-performance liquid chromatography (RP-HPLC) coupled to photodiode array detector (PDA) (Waters Alliance 2695 equipped with 2998 photodiode array detector). RP-HPLC analysis was carried out using a Symmetry 300 C 4 column (4.6 × 150 mm, 5 μm, 300 Å; Waters), a flow rate of 1 mL/min and a linear gradient from 0 to 60% B in 15 min (A = 0.045% trifluoroacetic acid (TFA) in water and B = 0.036% TFA in acetonitrile) at 60 °C. A calibration curve was generated based on Aβ40 and Aβ42 solutions that had previously been quantified by amino acid analysis.
Photo-Induced Cross-linking of Unmodified Proteins (PICUP)
The PICUP reaction was initially run following descriptions in the literature 33 . The experimental set-up consisted of a camera body and a 150-W slide projector. A PCR tube containing the reaction mixture to be cross-linked was placed inside the camera body for irradiation. The sample was irradiated via the slide projector for a short time, precisely controlled by the camera shutter. PICUP reactions were done using an Aβ/Ru(bpy) 3 2+ /APS ratio of 1:2:40. To this end, 4 μL of 3 mM Ru(bpy) 3 2+ and 4 μL of 60 mM APS were added to 40 μL of 150 μM LMW Aβ in 10 mM sodium phosphate buffer. The mixture was irradiated for 1 s at a distance of 10 cm and immediately quenched by adding 6.5 μL of 4 M dithiothreitol (DTT). Later on, reaction conditions were optimized to prevent Aβ oxidation ( Supplementary Fig. S1 ). The best conditions were found when using a lower proportion of APS, Aβ/Ru(bpy) 3 2+ /APS 1:2:5, a distance of 10 cm and an irradiation time of 1 s. Unless otherwise stated, we used an Aβ/Ru(bpy) 3 2+ /APS ratio of 1:2:5 to prevent formation of oxidized byproducts and to obtain chemically well-defined cross-linked oligomers. After the PICUP reaction, the reagents, which are incompatible with ESI-IM-MS and RP-HPLC analysis, were removed using Bio-Spin P30 columns (Bio-Rad) equilibrated in 10 mM ammonium acetate at pH 8.5. For comparative purposes, PICUP samples were also passed through Bio-Spin P30 columns when analyzed by SDS-PAGE.
10 μL of 3X sample buffer (SB) (150 μl 10% SDS, 75 μl 4 M DTT, 400 μl H 2 O, 375 μl 8X sample buffer −8 mL 1 M Tris pH 6.8, 9.3 mL 87% Glycerol, 5 mg Coomassie Brilliant Blue G, 2.8 mL H 2 O) was added to 20 μL of the sample to be analyzed by SDS-PAGE. The samples were boiled at 95 °C for 5 min, unless otherwise stated and kept at −20 °C until used. A 20-μL aliquot of each sample was electrophoresed in 0.75 mm-thick SDS-PAGE gels containing 15% acrylamide. Gels were run at 80–100 V and silver-stained.
RP-HPLC analysis of LMW_CL Aβ40 samples was done using a Symmetry 300 C 4 column (4.6 × 150 mm, 5 μm, 300 Å; Waters) at a flow rate of 1 mL/min and a linear gradient from 0 to 60% B in 15 min (A = 0.045% TFA in water and B = 0.036% TFA in acetonitrile) at 60 °C.
180 pmols of LMW_CL Aβ40 and LMW_CL Aβ42 ( Supplementary Fig. S14 ) were analyzed using a BioSuite pPhenyl 1000 analytical column (10 μm, 2 × 75 mm; Waters) at a flow rate of 100 μl/min comprising a linear gradient running from 5 to 80% B in 60 min (A = 0.1% formic acid (FA) in water, B = 0.1% FA in acetonitrile). The column outlet was directly connected to an Advion TriVersa NanoMate, which was used as a splitter and as the nanospray source of an LTQ-FT Ultra mass spectrometer (Thermo Scientific). Positive polarity was used with a spray voltage in the NanoMate source set to 1.7 kV. The capillary voltage, capillary temperature and tube lens on the LTQ-FT were tuned to 40 V, 200 °C and 100 V, respectively.
GdnHSCN-SEC
Although methionine oxidation was minimized by using Aβ/Ru(bpy) 3 2+ /APS ratios of 1:2:5, a small percentage of oxidized methionine was detected after isolation of CL Aβ dimers and trimers. To completely reduce all oxidized methionine side-chains, 500 μL of the quenched PICUP reaction were lyophilized and resuspended in TFA containing 30 equivalents of Me 2 S-NH 4 I and left for 2 hrs at 4 °C on a rotating wheel. Afterwards, TFA was evaporated under a stream of N 2 and 500 μL of H 2 O was added to the sample. The mixture was then lyophilized, resuspended in 6.8 M GdnHSCN at an Aβ concentration of 8.5 mg/mL and subsequently diluted with H 2 O to 5 mg/mL of peptide and 4M GdnHSCN. The resulting Aβ solution was injected into three columns in series, Superdex 75 HR 10/300-Superdex 75 HR 10/300-Superdex 200 HR10/300. The columns were equilibrated in 10 mM ammonium acetate pH 8.5 and the samples eluted at 4 °C at a flow rate of 0.5 mL/min.
ESI-IM-MS experiments were performed on a Synapt HDMS (Waters) quadrupole-traveling wave IMS-oaTOF mass spectrometer equipped with an Advion TriVersa NanoMate (Advion Biosciences). Positive ESI was used with a capillary voltage of 1.7 kV. A sampling cone voltage of 40 V and a backing pressure of 5.7 mbar were set for the observation of low-n Aβ oligomers. Although no signal was detected over 3000 m/z, data were acquired over the m/z range of 500 to 5000 for 2 min. The ion accelerating voltage in the trap T-wave device was 6 V unless otherwise stated; the ion accelerating voltage in the transfer T-wave device was kept constant at 4 V. The IM gas flow was kept at 23 mL/min. ESI-IM-MS data were obtained at three wave heights: 7, 7.5 and 8. The drift time Ω function was calibrated using denatured ubiquitin (charge states +9 to +11), myoglobin (charge states +15 to +22) and cytochrome C (charge states +11 to +18). Drift times were corrected for both mass-dependent and mass-independent times 48 , 49 . All unknown drift time values, for which a Ω was derived, fell within the calibration curve obtained using the 19 charge states of the three protein standards. The raw data were processed using Mass Lynx v4.1 software (Waters). The reported data are the average of three independent experiments. The considerations to assign ESI-IM-MS peaks to specific Aβ oligomers are described in Supplementary text . To determine the low-n Aβ oligomer distribution for all four samples under study, we used all charge state ions across the whole spectra. For those charge states that contained contributions from different Aβ species in the mobility dimension, the relative contribution of each one was determined by fitting the mobilogram to a sum of Gaussian functions g (x c , w, A), where x c , w and A correspond to the center, the width at half-height and the area of the Gaussian peak, respectively. The fitted function obtained for each mobilogram, f_im, can be written as:
where k_im is the baseline intensity of the mobilogram, Σ g (x c N , w N , A N ) is the sum of Gaussians contributing to each mobilogram and N is the number of Gaussian/species used to fit each mobilogram. The relative population of a species contributing to a given charge state was obtained by dividing the area of the peak representing that species by the sum of the areas of each of the other species contributing to the given charge state. The contribution of a species to the overall intensity of a charge state ion was obtained by multiplying it by its relative population. The population of a species was obtained by summing the intensity of all charge states specific for the particular species and dividing it by the sum of the intensities of all charge state ions across the whole spectra.
Modeling Aβ dimer structure
Construction of aβ dimer models.
Three structural models for Aβ40 and Aβ42 dimers were built, taking into account reported structural restraints for Aβ aggregates (nuclear 36 , fibrillar 37 and compact 9 , 38 ) ( Fig. 4b for Aβ40, Supplementary Fig. S10a for Aβ42). The sequence of Aβ was divided into three segments: the N-terminus (residues 1 to 11), the middle segment (residues 12 to 26) and the C-terminus (residues 27 to 40 for Aβ40, or 27 to 42 for Aβ42). In all the models, the two Aβ units interact through β-strand regions defined within each Aβ unit to form parallel β-sheets through intermolecular hydrogen bonds. Previous reports have proposed that the region comprising residues 17 to 21 nucleates Aβ self-assembly 36 . In the nuclear model, residues 12 to 24 adopt a β-strand conformation. In the fibrillar model, the regions spanning residues 12 to 24 and residues 30 to 40 (for Aβ40) or 42 (for Aβ42) adopt a β-strand conformation. Residues 25–29 contain a bend that brings the two β-strands into contact through side chain-side chain interactions. This model corresponds to the structure of Aβ when it is incorporated into the fibril 37 . The compact model was constructed taking into account structural models of specific Aβ oligomer preparations that reveal a stable N-terminal β-strand 9 , 38 . Thus, each Aβ unit contained three β-strand regions comprising residues 1 to 6, 12 to 24 and 30 to 40 (for Aβ40) or 42 (for Aβ42). Residues 7 to 11 and 25 to 29 include a bend of the peptide that brings the three β-strands into contact through side chain-side chain interactions. Each of the six structural models, three for Aβ40 and three for Aβ42, were constructed using PyMol and Modeller tools starting from the Aβ40 fibril structure derived from solid-state Nuclear Magnetic Resonance Spectroscopy (ssNMR) data (Protein Data Bank ID is 2LMN) 37 .
Structure equilibration in water
The six structural models were built, optimized, solvated in a truncated octahedron box and then subsequently neutralized with six sodium ions 50 . Transferable Interaction Potential 3 point (TIP3P) 51 parameters were used for water and AMBER99SB-ILDN force field 52 for proteins. Simulations in water were done using periodic boundary conditions and the particle mesh Ewald method 53 for long-range electrostatic treatment (0.12 nm grid size), combined with a cut-off radius of 1 nm for Lennard-Jones interactions. All aqueous simulations were done at the isothermal-isobaric ensemble (T = 300 K and P = 1 atm) using a Berendsen thermostat and barostat 54 . The SHAKE algorithm 55 was used to constrain all bond distances to their equilibrium value, allowing a 2-fs integration time step for solution conditions. We performed 5 ns of equilibration in water and computed and minimized the averaged structure of snapshots collected in the last ns. Ω values for each of the structural models were computed with the exact hard sphere scattering method (EHSS), which consists of a trajectory calculation using hard spheres centered at the position of each atom 56 .
REMD simulation in the gas phase
As a starting structure for REMD simulation, water molecules and counter ions were removed. We used the REMD method 57 to perform extended simulations at a range of temperatures for the three models of Aβ40 and Aβ42 dimers in the gas phase. The simulations were done considering a charge state +5, which is the most populated charge state for dimers in the ESI-MS profile. We chose 19 simulation temperatures for each structure, covering a range from 300 K to 614 K, which corresponds to the temperature variability range in the mobility measurements 58 . For gas phase simulations, we did not use any cut-off for non-bonded interactions. We used the SHAKE algorithm for constraining bonds and a 1-fs integration time step. For each Aβ dimer structure, we performed 1 μs of REMD simulation. For the analysis, we considered the trajectories at 300 K, a temperature that simulates ideally mild vaporization conditions and used the Gromacs.4.5.3 package. Ω values for the ensemble of structures generated during the last 500 ns of REMD, were also computed with the exact hard sphere scattering method (EHSS) 56 .
Far-UV CD Spectroscopy
CD spectra were recorded on a Jasco 815 spectrometer from 190 to 250 nm with a data pitch of 0.2 nm, a bandwidth of 2 nm and a scan speed of 50 nm/min with a 4-s response time. A 1-cm cell was used. After GdnHSCN-SEC fractionation using three columns in series equilibrated in 10 mM sodium phosphate at pH 8.5, spectra for CL Aβ40 and Aβ42 dimers and trimers were acquired at 4 °C. CD data were analyzed using the SpectraManager program.
Summary of statistical analysis
GraphPad Prism was used for all statistical analyses. The data are presented as mean ± s.d. except when stated otherwise. The Student’s t-test (unpaired, two-tailed) was used to calculate statistical significance. The images shown are representative of those obtained in at least three independent experiments.
Additional Information
How to cite this article : Pujol-Pina, R. et al. SDS-PAGE analysis of Aβ oligomers is disserving research into Alzheimer’s disease: appealing for ESI-IM-MS. Sci. Rep. 5 , 14809; doi: 10.1038/srep14809 (2015).
Haass, C. & Selkoe, D. J. Soluble protein oligomers in neurodegeneration: lessons from the Alzheimer’s amyloid beta-peptide. Nat. Rev. Mol. Cell Biol. 8, 101–112 (2007).
Article CAS PubMed Google Scholar
Hefti, F. et al. The case for soluble Abeta oligomers as a drug target in Alzheimer’s disease. Trends Pharmacol. Sci. 34, 261–266 (2013).
Editorial. State of aggregation. Nat. Neurosci. 14, 399 (2011).
Benilova, I., Karran, E. & De Strooper, B. The toxic Abeta oligomer and Alzheimer’s disease: an emperor in need of clothes. Nat. Neurosci. 15, 349–357 (2012).
Bitan, G. et al. Amyloid beta-protein (Abeta) assembly: Abeta 40 and Abeta 42 oligomerize through distinct pathways. Proc. Natl. Acad. Sci. USA 100, 330–335 (2003).
Article CAS ADS PubMed Google Scholar
Necula, M., Kayed, R., Milton, S. & Glabe, C. G. Small molecule inhibitors of aggregation indicate that amyloid beta oligomerization and fibrillization pathways are independent and distinct. J. Biol. Chem. 282, 10311–10324 (2007).
Bernstein, S. L. et al. Amyloid-beta protein oligomerization and the importance of tetramers and dodecamers in the aetiology of Alzheimer’s disease. Nat. Chem. 1, 326–331 (2009).
Article CAS PubMed PubMed Central Google Scholar
Yu, L. et al. Structural characterization of a soluble amyloid beta-peptide oligomer. Biochemistry 48, 1870–1877 (2009).
Ahmed, M. et al. Structural conversion of neurotoxic amyloid-beta(1-42) oligomers to fibrils. Nat. Struct. Mol. Biol. 17, 561–567 (2010).
Kloniecki, M. et al. Ion mobility separation coupled with MS detects two structural states of Alzheimer’s disease Abeta1-40 peptide oligomers. J. Mol. Biol. 407, 110–124 (2011).
Lee, J., Culyba, E. K., Powers, E. T. & Kelly, J. W. Amyloid-beta forms fibrils by nucleated conformational conversion of oligomers. Nat. Chem. Biol. 7, 602–609 (2011).
Narayan, P. et al. The extracellular chaperone clusterin sequesters oligomeric forms of the amyloid-beta(1–40) peptide. Nat. Struct. Mol. Biol. 19, 79–83 (2012).
Article CAS Google Scholar
Bitan, G. & Teplow, D. B. Rapid photochemical cross-linking–a new tool for studies of metastable, amyloidogenic protein assemblies. Acc. Chem. Res. 37, 357–364 (2004).
Citron, M. et al. Mutant presenilins of Alzheimer’s disease increase production of 42-residue amyloid beta-protein in both transfected cells and transgenic mice. Nat. Med. 3, 67–72 (1997).
Blennow, K., Zetterberg, H. & Fagan, A. M. Fluid biomarkers in Alzheimer disease. Cold Spring Harb. Perspect. Med. 2, a006221 (2012).
Article PubMed PubMed Central Google Scholar
Ono, K., Condron, M. M. & Teplow, D. B. Structure-neurotoxicity relationships of amyloid beta-protein oligomers. Proc. Natl. Acad. Sci. USA 106, 14745–14750 (2009).
Article CAS ADS PubMed PubMed Central Google Scholar
Paravastu, A. K., Qahwash, I., Leapman, R. D., Meredith, S. C. & Tycko, R. Seeded growth of beta-amyloid fibrils from Alzheimer’s brain-derived fibrils produces a distinct fibril structure. Proc. Natl. Acad. Sci. USA 106, 7443–7448 (2009).
Roychaudhuri, R., Yang, M., Hoshi, M. M. & Teplow, D. B. Amyloid beta-protein assembly and Alzheimer disease. J. Biol. Chem. 284, 4749–4753 (2009).
Bitan, G., Fradinger, E. A., Spring, S. M. & Teplow, D. B. Neurotoxic protein oligomers–what you see is not always what you get. Amyloid 12, 88–95 (2005).
Article PubMed Google Scholar
Watt, A. D. et al. Oligomers, fact or artefact? SDS-PAGE induces dimerization of beta-amyloid in human brain samples. Acta Neuropathol. 125, 549–564 (2013).
Bernstein, S. L. et al. Amyloid beta-protein: monomer structure and early aggregation states of Abeta42 and its Pro19 alloform. J. Am. Chem. Soc. 127, 2075–2084 (2005).
Smith, D. P., Radford, S. E. & Ashcroft, A. E. Elongated oligomers in beta2-microglobulin amyloid assembly revealed by ion mobility spectrometry-mass spectrometry. Proc. Natl. Acad. Sci. USA 107, 6794–6798 (2010).
Sitkiewicz, E., Oledzki, J., Poznanski, J. & Dadlez, M. Di-tyrosine cross-link decreases the collisional cross-section of Abeta peptide dimers and trimers in the gas phase: an ion mobility study. PloS one 9, e100200 (2014).
Article ADS PubMed PubMed Central Google Scholar
Sitkiewicz, E., Kloniecki, M., Poznanski, J., Bal, W. & Dadlez, M. Factors influencing compact-extended structure equilibrium in oligomers of Abeta1-40 peptide–an ion mobility mass spectrometry study. J. Mol. Biol. 426, 2871–2885 (2014).
Hernandez, H. & Robinson, C. V. Determining the stoichiometry and interactions of macromolecular assemblies from mass spectrometry. Nat. Protoc. 2, 715–726 (2007).
Ayed, A., Krutchinsky, A. N., Ens, W., Standing, K. G. & Duckworth, H. W. Quantitative evaluation of protein-protein and ligand-protein equilibria of a large allosteric enzyme by electrospray ionization time-of-flight mass spectrometry. Rapid Commun. Mass Spectrom. 12, 339–344 (1998).
Gabelica, V., Galic, N., Rosu, F., Houssier, C. & De Pauw, E. Influence of response factors on determining equilibrium association constants of non-covalent complexes by electrospray ionization mass spectrometry. J. Mass Spectrom. 38, 491–501 (2003).
Chitta, R. K., Rempel, D. L. & Gross, M. L. Determination of affinity constants and response factors of the noncovalent dimer of gramicidin by electrospray ionization mass spectrometry and mathematical modeling. J. Am. Soc. Mass Spectrom. 16, 1031–1038 (2005).
Liu, J. & Konermann, L. Protein-protein binding affinities in solution determined by electrospray mass spectrometry. J. Am. Soc. Mass Spectrom. 22, 408–417 (2011).
Boeri Erba, E., Barylyuk, K., Yang, Y. & Zenobi, R. Quantifying protein-protein interactions within noncovalent complexes using electrospray ionization mass spectrometry. Anal. Chem. 83, 9251–9259 (2011).
Teplow, D. B. Preparation of amyloid beta-protein for structural and functional studies. Methods Enzymol. 413, 20–33 (2006).
Jan, A., Hartley, D. M. & Lashuel, H. A. Preparation and characterization of toxic Abeta aggregates for structural and functional studies in Alzheimer’s disease research. Nat. Protoc. 5, 1186–1209 (2010).
Bitan, G. Structural study of metastable amyloidogenic protein oligomers by photo-induced cross-linking of unmodified proteins. Methods Enzymol. 413, 217–236 (2006).
Selkoe, D. J., Abraham, C. R., Podlisny, M. B. & Duffy, L. K. Isolation of low-molecular-weight proteins from amyloid plaque fibers in Alzheimer’s disease. J. Neurochem. 46, 1820–1834 (1986).
Fancy, D. A. & Kodadek, T. Chemistry for the analysis of protein-protein interactions: rapid and efficient cross-linking triggered by long wavelength light. Proc. Natl. Acad. Sci. USA 96, 6020–6024 (1999).
Reinke, A. A., Ung, P. M., Quintero, J. J., Carlson, H. A. & Gestwicki, J. E. Chemical probes that selectively recognize the earliest Abeta oligomers in complex mixtures. J. Am. Chem. Soc. 132, 17655–17657 (2010).
Petkova, A. T., Yau, W. M. & Tycko, R. Experimental constraints on quaternary structure in Alzheimer’s beta-amyloid fibrils. Biochemistry 45, 498–512 (2006).
Haupt, C. et al. Structural basis of beta-amyloid-dependent synaptic dysfunctions. Angew. Chem. Int. Ed. Engl. 51, 1576–1579 (2012).
Arcella, A. et al. Structure and dynamics of oligonucleotides in the gas phase. Angew. Chem. Int. Ed. Engl. 54, 467–471 (2015).
CAS PubMed Google Scholar
Meyer, T., Gabelica, V., Grubmüller, H. & Orozco, M. Proteins in the gas phase. WIRES Comput. Mol. Sci. 3, 408–425 (2012).
Article Google Scholar
Murray, M. M. et al. Amyloid beta protein: Abeta40 inhibits Abeta42 oligomerization. J. Am. Chem. Soc. 131, 6316–6317 (2009).
Gessel, M. M., Bernstein, S., Kemper, M., Teplow, D. B. & Bowers, M. T. Familial Alzheimer’s disease mutations differentially alter amyloid beta-protein oligomerization. ACS Chem. Neurosci. 3, 909–918 (2012).
Gessel, M. M. et al. Abeta(39–42) modulates Abeta oligomerization but not fibril formation. Biochemistry 51, 108–117 (2012).
Zheng, X. et al. Z-Phe-Ala-diazomethylketone (PADK) disrupts and remodels early oligomer states of the Alzheimer disease Abeta42 protein. J. Biol. Chem. 287, 6084–6088 (2012).
Zheng, X. et al. Amyloid beta-protein assembly: The effect of molecular tweezers CLR01 and CLR03. J. Phys Chem. B 119, 4831–4841 (2015).
Cukalevski, R. et al. The Aβ40 and Aβ42 peptides self-assemble into separate homomolecular fibrils in binary mixtures but cross-react during primary nucleation. Chem. Sci. 6, 4215–4233 (2015).
Lendel, C. et al. A hexameric peptide barrel as building block of amyloid-beta protofibrils. Angew. Chem. Int. Ed. Engl. 53, 12756–12760 (2014).
Ruotolo, B. T., Benesch, J. L., Sandercock, A. M., Hyung, S. J. & Robinson, C. V. Ion mobility-mass spectrometry analysis of large protein complexes. Nat. Protoc. 3, 1139–1152 (2008).
Smith, D. P. et al. Deciphering drift time measurements from travelling wave ion mobility spectrometry-mass spectrometry studies. Eur. J. Mass. Spectrom. 15, 113–130 (2009).
Smith, D. E. & Dang, L. X. Computer simulations of NaCl association in polarizable water. J. Chem. Phys. 100, 3757–3766 (1994).
Article CAS ADS Google Scholar
Jorgensen, W. L., Chandrasekhar, J., Madura, J. D., Impey, R. W. & Klein, M. L. Comparison of Simple Potential Functions for Simulating Liquid Water. J. Chem. Phys. 79, 926–935 (1983).
Lindorff-Larsen, K. et al. Improved side-chain torsion potentials for the Amber ff99SB protein force field. Proteins 78, 1950–1958 (2010).
CAS PubMed PubMed Central Google Scholar
York, D. M., Darden, T. A. & Pedersen, L. G. The effect of long‐range electrostatic interactions in simulations of macromolecular crystals: A comparison of the Ewald and truncated list methods. J. Chem. Phys. 99, 8345–8348 (1993).
Berendsen, H. J. C., Postma, J. P. M., van Gunsteren, W. F., DiNola, A. & Haak, J. R. Molecular dynamics with coupling to an external bath. J. Chem. Phys. 81, 3684–3690 (1984).
Ryckaert, P., Cicotti, G. & Berendsen, H. J. C. Numerical Integration of the Cartesian Equations of Motion of a System with Constraints: Molecular Dynamics of n-Alkanes. J. Comput. Phys. 23, 327–341 (1977).
Shvartsburg, A. A. & Jarrold, M. F. An exact hard-spheres scattering model for the mobilities of polyatomic ions. Chem. Phys. Lett. 261, 86–91 (1996).
Sugita, Y. & Okamoto, Y. Replica-exchange molecular dynamics method for protein folding. Chem. Phys. Lett. 314, 141–151 (1999).
Morsa, D., Gabelica, V. & De Pauw, E. Effective temperature of ions in traveling wave ion mobility spectrometry. Anal. Chem. 83, 5775–5782 (2011).
Download references
Acknowledgements
R.P. and S.V. acknowledge IRB Barcelona and the Spanish Government FPI programs, respectively, for predoctoral fellowships. We thank Prof. Ernest Giralt for helpful discussions and critical reading of the manuscript, Dr. Sergio Madurga for generating preliminary models for comparison against experimental Ω and helping with Gaussian fitting, Dr. Marina Gay and Mar Vilanova for mass spectrometry support and T. Yates for editorial help. N.C. and M.V. are active participants and MC members of the European COST Action BM 1403. IRB Barcelona Mass Spectrometry Core Facility is a ProteoRed laboratory, part of PRB2-ISCIII, supported by grant PT13/0001. This work was supported by grants from MINECO-FEDER (SAF2012-35226) and from the Alzheimer´s Association (NIRP-12-256641), both to N.C.
Author information
Pujol-Pina Rosa and Vilaprinyó-Pascual Sílvia contributed equally to this work.
Authors and Affiliations
Institute for Research in Biomedicine (IRB Barcelona), Baldiri Reixac 10, Barcelona, 08028, Spain
Rosa Pujol-Pina, Sílvia Vilaprinyó-Pascual, Roberta Mazzucato & Natàlia Carulla
Joint IRB-BSC Research Program in Computational Biology, Institute for Research in Biomedicine (IRB Barcelona), Baldiri Reixac 10, Barcelona, 08028, Spain
Annalisa Arcella & Modesto Orozco
Mass Spectrometry Core Facility, Institute for Research in Biomedicine (IRB Barcelona), Baldiri Reixac 10, Barcelona, 08028, Spain
Marta Vilaseca
Department of Biochemistry and Molecular Biology, University of Barcelona, Diagonal 647, Barcelona, 08028, Spain
Modesto Orozco
You can also search for this author in PubMed Google Scholar
Contributions
R.P. and S.V. optimized PICUP conditions for preparing chemically well-defined LMW_CL Aβ samples. S.V. designed, performed and analyzed the results of SDS-PAGE analysis. S.V. and R.M. designed and implemented the GdnHSCN-SEC protocol. R.P. designed, performed and analyzed ESI-IM-MS experiments. S.V. obtained oligomer distribution from ESI-IM-MS data. Regarding the construction of Aβ dimer models and atomistic MD simulations, A.A. and M.O. designed the experiments, A.A. performed them and A.A. and M.O. analyzed the data. M.V. performed LC-HRMS and ESI-MS experiments and gave technical support and conceptual advice on the ESI-IM-MS experiments. S.V. and R.M. designed, performed and analyzed the results of CD experiments. N.C. conceived the study, designed and oversaw all experiments and wrote the manuscript. All the authors discussed the results and commented on and contributed to sections of the manuscript.
Ethics declarations
Competing interests.
The authors declare no competing financial interests.
Electronic supplementary material
Supplementary information, rights and permissions.
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
Reprints and permissions
About this article
Cite this article.
Pujol-Pina, R., Vilaprinyó-Pascual, S., Mazzucato, R. et al. SDS-PAGE analysis of Aβ oligomers is disserving research into Alzheimer´s disease: appealing for ESI-IM-MS. Sci Rep 5 , 14809 (2015). https://doi.org/10.1038/srep14809
Download citation
Received : 21 May 2015
Accepted : 09 September 2015
Published : 09 October 2015
DOI : https://doi.org/10.1038/srep14809
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
This article is cited by
Screening carbon nano materials for preventing amyloid protein aggregation by adopting a facile method.
- Daisy L. Wilson
- Ana Carreon
- Mahesh Narayan
Cell Biochemistry and Biophysics (2024)
A Brain-Targeting Bispecific-Multivalent Antibody Clears Soluble Amyloid-Beta Aggregates in Alzheimer's Disease Mice
- Silvio R. Meier
- Greta Hultqvist
Neurotherapeutics (2022)
An evaluation of the self-assembly enhancing properties of cell-derived hexameric amyloid-β
- Devkee M. Vadukul
- Céline Vrancx
- Pascal Kienlen-Campard
Scientific Reports (2021)
Production of seedable Amyloid-β peptides in model of prion diseases upon PrPSc-induced PDK1 overactivation
- Juliette Ezpeleta
- Vincent Baudouin
- Benoit Schneider
Nature Communications (2019)
Solvent-Assisted Paper Spray Ionization Mass Spectrometry (SAPSI-MS) for the Analysis of Biomolecules and Biofluids
- Nicoló Riboni
- Alessandro Quaranta
- Leopold L. Ilag
Scientific Reports (2019)
By submitting a comment you agree to abide by our Terms and Community Guidelines . If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily.
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
- My Bibliography
- Collections
- Citation manager
Save citation to file
Email citation, add to collections.
- Create a new collection
- Add to an existing collection
Add to My Bibliography
Your saved search, create a file for external citation management software, your rss feed.
- Search in PubMed
- Search in NLM Catalog
- Add to Search
SDS-PAGE and Western Blotting
Affiliation.
- 1 Department of Immunology and Medical Microbiology, Alfatah University for Medical Sciences, Tripoli, Libya.
- PMID: 21337110
- DOI: 10.1385/1-59259-076-4:391
Proteins can be separated according to their molecular sizes and charges, since these factors will determine the speed at which they will travel through a gel. The SDS-PAGE method involves the denaturation of proteins with the detergent sodium dodecyl sulfate (SDS) and the use of an electric current to pull them through a polyacrylamide gel, a process termed polyacrylamide gel electrophoresis (PAGE). SDS binds strongly to proteins, with approximately one detergent molecule binding to two amino acids when SDS is present at 0.1% (1,2). When boiled with SDS, proteins gain a negative charge in proportion to their molecular size, and thus travel in the acrylamide gel according to their molecular sizes. The smaller the size of the running protein, the faster it travels through the pores of the gel Fig. 1 ).
PubMed Disclaimer
Similar articles
- SDS-PAGE under focusing conditions: an electrokinetic transport phenomenon based on charge compensation. Zilberstein G, Korol L, Antonioli P, Righetti PG, Bukshpan S. Zilberstein G, et al. Anal Chem. 2007 Feb 1;79(3):821-7. doi: 10.1021/ac0615091. Anal Chem. 2007. PMID: 17263306
- Acetic Acid-urea polyacrylamide gel electrophoresis of proteins. Smith BJ. Smith BJ. Methods Mol Biol. 1984;1:63-73. doi: 10.1385/0-89603-062-8:63. Methods Mol Biol. 1984. PMID: 20512675
- Native polyacrylamide gels. Arndt C, Koristka S, Bartsch H, Bachmann M. Arndt C, et al. Methods Mol Biol. 2012;869:49-53. doi: 10.1007/978-1-61779-821-4_5. Methods Mol Biol. 2012. PMID: 22585476
- Comparison of the separation of proteins by sodium dodecyl sulfate-slab gel electrophoresis and capillary sodium dodecyl sulfate-gel electrophoresis. Guttman A, Nolan J. Guttman A, et al. Anal Biochem. 1994 Sep;221(2):285-9. doi: 10.1006/abio.1994.1413. Anal Biochem. 1994. PMID: 7810868
- Lectin blotting. Nonaka M, Kawasaki T. Nonaka M, et al. 2021 Sep 10 [updated 2022 Mar 21]. In: Nishihara S, Angata K, Aoki-Kinoshita KF, Hirabayashi J, editors. Glycoscience Protocols (GlycoPODv2) [Internet]. Saitama (JP): Japan Consortium for Glycobiology and Glycotechnology; 2021–. 2021 Sep 10 [updated 2022 Mar 21]. In: Nishihara S, Angata K, Aoki-Kinoshita KF, Hirabayashi J, editors. Glycoscience Protocols (GlycoPODv2) [Internet]. Saitama (JP): Japan Consortium for Glycobiology and Glycotechnology; 2021–. PMID: 37590714 Free Books & Documents. Review. No abstract available.
- Unveiling the power of proteomics in advancing tropical animal health and production. Adnane M, de Almeida AM, Chapwanya A. Adnane M, et al. Trop Anim Health Prod. 2024 Jun 3;56(5):182. doi: 10.1007/s11250-024-04037-4. Trop Anim Health Prod. 2024. PMID: 38825622 Review.
- Horseradish peroxidase-catalyzed polyacrylamide gels: monitoring their polymerization with BSA-stabilized gold nanoclusters and their functional validation in electrophoresis. Liao C, Li T, Chen F, Yan S, Zhu L, Tang H, Wang D. Liao C, et al. RSC Adv. 2024 Jan 10;14(4):2182-2191. doi: 10.1039/d3ra07208h. eCollection 2024 Jan 10. RSC Adv. 2024. PMID: 38213962 Free PMC article.
- Derazantinib Inhibits the Bioactivity of Keloid Fibroblasts via FGFR Signaling. Xu S, Zhu Y, Wang P, Qi S, Shu B. Xu S, et al. Biomedicines. 2023 Dec 5;11(12):3220. doi: 10.3390/biomedicines11123220. Biomedicines. 2023. PMID: 38137441 Free PMC article.
- Toxic effects of sodium dodecyl sulfate on planarian Dugesia japonica . Feng M, Xu Z, Yin D, Zhao Z, Zhou X, Song L. Feng M, et al. PeerJ. 2023 Jul 10;11:e15660. doi: 10.7717/peerj.15660. eCollection 2023. PeerJ. 2023. PMID: 37456884 Free PMC article.
- Increased expression of 6-phosphofructo-2-kinase/fructose 2,6-bisphosphatase-3 is required for growth of mouse embryonic stem cells that are undergoing differentiation. Guzel S, Gurpinar Y, Altunok TH, Yalcin A. Guzel S, et al. Cytotechnology. 2023 Feb;75(1):27-38. doi: 10.1007/s10616-022-00557-9. Epub 2022 Nov 10. Cytotechnology. 2023. PMID: 36713065 Free PMC article.
Related information
- Citation Manager
NCBI Literature Resources
MeSH PMC Bookshelf Disclaimer
The PubMed wordmark and PubMed logo are registered trademarks of the U.S. Department of Health and Human Services (HHS). Unauthorized use of these marks is strictly prohibited.

IMAGES
COMMENTS
Sodium dodecyl-sulfate polyacrylamide gel electrophoresis (SDS-PAGE) is commonly used to obtain high resolution separation of complex mixtures of proteins. The method initially denatures the proteins that will undergo electrophoresis. Although covalent ...
This Journal of Biological Chemistry (JBC) Classic on using sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) to determine the molecular weight of proteins is one of our most highly cited articles. According to the Thomson Scientific Web of Science it was the 13th most cited article in 2004, with 23,167 total citations.
The migration rate of the proteins during SDS-PAGE is determined by the pore size of the gel matrix and charge, size, and shape of the protein. In this unit, the protocol covers the casting of gels, preparation of the protein samples, staining and drying of the gels, and calculation of molecular mass of the proteins based on electrophoretic ...
This protocol describes a denaturing polyacrylamide gel system utilizing sodium dodecyl sulfate (SDS) to separate protein molecules based on size as first described by Laemmli (1970). SDS-PAGE can be used to monitor protein purifications, check the purity of samples, and to estimate molecular weight …
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) is a standard method for protein analysis. However, the stacking gel employed for…
A very common method for separating proteins by electrophoresis uses a discontinuous polyacrylamide gel as a support medium and sodium dodecyl sulfate (SDS) to denature the proteins.
In order to accelerate Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), here we propose an optimized version of the technique enabled by experimental tuning reinforced by theoret...
Explore the latest full-text research PDFs, articles, conference papers, preprints and more on SDS-PAGE. Find methods information, sources, references or conduct a literature review on SDS-PAGE
Tricine-SDS-PAGE is commonly used to separate proteins in the mass range 1-100 kDa. It is the preferred electrophoretic system for the resolution of proteins smaller than 30 kDa.
1. Introduction Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE, see Table I for a list of acronyms used in this paper) has been used for size-based separations of proteins for over four decades [ 1, 2 ], and it is still the workhorse for protein separations and analyses in most biological research laboratories.
In this research, to take advantage of both zymography and unstained SDS-PAGE, each band in an unstained SDS-PAGE gel was cut and transferred to a zymography gel. Through this method, bands on the SDS-PAGE gel and the accompanying electroblotted membrane could be used to confirm the target proteolytic activity.
Abstract The separation of mixtures of proteins by SDS-polyacrylamide gel electrophoresis (SDS-PAGE) is a technique that is widely used—and, indeed, this technique underlies many of the assays and analyses that are described in this book.
SDS-PAGE is considered to be a universal method for size-based separation and analysis of proteins. In this study, we applied the principle of SDS-PAGE to the analysis of new entirely uncharged nucleic acid (NA) analogues, - phosphoryl guanidine oligonucleotides (PGOs).
Abstract Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and Western blotting to detect proteins and glycoproteins is one of the most widely used and broadly useful techniques in cancer research, allowing the proteins in a complex sample--such as a blood sample, aspirate, or solid tumor homogenate--to be separated according to molecular weight and visualized within a gel ...
Western blotting is an important analytical technique used in cell and molecular biology for last four decades. It involves separation of proteins in SDS-PAGE and then transfer of proteins to a membrane followed by detection. By using a western blot, one can identify...
Sodium dodecyl-sulfate polyacrylamide gel electrophoresis (SDS-PAGE) is commonly used to obtain high resolution separation of complex mixtures of proteins. The method initially denatures the proteins that will undergo electrophoresis. Although covalent structural features of resolved proteins can be determin
Analysis of these samples by SDS-PAGE revealed that SDS affects the oligomerization state of Aβ42 oligomers, thus providing flawed information on their order and distribution.
Abstract and Figures SDS-PAGE is considered to be a universal method for size-based separation and analysis of proteins. In this study, we applied the principle of SDS-PAGE to the analysis of new ...
The SDS-PAGE method involves the denaturation of proteins with the detergent sodium dodecyl sulfate (SDS) and the use of an electric current to pull them through a polyacrylamide gel, a process termed polyacrylamide gel electrophoresis (PAGE). SDS binds strongly to proteins, with approximately one detergent molecule binding to two amino acids ...
Laemmli showed that proteins could be reliably fractionated by SDS-PAGE, which he described in a figure legend in a Nature paper [2]. SDS-polyacrylamide gel electrophoresis involves the separation of protein based on their size.
The method is called sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE). The most commonly used system is also called the Laemmli method after U.K. Laemmli, who was the first to publish a paper employing SDS-PAGE in a scientific study.
Request PDF | A Practical Approach on SDS PAGE for Separation of Protein | Polyacrylamide gel electrophoresis (PAGE), describes a technique widely used in biochemistry, forensics, genetics ...
SDS-PAGE ( sodium dodecyl sulfate-polyacrylamide gel electrophoresis) is a discontinuous electrophoretic system developed by Ulrich K. Laemmli which is commonly used as a method to separate proteins with molecular masses between 5 and 250 kDa. [1] [2] The combined use of sodium dodecyl sulfate (SDS, also known as sodium lauryl sulfate) and polyacrylamide gel eliminates the influence of ...
Ubiquitin-Carrier Protein H7 human ≥95% (SDS-PAGE), recombinant, expressed in E. coli, liquid; Synonyms: UbcH7; find Sigma-Aldrich-U9132 MSDS, related peer-reviewed papers, technical documents, similar products & more at Sigma-Aldrich
A) Whole cell lysate from primary myoblasts and H2K myogenic progenitors were separated by SDS-PAGE and western-blotted for Kat2b, GSK3β, Myf5 and γ-tubulin. B) Densitometry was performed on ...