

Login into your account
Please enter username and password bellow!
Forgotten Password
Don't have an account? Register here
Our Pharmacy Blog
Mastering pharmacy case studies.

Introduction
If you are training to become a pharmacist, you will have had experience with pharmacy case studies. But why are pharmacy case studies so important?
As a qualifying pharmacist, case studies bring together the threads of study over the past four years. This includes your study of subjects such as:
- Pharmacology
- Pharmaceutical chemistry
- Pharmaceutics
- Clinical pharmacy practice
In practice, pharmacists are expected to draw on this knowledge and clinically apply it where necessary. These subjects feed into one another where knowledge of one subject became necessary to advance in a second subject and so forth. University staff overseeing the course structure put that structure together with these factors in mind. Pharmacy case studies are an important component, often toward the end of your pharmacy degree, that aim to establish the most relevant details that play a role in the career of a qualified pharmacist.
Case studies give pharmacy students an opportunity to test their understanding of a specialist topic. This may be anything from the formulation and dosing of medicines; to a drug’s mechanism of action, drug interactions, and clinical appropriateness for a medicine in a given scenario for a patient with specific factors to keep in mind. Evidently, this takes practice. There are many possible case study scenarios to consider. It can be difficult to always get things right.
Case studies are, then, a special kind of barometer through which we measure the professional competency of pharmacy students .
That is why pharmacy case studies are popular in degree programs – forcing students to think critically about a given topic – whether it be blood diagnostics, epidemiology, treatment options, or drug monitoring – tying together their past year’s study and how to apply this knowledge to (potentially) real-life situations.
Below, we’ve put together an introductory case study to provide you with a clear example of what kinds of questions can be asked and how best you should approach each question. With enough practice, clinical case studies become that much easier. And with time, students learn to enjoy case studies – as they are often your first direct experience of learning real and relevant facts that have an impact on your long-term professional career.
Pharmacy Case Study – Osteoporosis
A 49-year old woman with osteoporosis has been taking Fosamax for 6-months. She visits her GP complaining of acid reflux and pain radiating down her esophagus.
- What is the active ingredient of Fosamax?
- What is the mechanism of action of this medicine?
- Suggest a reason why this patient is taking Fosamax.
- How should the GP respond to the patient’s symptoms?
- What foods and/or medicines should the patient avoid?
Explanation
The questions ask more about the medicine – how it works, what it’s indicated for, how the GP should respond to patient symptoms and what interactions, from both food and drug sources, the prescriber and pharmacist must consider.
A – The active ingredient of Fosamax is alendronate; a bisphosphonate drug.
B – Alendronate works by inhibiting osteoclast-mediated bone resorption (the process whereby bone is broken down and minerals are released into the blood).
C – As a 49-year old woman, the patient is likely post-menopausal. Bisphosphonates are routinely prescribed to prevent osteoporosis in these patients.
D – The patient may be improperly administering the medicine. Patients who do not follow the correct protocol of administering bisphosphonates are likely to experience specific symptoms, particularly relating to the esophagus and GI tract. Patients should be counseled to take the medicine in the morning on an empty stomach, whilst remaining upright, and taken with a full glass of water. This eases the bisphosphonate through the digestive tract without irritating the esophageal wall. Patients should avoid taking and food or medicines, both before and for at least 30-minutes after taking the bisphosphonate.
E – Two groups of medicines should be avoided. First, NSAIDs should be avoided; as they increase the risk of gastrointestinal side effects. Second, patients should avoid foods or supplements that contain multivalent ions such as magnesium, aluminum, or calcium. This category includes dairy products and antacids. As we learned above, bisphosphonates should be avoided with these medicines/foods for at least 30-minutes after the bisphosphonate has been taken (on an empty stomach).
Practice More Pharmacy Case Studies
The more pharmacy case studies you practice , the better prepared you are for the needs and demands that present during the licensing end of your pharmacy program. Pharmacy case studies help guide students through the must-know clinical facts about drugs and medicines; both theoretical and practical knowledge.
Clinical case studies are one of the ways in which students make the transition between an experienced, knowledgeable student and a clinical professional whose expertise can be trusted in the real world. Case studies bring pharmacy students to the next level. The more practice you put in, the better results you can expect as you progress through the licensing stage of your nascent career. That, in the end, is what matters.
That’s about it for our discussion of case studies! Check back to our pharmacy blog soon for more exclusive content to help you master the science of drugs and medicines and build your long-term career.
- Anticancer Pharmacology
- Antimicrobial Drugs
- Cardiovascular Pharmacology
- Clinical Case Study
- Clinical Pharmacy
- General Pharmacology
- GI Pharmacology
- Immune System Pharmacology
- Nervous System Pharmacology
- Respiratory Pharmacology
- Study Tips and Tricks
Join Our Mailing List For Even More Facts!
Don't stop learning now, you may also like, drugs used to treat heart failure, diabetes pharmacology, triptans pharmacology.

- Clinical Pharmacy and Pharmacology
Explore the latest in clinical pharmacy and pharmacology, including topics in drug safety, development, pharmacogenetics, and pharmacoeconomics.
Publication
Article type.
This cohort study compares outcomes of patients with proliferative diabetic retinopathy treated with panretinal photocoagulation (PRP) and subsequent anti–vascular endothelial growth factor (VEGF) injections to a matched cohort of patients treated with anti-VEGF injections and subsequent PRP.
This systematic review addresses the question of whether and to what extent the methods of real-world data studies compare to standard methods used in randomized clinical trials.
This cohort study uses a case-crossover design to investigate the association between prescription of first-generation antihistamines and seizures in young children using a nationwide population-based claims dataset.
- First-Generation Antihistamines and Seizures in Young Children JAMA Network Open Opinion August 28, 2024 Neurology Toxicology Adverse Drug Events Pharmacoepidemiology Pharmacy and Clinical Pharmacology Full Text | pdf link PDF open access
This mendelian randomization study uses mendelian randomization to investigate potential causal relationships between perturbing maternal genetic variants influencing antihypertensive drug targets and perinatal outcomes among offspring.
This cohort study assesses the association between the risk of fall-related injuries and first-line treatments for depression among older US adults with depression.
This cross-sectional study examines the association of availability of primary care practitioners and level of socioeconomic vulnerability with risk of pharmacy deserts in regions of the US.
This nonrandomized controlled trial aims to determine the activity of olaparib monotherapy among patients with high-risk biochemically recurrent prostate cancer after radical prostatectomy.
This systematic review and meta-analysis updates data on the incidence of cardiovascular adverse events in clinical trials of immune checkpoint inhibitors alone and in combination therapies and provides recommendations for management among patients treated for cancer.
This case report describes cutaneous collagenous vasculopathy associated with loncastuximab tesirine, an antibody-drug conjugate treatment.
This case control study evaluates potential signals for suicidal and self-injurious adverse drug reactions associated with semaglutide and liraglutide.
- GLP-1 Receptor Agonists and Suicidality—Caution Is Needed JAMA Network Open Opinion August 20, 2024 Global Health Adverse Drug Events Pharmacy and Clinical Pharmacology Diabetes Diabetes and Endocrinology Full Text | pdf link PDF open access
This cohort study evaluates time to initiation of exogenous sex steroid hormones among adolescents and young adults with gender dysphoria receiving health care through the US military.
This cross-sectional study examines the association between prescribing clinician specialty and patients not picking up their initial PrEP prescription.
This randomized, adaptive phase 2 to 3 clinical trial assesses the efficacy and safety of HSK16149 capsules, an oral γ-aminobutyric acid (GABA) analogue, to treat patients with diabetic peripheral neuropathic pain.
This special communication examines the benefits and risks of using large language models to support medical product postmarket surveillance.
- New Cardiovascular Risk Calculator Could Reduce Blood Pressure Medication Prescribing JAMA News August 16, 2024 Cardiology Full Text | pdf link PDF free
This secondary analysis of adult patients in the Penicillin Allergy Clinical Decision Rule (PALACE) Study investigates the risk of self-reported penicillin allergy despite removal of penicillin allergy label.
This cohort study evaluates the association of a US state copayment cap with out-of-pocket spending, medication adherence, and health care services utilization for diabetes-related complications among individuals with type 1 diabetes.
This Viewpoint describes new maximum contaminant levels set by the Environmental Protection Agency for specific perfluoroalkyl and polyfluoroalkyl substances (PFASs) and discusses the role clinicians can play in addressing their patients’ PFAS health concerns.
Select Your Interests
Customize your JAMA Network experience by selecting one or more topics from the list below.
- Academic Medicine
- Acid Base, Electrolytes, Fluids
- Allergy and Clinical Immunology
- American Indian or Alaska Natives
- Anesthesiology
- Anticoagulation
- Art and Images in Psychiatry
- Artificial Intelligence
- Assisted Reproduction
- Bleeding and Transfusion
- Caring for the Critically Ill Patient
- Challenges in Clinical Electrocardiography
- Climate and Health
- Climate Change
- Clinical Challenge
- Clinical Decision Support
- Clinical Implications of Basic Neuroscience
- Complementary and Alternative Medicine
- Consensus Statements
- Coronavirus (COVID-19)
- Critical Care Medicine
- Cultural Competency
- Dental Medicine
- Dermatology
- Diabetes and Endocrinology
- Diagnostic Test Interpretation
- Drug Development
- Electronic Health Records
- Emergency Medicine
- End of Life, Hospice, Palliative Care
- Environmental Health
- Equity, Diversity, and Inclusion
- Facial Plastic Surgery
- Gastroenterology and Hepatology
- Genetics and Genomics
- Genomics and Precision Health
- Global Health
- Guide to Statistics and Methods
- Hair Disorders
- Health Care Delivery Models
- Health Care Economics, Insurance, Payment
- Health Care Quality
- Health Care Reform
- Health Care Safety
- Health Care Workforce
- Health Disparities
- Health Inequities
- Health Policy
- Health Systems Science
- History of Medicine
- Hypertension
- Images in Neurology
- Implementation Science
- Infectious Diseases
- Innovations in Health Care Delivery
- JAMA Infographic
- Law and Medicine
- Leading Change
- Less is More
- LGBTQIA Medicine
- Lifestyle Behaviors
- Medical Coding
- Medical Devices and Equipment
- Medical Education
- Medical Education and Training
- Medical Journals and Publishing
- Mobile Health and Telemedicine
- Narrative Medicine
- Neuroscience and Psychiatry
- Notable Notes
- Nutrition, Obesity, Exercise
- Obstetrics and Gynecology
- Occupational Health
- Ophthalmology
- Orthopedics
- Otolaryngology
- Pain Medicine
- Palliative Care
- Pathology and Laboratory Medicine
- Patient Care
- Patient Information
- Performance Improvement
- Performance Measures
- Perioperative Care and Consultation
- Pharmacoeconomics
- Pharmacoepidemiology
- Pharmacogenetics
- Pharmacy and Clinical Pharmacology
- Physical Medicine and Rehabilitation
- Physical Therapy
- Physician Leadership
- Population Health
- Primary Care
- Professional Well-being
- Professionalism
- Psychiatry and Behavioral Health
- Public Health
- Pulmonary Medicine
- Regulatory Agencies
- Reproductive Health
- Research, Methods, Statistics
- Resuscitation
- Rheumatology
- Risk Management
- Scientific Discovery and the Future of Medicine
- Shared Decision Making and Communication
- Sleep Medicine
- Sports Medicine
- Stem Cell Transplantation
- Substance Use and Addiction Medicine
- Surgical Innovation
- Surgical Pearls
- Teachable Moment
- Technology and Finance
- The Art of JAMA
- The Arts and Medicine
- The Rational Clinical Examination
- Tobacco and e-Cigarettes
- Translational Medicine
- Trauma and Injury
- Treatment Adherence
- Ultrasonography
- Users' Guide to the Medical Literature
- Vaccination
- Venous Thromboembolism
- Veterans Health
- Women's Health
- Workflow and Process
- Wound Care, Infection, Healing
- Register for email alerts with links to free full-text articles
- Access PDFs of free articles
- Manage your interests
- Save searches and receive search alerts

Clinical Pharmacology: Current Topics and Case Studies
- © 2016
- Latest edition
- Markus Müller 0
Universitätsklinik für Klinische, Medizinische Universität Wien, Wien, Austria
You can also search for this editor in PubMed Google Scholar
Updated and revised 2nd edition
Summarizes the latest topics, tools and clinical trials in clinical pharmacology
Added value for employees in drug research and development
61k Accesses
13 Citations
7 Altmetric
This is a preview of subscription content, log in via an institution to check access.
Access this book
Subscribe and save.
- Get 10 units per month
- Download Article/Chapter or eBook
- 1 Unit = 1 Article or 1 Chapter
- Cancel anytime
- Available as EPUB and PDF
- Read on any device
- Instant download
- Own it forever
- Compact, lightweight edition
- Dispatched in 3 to 5 business days
- Free shipping worldwide - see info
- Durable hardcover edition
Tax calculation will be finalised at checkout
Other ways to access
Licence this eBook for your library
Institutional subscriptions
About this book
Similar content being viewed by others.
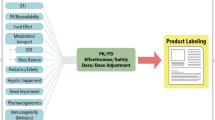
Clinical Pharmacology Regulatory Sciences in Drug Development and Precision Medicine: Current Status and Emerging Trends

Clinical Drug Trials: The Path to the Patient

Clinical Research in Pharmaceutical Drug Development
- drug development
- clinical trial
- gene therapy
- pharmacodynamics
- drug research
- pharmacotherapy
Table of contents (23 chapters)
Front matter, introduction, the discipline of clinical pharmacology.
Markus Müller
Current Issues in Drug Development
Current issues in drug regulation.
- Christa Wirthumer-Hoche, Brigitte Bloechl-Daum
Current Topics in Drug Reimbursement
- Anna Bucsics, Robert Sauermann, Valerie Nell-Duxneuner
Clinical Trials
Ethics in clinical research.
- Ernst Singer, Christiane Druml
Good Clinical Practice (GCP) and Scientific Misconduct
- Brigitte Bloechl-Daum
Phase I Studies and First-In-Human Trials
- Ulla Derhaschnig, Bernd Jilma
Clinical Trials: Interventional Studies
- Michael Wolzt, Stefan Aschauer
Observational Studies
- Harald Herkner, Christoph Male
Tools in Clinical Pharmacology
Tools in clinical pharmacology: imaging techniques.
- Martin Bauer, Oliver Langer
Pharmacokinetics II: 14C-Labelled Microdosing in Assessing Drug Pharmacokinetics at Phase 0
- Graham Lappin
Current Concepts of Pharmacogenetics, Pharmacogenomics, and the “Druggable” Genome
- Wolfgang M. Schmidt, Robert M. Mader
Pharmacokinetics I: PK-PD Approach, the Case of Antibiotic Drug Development
- Sherwin K. B. Sy, Hartmut Derendorf
Epidemiology and Biostatistics
- Gerhard Garhöfer, Leopold Schmetterer
Placebo Effects and Placebo Control in Clinical Trials
- Johannes Pleiner-Duxneuner
Topics in Clinical Pharmacology
“The aim is to describe the role of the discipline of clinical pharmacology in drug discovery. … The intended audience is faculty, researchers, and advanced students from academia and the pharmaceutical industry who are learning about or involved in the process of drug discovery. … This book provides a multitude of very useful insights about the successes and failures in drug development for scientists who are, or will be, involved in the process of drug discovery.” (Thomas L. Pazdernik, Doody's Book Reviews, May, 2016)
Editors and Affiliations
About the editor, bibliographic information.
Book Title : Clinical Pharmacology: Current Topics and Case Studies
Editors : Markus Müller
DOI : https://doi.org/10.1007/978-3-319-27347-1
Publisher : Springer Cham
eBook Packages : Biomedical and Life Sciences , Biomedical and Life Sciences (R0)
Copyright Information : Springer International Publishing Switzerland 2016
Hardcover ISBN : 978-3-319-27345-7 Published: 25 March 2016
Softcover ISBN : 978-3-319-80118-6 Published: 25 April 2018
eBook ISBN : 978-3-319-27347-1 Published: 15 March 2016
Edition Number : 2
Number of Pages : VI, 405
Number of Illustrations : 14 b/w illustrations, 16 illustrations in colour
Topics : Pharmacology/Toxicology , Pharmacy , Pharmacotherapy
- Publish with us
Policies and ethics
- Find a journal
- Track your research
- Remote Access
- Save figures into PowerPoint
- Download tables as PDFs
Top 125 Drug Card Case Quiz
- Katzung Pharmacology Cases
- Pharmacotherapy Casebook and Care Plans
- Standardized Patient Cases
- Pharmacogenomics: A Primer for Clinicians
- Transitions of Care in Pharmacy Casebook
- Pharmacy Practice and Tort Law
- Case Files®: Pharmacology
- G&G Pharm Cases
- Pathophysiology Cases
- Infectious Diseases: A Case Study Approach
- Harrison’s Visual Case Challenge

Author(s): Jill M. Kolesar, PharmD, MS, BCPS, FCCP; Lee C. Vermeulen, BSPharm, MS, FCCP, FFIP
- 001 Case 001-Surgical Care
- 002 Case 002-Hypertension Case # 1
- 003 Case 003-Hypertension Case # 2
- 004 Case 004-Hypertension Case # 3
- 005 Case 005-Hypertension Case # 4
- 006 Case 006-Hypertension Case # 5
- 007 Case 007-Dyslipidemia Case # 1
- 008 Case 008-Dyslipidemia Case # 2
- 009 Case 009-Stable Ischemic Heart Disease
- 010 Case 010-Acute Coronary Syndromes
- 011 Case 011-Cardiovascular Respiratory Decompensation
- 012 Case 012-Chronic Heart Failure Case # 1
- 013 Case 013-Chronic Heart Failure Case # 2
- 014 Case 014-Venous Thromboembolism-Pulmonary Embolism Case # 1
- 015 Case 015-Venous Thromboembolism-Pulmonary Embolism Case # 2
- 016 Case 016-Stroke
- 017 Case 017-Arrhythmias
- 018 Case 018-Cardiac Arrest, Resuscitation
- 019 Case 019-Acne and Pediatric Dermatology Disorders
- 020 Case 020-Psoriasis
- 021 Case 021-Atopic Dermatitis
- 022 Case 022-Miscellaneous Dermatology
- 023 Case 023-Peptic Ulcer Disease, GERD Case # 1
- 024 Case 024-Peptic Ulcer Disease, GERD Case # 2
- 025 Case 025-Irritable Bowel Syndrome
- 026 Case 026-Diarrhea
- 027 Case 027-Constipation
- 028 Case 028-Crohn Disease
- 029 Case 029-Anemia
- 030 Case 030-CNS Infections
- 031 Case 031-Lower Respiratory Tract Infection
- 032 Case 032-Upper Respiratory Tract Infection
- 033 Case 033-Skin and Soft-Tissue Infections; Fungal (Multiple Patient Case)
- 034 Case 034-Infective Endocarditis
- 035 Case 035-Gastrointestinal Infections
- 036 Case 036-Intra-Abdominal Infections
- 037 Case 037-Urinary Tract Infection
- 038 Case 038-Skin and Soft Tissue Infection (Bacterial), Bone and Joint Infections
- 039 Case 039-Miscellaneous Bacterial Infections
- 040 Case 040-Sexually Transmitted Infections
- 041 Case 041-Sepsis and Septic Shock
- 042 Case 042-Fungal Infections Case # 1
- 043 Case 043-Fungal Infections Case # 2
- 044 Case 044-Viral Infections
- 045 Case 045-Parasitic Infections
- 046 Case 046-Infectious Endocarditis
- 047 Case 047-Viral Hepatitis, Hepatitis Vaccination
- 048 Case 048-Influenza and Influenza Vaccination (Multiple Patient Case)
- 049 Case 049-Foot and Nail Fungal Infection
- 050 Case 050-Vaccines Case # 1
- 051 Case 051-Vaccines Case # 2
- 052 Case 052-Travel Health (Multiple Patient Case)
- 053 Case 053-Human Immunodeficiency Virus Infection
- 054 Case 054-Systemic Lupus Erythematosus
- 055 Case 055-Solid Organ Transplantation
- 056 Case 056-Erectile Dysfunction
- 057 Case 057-Benign Prostatic Hyperplasia
- 058 Case 058-Attention Deficit Hyperactivity Disorder
- 059 Case 059-Substance Use Disorders (Opioids)
- 060 Case 060-Substance-Related Disorders (Alcohol, Nicotine)
- 061 Case 061-Schizophrenia
- 062 Case 062-Depression Case # 1
- 063 Case 063-Depression Case # 2
- 064 Case 064-Panic Disorder
- 065 Case 065-Bipolar Disorder
- 066 Case 066-Anxiety Disorders Case # 1
- 067 Case 067-Anxiety Disorders Case # 2
- 068 Case 068-Sleep-Wake Disorders
- 069 Case 069-Diabetes Mellitus (Type 2) Case # 1
- 070 Case 070-Diabetes Mellitus (Type 2) Case # 2
- 071 Case 071-Diabetes Mellitus (Type 2) Case # 3
- 072 Case 072-Thyroid Disorders
- 073 Case 073-Obesity
- 074 Case 074-Migraine Headache
- 075 Case 075-Critical Care Pain, Agitation, Delirium
- 076 Case 076-Alzheimer Disease
- 077 Case 077-Multiple Sclerosis
- 078 Case 078-Epilepsy Case # 1
- 079 Case 079-Epilepsy Case # 2
- 080 Case 080-Parkinson Disease
- 081 Case 081-Pain Management Case # 1
- 082 Case 082-Pain Management Case # 2
- 083 Case 083-Pain Management Case # 3
- 084 Case 084-Brain Cancer
- 085 Case 085-Breast Cancer
- 086 Case 086-Lung Cancer
- 087 Case 087-Colorectal Cancer
- 088 Case 088-Prostate Cancer
- 089 Case 089-Hodgkin Lymphoma
- 090 Case 090-Lymphoma
- 091 Case 091-Acute Myeloid Leukemia
- 092 Case 092-Chronic Lymphocytic Leukemia
- 093 Case 093-Multiple Myeloma
- 094 Case 094-Myelodysplastic Syndrome
- 095 Case 095-Renal Cell Cancer
- 096 Case 096-Melanoma
- 097 Case 097-Ovarian Cancer
- 098 Case 098-Chemotherapy-Induced Emesis
- 099 Case 099-Cancer Pain
- 100 Case 100-Stem Cell Transplant
- 101 Case 101-Idiopathic Thrombocytopenic Purpura, Thrombotic Thrombocytopenic Purpura
- 102 Case 102-Glaucoma
- 103 Case 103-Bacterial Conjunctivitis
- 104 Case 104-Allergic Conjunctivitis
- 105 Case 105-Asthma Case # 1
- 106 Case 106-Asthma Case # 2
- 107 Case 107-Allergic Rhinitis Case # 1
- 108 Case 108-Allergic Rhinitis Case # 2
- 109 Case 109-Chronic Obstructive Pulmonary Disease
- 110 Case 110-Osteoarthritis
- 111 Case 111-Rheumatoid Arthritis
- 112 Case 112-Osteoporosis
- 113 Case 113-Gout
- 114 Case 114-Urinary Disorders – Male
- 115 Case 115-Urinary Disorders – Female
- 116 Case 116-Menopause Care
- 117 Case 117-Menstruation-Related Disorders
- 118 Case 118-Contraception
- 119 Case 119-Obstetric Care
- 120 Case 120-Family Medicine Case # 1 (Multiple Patient Case)
- 121 Case 121-Family Medicine Case # 2 (Multiple Patient Case)
- 122 Case 122-Family Medicine Case # 3 (Multiple Patient Case)
- 123 Case 123-Family Medicine Case # 4 (Multiple Patient Case)
- 124 Case 124-Family Medicine Case # 5 (Multiple Patient Case)
- 125 Case 125-Family Medicine Case # 6 (Multiple Patient Case)
| Date | Medical Condition | Therapeutic Goals | Drug-Therapy Problem | Recommendations and Interventions | Monitoring Parameters, Desired Endpoints, and Frequency | Follow-up plan |
|---|
Academia.edu no longer supports Internet Explorer.
To browse Academia.edu and the wider internet faster and more securely, please take a few seconds to upgrade your browser .
Enter the email address you signed up with and we'll email you a reset link.
- We're Hiring!
- Help Center


Drugs in Use Clinical Case Study for Pharmacist

Related Papers
Australian journal of general practice
Louise Deeks
Non-dispensing pharmacists are being suggested as a useful addition to the workforce in general practice. The aim of this study was to describe the activities of three general practice pharmacists over six months in a pilot trial. Three general practices integrated a part-time (15.2-16 hours per week) non-dispensing pharmacist to be employed according to their individual skillset and local workplace needs. Each general practice pharmacist maintained a daily activity diary, which was subsequently analysed. The general practice pharmacists' activities were categorised as quality of practice (37%), administration (34%), medication review (19%) and patient education (11%). Within the quality of practice category, most time was spent conducting clinical audits (47%). Over the course of the six months, time spent on administration decreased, while time communicating with general practitioners (GPs) on clinical issues increased. The general practice pharmacists conducted a range of pre...
Integrated Pharmacy Research and Practice
Financial Accountability & Management
Audrey Paterson
Judy Mullan
Presentation at 2015 Primary Health Care Research Conference, Adelaide, Australia, 29-31 July
Journal of Pharmaceutical Policy and Practice
Elisha Chopra
Background General practices in primary care across England are increasingly employing clinical pharmacists to help tackle the workforce crisis and alleviate pressure. Clinical pharmacists can provide administrative and clinical duties, including non-medical prescribing, advice on polypharmacy and medicines optimisation. The aim of this study was to investigate the distribution of clinical pharmacists in general practice across England, and explore the relationship between the distribution and regional demography. Methods This study used publicly available government database from various sources pertaining to primary care general practice workforce and population demographics of England. The number and distribution of pharmacists working within general practices in England were analysed and compared across practices considering general practitioner (GP), nurse and patient population in the practices, patients age ≥ 65 years and over and the Index of Multiple Deprivation (IMD) score...
Delivering primary healthcare: Pharmacists taking the next leap forward
ecehan balta
Internal and Emergency Medicine
Anna Padula
Claire Mann
Journal of Pharmaceutical Care & Health Systems
Fatima Fasih
Saudi Pharmaceutical Journal
suja abraham
Loading Preview
Sorry, preview is currently unavailable. You can download the paper by clicking the button above.
RELATED PAPERS
American Journal of Biomedical Science & Research
Abdul Kader Mohiuddin
Archives in Biomedical Engineering & Biotechnology
Journal of Applied Pharmaceutical Sciences and Research
Tarun Virmani
Alan Kearney
British Journal of Clinical Pharmacology
James Mclay
Handbook for the Hospital and Community Pharmacists
Australasian Medical Journal
Jeff Hughes
Prafull Agarwal
Pharmacy World & Science
Journal of Primary Health Care
June Tordoff
Book Review
INNOVATIONS in pharmacy
Jeffrey Aronson
Annals of Pharmacotherapy
IP innovative publication pvt. ltd
IP Innovative Publication Pvt. Ltd. , Abdul Kader Mohiuddin
Nataly Martini
American Journal of Health-system Pharmacy
Craig Pedersen
The British journal of general practice : the journal of the Royal College of General Practitioners
Primary Care: Clinics in Office Practice
Michelle Bholat
Sociology of Health & Illness
Marjorie Weiss , Jane Sutton
International Journal of Pharmacy Practice
Amanda Evans
Olihe Okoro
- We're Hiring!
- Help Center
- Find new research papers in:
- Health Sciences
- Earth Sciences
- Cognitive Science
- Mathematics
- Computer Science
- Academia ©2024
- Open access
- Published: 27 August 2024
Developing a validated methodology for identifying clozapine treatment periods in electronic health records
- Aviv Segev ORCID: orcid.org/0000-0002-9550-3895 1 , 2 , 3 , 4 na1 ,
- Risha Govind ORCID: orcid.org/0000-0001-9925-7866 1 , 5 na1 ,
- Ebenezer Oloyede ORCID: orcid.org/0000-0003-1352-4017 6 , 7 ,
- Hamilton Morrin ORCID: orcid.org/0000-0002-7801-0212 2 , 8 ,
- Amelia Jewell ORCID: orcid.org/0000-0002-0887-2159 1 ,
- Rowena Jones ORCID: orcid.org/0000-0002-3426-1394 9 , 10 ,
- Laura Mangiaterra 1 , 2 , 11 ,
- Stefano Bonora 1 , 2 , 12 ,
- Ehtesham Iqbal ORCID: orcid.org/0000-0001-9477-9745 5 ,
- Robert Stewart ORCID: orcid.org/0000-0002-4435-6397 1 , 5 ,
- Matthew Broadbent 1 &
- James H. MacCabe ORCID: orcid.org/0000-0002-6754-1018 2 , 13
BMC Psychiatry volume 24 , Article number: 584 ( 2024 ) Cite this article
13 Accesses
Metrics details
Clozapine is the only recommended antipsychotic medication for individuals diagnosed with treatment-resistant schizophrenia. Unfortunately, its wider use is hindered by several possible adverse effects, some of which are rare but potentially life threatening. As such, there is a growing interest in studying clozapine use and safety in routinely collected healthcare data. However, previous attempts to characterise clozapine treatment have had low accuracy.
To develop a methodology for identifying clozapine treatment dates by combining several data sources and implement this on a large clinical database.
Non-identifiable electronic health records from a large mental health provider in London and a linked database from a national clozapine blood monitoring service were used to obtain information regarding patients' clozapine treatment status, blood tests and pharmacy dispensing records. A rule-based algorithm was developed to determine the dates of starting and stopping treatment based on these data, and more than 10% of the outcomes were validated by manual review of de-identified case note text.
A total of 3,212 possible clozapine treatment periods were identified, of which 425 (13.2%) were excluded due to insufficient data to verify clozapine administration. Of the 2,787 treatments remaining, 1,902 (68.2%) had an identified start-date. On evaluation, the algorithm identified treatments with 96.4% accuracy; start dates were 96.2% accurate within 15 days, and end dates were 85.1% accurate within 30 days.
Conclusions
The algorithm produced a reliable database of clozapine treatment periods. Beyond underpinning future observational clozapine studies, we envisage it will facilitate similar implementations on additional large clinical databases worldwide.
Peer Review reports
Introduction
Treatment-resistant schizophrenia (TRS) is associated with poor prognosis, long-term disability, and increased mortality [ 1 ]. The introduction of clozapine in the late 1950s provided clinicians with a unique option in the pharmacological treatment of individuals with TRS [ 2 , 3 ]. Despite its discovery many decades ago and the development of many drugs since then, clozapine remains the treatment of choice in TRS due to its superior efficacy [ 4 ]. Current evidence indicates that of the 30% of patients diagnosed with schizophrenia who do not respond to conventional antipsychotics, 50% will respond to clozapine [ 5 ]. Moreover, several studies have shown that clozapine yields the best prognosis versus other antipsychotics, not only for psychiatric clinical scales but also for broader health outcomes, including all-cause mortality [ 6 , 7 , 8 , 9 ].
Unfortunately, despite a considerable evidence-base for therapeutic benefits, clozapine is associated with a range of adverse effects, including potentially life-threatening events such as myocarditis, ileus and blood dyscrasias, mandating regular blood tests [ 10 , 11 ]. As such, there has been much interest in the study of clozapine: basic-science research, that attempts to elucidate the reasons for its superior efficacy or the mechanisms underlying its side effects [ 12 ], clinical and laboratory biomarkers to predict its efficacy [ 13 ] and clinical studies to better understand, detect and manage its adverse events [ 14 , 15 ]. Such insights may help to diminish underutilization of clozapine [ 16 ] and to prevent unnecessary clozapine cessation and the associated increased risk of relapse [ 17 ]. Many of these clinical observational studies rely on small, biased samples, and as such are disadvantaged by low statistical power and uncertainty around the generalizability of findings. In view of this, it is important to enable investigators to reliably study large, unbiased cohorts of patients prescribed clozapine, with accurate data on the dates when treatment was started and stopped.
South London and Maudsley NHS Foundation Trust (SLaM) is one of the largest mental health providers in Europe, catering to all secondary mental health care needs of over 1.3 million people spanning four London boroughs (Lambeth, Southwark, Lewisham, and Croydon). It contains clinical records of over 500,000 patients, including many individuals diagnosed with psychotic spectrum disorders, and patients who were or are currently prescribed clozapine. In the 2000s, SLaM records became digital and complete electronic health records (EHR) became available during 2006. In 2008, data from the SLaM EHR were made available to researchers through the Clinical Record Interactive Search (CRIS), which is a de-identified copy of the entire SLaM EHR [ 18 ]. The granularity of this type of data resource presents valuable opportunities for novel and informative observational studies. However, as with all real-world databases, there is the potential for input errors or missing data. Therefore, when using such data for research, data cleaning, validating, and processing of the desired cohort are required. Overcoming these challenges for clozapine pharmacoepidemiology requires a collaboration of clinicians, familiar with the patterns and protocols surrounding the usage of the medication, alongside informaticians, proficient in handling and analysing real-world big data. This paper describes the rationale, process and heuristics-based algorithms used to create a database of clozapine treatment periods, derived from CRIS at SLaM, to serve as a resource for large-scale retrospective clozapine studies. The generation of this database provides great potential for upcoming observational studies on clozapine. Beyond enabling studies on SLaM users, the heuristics and algorithms outlined in this paper can be adapted, with appropriate modifications, to suit any other extensive clinical database resembling CRIS in terms of data sources on an international scale. Consequently, it will facilitate the development of additional databases on clozapine treatment periods, thereby laying the groundwork for further research in diverse countries and psychiatric services.
The data sources – CRIS and ZTAS databases
The Clinical Record Interactive Search (CRIS), previously described, makes available all SLaM electronic health records for secondary analysis within a robust data security and governance framework [ 19 ].
The Zaponex Treatment Access System (ZTAS) is one of three mandatory blood monitoring service providers in the UK. All patients prescribed clozapine at SLaM are registered with ZTAS [ 20 ]. ZTAS has a database of all the mandatory blood test results and all the clozapine treatment-related statuses (e.g., on-treatment, discontinued etc.) assigned to each patient.
SLaM’s Clinical Data Linkage Service (CDLS) provides a secure data environment that allows CRIS to be linked with other external clinical and non-clinical databases, including ZTAS data, using individual matching but then discarding the identifiers, allowing the data to be made available in the same de-identified format as CRIS [ 21 ].
The linkage between CRIS and ZTAS, facilitated through CLDS, is the foundation of this cohort. The two databases were first linked in May 2016, followed by a refresh in October 2019. Therefore, the time frame for the current study starts with the establishment of ZTAS in 2004, and ends with its most recent linkage to CRIS, in October 2019.
Clinical aspects of clozapine prescription
There are several aspects of clozapine treatment that make it challenging to determine if and when clozapine treatments begin and end from the aforementioned databases. In clinical practice, there is often extensive discussion with the patient and treating team regarding the possibility of starting clozapine, for months or even years before the treatment is started. Thus, relying on natural language processing tools, which have shown success in identifying medications through textual references in medical records, may result in numerous false positives, particularly in the case of clozapine.
Patients may have single or multiple periods of clozapine treatment. Due to the adverse-effects profile of clozapine and its mandatory monitoring, any cessation of clozapine lasting more than 48 h requires re-initiation of the drug and blood monitoring as though for the first time [ 22 ]. Our algorithm aimed to identify each clozapine treatment period, even when several were recorded for the same patient. This was further complicated by the fact that patients may be prescribed clozapine for long periods but with infrequent clinical contacts, so the algorithm must infer whether there was a treatment break between two clinical contacts.
Another complication is that patients are sometimes registered with ZTAS but are ultimately not prescribed clozapine for various reasons (e.g., non-adherence, medical contraindications), or there may be a long delay between registration and receipt of the first dose.
Outline of algorithm
The first step was designed to confirm the validity of the treatment period, meaning that clozapine was indeed administered, rather than just intended to be prescribed. In addition, data were collected to define each treatment period, which involved identifying start- and end-dates. At the second stage, we used data from adjacent periods to further confirm clozapine administration, and to determine when two apparently separated treatments, were in fact one continuous treatment. Three data sources were used for this purpose (described in detail below): i) patients' recorded status, ii) blood test monitoring records, and iii) pharmacy dispensing records.
When devising the algorithm, it was decided to value precision over recall. Thus, the algorithm takes a conservative approach, even at the expense of missing potential treatment periods.
As part of the algorithm development, each heuristic implemented in this algorithm was examined separately. However, the validation and verification of the entire algorithm was done as a whole.
First data source – patient status
In clinical practice, registration with ZTAS is required for clozapine to be dispensed and administered. ZTAS receives notification and grants approval for each initiation of clozapine. When a clozapine treatment ends, the hospital pharmacy will report it to the ZTAS team, and if an additional clozapine treatment attempt is planned, re-registration with ZTAS is required. Possible patient statuses include "on-treatment", "interrupted", "discontinued", "transferred" or "non-rechallengeable" (and several variations of these). A patient’s status changes over time, and the dates of change are recorded, thus a history of dated status changes is stored. Thus, the status of the patient appears at face value to be a relatively robust and reliable dataset.
However, status was found to be inconsistently recorded in practice: some patients had multiple "on-treatment" entries, or multiple redundant "discontinuation" entries, or a confusing sequence of statuses. For example, if a patient's blood test returns with abnormal results, often a status of "interrupted" would appear on that day, as clozapine administration is paused. If an additional abnormal result re-occurs the following day, the patient's status would change to "discontinued". On the same day, or within a few days, usually after consultation between the ZTAS and clinical teams, the status would then change to "discontinued – final", and then "non-re-challengeable". As a result, each clozapine treatment period could be surrounded by many redundant and sometimes contradictory status entries. Accordingly, we classified all possible statuses to one of two groups – start-signals (e.g., "on-treatment") or stop-signals (e.g., "discontinued").
To overcome the problem of multiple and redundant entries, clozapine treatments were initially identified by locating the first start-signal status (per date), either as the first entry for the patient, or following a previous stop-signal. In the same manner, the end of the treatment was identified as the first stop-signal after a previous start-signal. Stop-signals were ignored if on the same day there was an additional start-signal, during an ongoing clozapine treatment period. The periods between the start- and stop-signals were defined as "tentative clozapine treatment periods", that need to be validated and examined. Tentative treatment periods of less than 7 days were excluded from the analysis. The rational for this exclusion stemmed from several reasons: it is likely that such very short treatment periods would not be significant to the study of clozapine; such a short window of treatment is more likely to represent the intention to administer clozapine, without the patient starting the treatment (or taking very few doses); and difficulty to identify markers for an automated verification for clozapine being administered.
There were several reasons why the start-signals and stop-signals could not be considered reliable on their own. Though the start-signals were designed to be assigned at the start of clozapine initiation, relying on patient status had limitations. Patients who were prescribed clozapine prior to the start date of the ZTAS database at our disposal, and who therefore were added to ZTAS during their clozapine treatment, had an inaccurate "start-signal". Similar problems occurred with patients who were registered for SLaM care after a transfer from another Trust in the UK or a different country whilst already receiving clozapine treatment. Another limitation was that the start-signal was an indicator of ZTAS approving a patient's clozapine treatment but did not necessarily indicate that the clozapine treatment was initiated. Delays in clozapine initiation could stem from different reasons, such as a patient’s refusal, physical deterioration, improvement in mental status, etc., and the actual commencement of clozapine dose titration might start weeks after a start-signal appeared in the status field. While clozapine treatment occurred outside the windows defined by the patient start- and stop-signals only in specific circumstances (described later), the presence of the window did not guarantee that clozapine was in fact administered, or that the start-signal corresponded to the actual administration start-date.
Another caveat was clerical errors of omission or commission. Errors of omission were particularly abundant in older patient records, where recording was less systematic. In such cases, a treatment could be evident in the clinical notes but have no preceding start-signal and therefore potentially missed in an algorithm relying on this. Errors of commission included incorrect status entries recorded. An example was a status entry of "transferred", despite the patient's records clearly showing that they remained under the care of SLaM, or "interrupted" despite the clinical records not indicating any problem or change in clozapine administration. Due to these limitations, it was necessary to address and integrate additional datasets.
Second data source – blood test monitoring
Blood monitoring information was used both for confirming the authenticity of the treatment period and for re-affirming actual start-dates. For each tentative clozapine treatment period, we established the pattern of blood test monitoring. To identify these patterns, we relied on the UK mandatory monitoring guidelines, which require weekly blood monitoring for 18 weeks, followed by fortnightly monitoring for an additional 34 weeks, after which monitoring is reduced to a monthly basis until the treatment is stopped [ 22 ]. Using the timing of blood tests, we aimed to identify several possible patterns of monitoring, with the following hierarchy: (1) Sustained weekly pattern (longer than 5 weeks); (2) Short weekly pattern (5 weeks or shorter); (3) Monthly pattern (of over 6 months); and (4) No pattern. The detailed criteria are elaborated in the supplementary material (S1).
ZTAS contains the results of blood tests and the date they were taken, but also the type of blood test in relation to the clozapine treatment period. The blood test that precedes actual administration is defined as "Baseline" (required for ZTAS approval of clozapine treatment). Tests during the clozapine treatment period are named "New". Tests that were entered retrospectively are defined as "Historical". Therefore, we used this information to further verify the actual start-date of the treatment period. When a "Baseline" blood test was recorded ± 10 days from a start-signal, it was regarded as re-affirming the actual start-date (as opposed to artificial start that, a label given to those starting clozapine prior to 2004 or having started this elsewhere prior to being transferred to SLaM).
Blood test monitoring records, when present, were considered a robust source of information. However, several caveats needed to be taken into consideration. The recording of blood tests in ZTAS did not systematically start with the establishment of ZTAS, and for several years was inconsistent between different service providers within SLaM. As such, a lack of blood test records did not mean blood was not taken. An additional problem was that the type of blood test was recorded improperly at times, and a "baseline" test might be labelled as "new". A third problem, limiting our ability to rely on blood test monitoring, was that blood could be drawn several times prior to clozapine initiation (due to the patient changing their mind about treatment, problematic results, etc.), or after clozapine cessation (mainly when attempting to verify neutrophil count normalization following neutropenia, per UK mandatory guidelines). However, the presence of blood test results outside the "treatment window" of start–end signals (as derived from a patient status) would help to detect errors of omission or commission in status records. A common example was lack of status for patients who were entered into ZTAS in the early days of the system when clerical errors were more likely to occur. In these cases, months and even years of repeated blood tests preceded the first status record. Therefore, in cases where blood tests were recorded more than three times prior to the first status, a new tentative "pre-status" treatment period was defined (though start and stop signals were missing). The reason for omitting cases with three or less blood tests was that it was common to see preparatory blood-results before commencing clozapine, preceding the start signal. In addition, analysing the authenticity of these very short periods was extremely challenging. On the other hand, even if those three blood tests represented part of a genuine clozapine treatment period, the inferred treatment period would not have been underestimated substantially. The pre-treatment period was defined as starting at the first blood test and ending at the last blood test preceding the first start-signal. Additional use of blood-monitoring was conducted to ascertain redundant stop signals. As per UK clozapine protocols, clozapine cessation should be followed by four follow-up blood tests. As such, 5 blood tests or less post a stop-signal was considered a per-protocol follow-up. Instances where more than five blood tests were identified outside of a tentative period were flagged and examined manually. Since there were only three such occurrences, it was determined that developing a specific algorithm to analyse these cases would be unnecessary, and thus they were disregarded.
Third data source – pharmacy dispensary records
SLaM Pharmacy records of clozapine dispensing, as with all other medications, are incorporated in the CRIS database and are completed both for inpatients and outpatients. Again, these had face validity as an ideal indication of clozapine administration; however, as with other data resources, they came with several caveats. Pharmacy records began inconsistently during the first years of wider SLaM record digitalization and records were consequently often missing in the first years of CRIS. Moreover, records were sometimes omitted due to technical or human errors. Conversely, dispensary records may exist even in cases where the patient did not receive the prescribed medication, often attributable to reasons such as patient non-compliance, although not limited to this factor alone. Therefore, the dispensary records could only serve as supporting evidence and were not sufficient to be used alone. We regarded dispensary records as re-affirming when at least 3 records of clozapine were recorded at 3 different dates, as a single dispensation might occur when a clozapine treatment did not commence (for example, due to patient reluctance).
Combining the three datasets
Using the described tiers of information, we devised an algorithm (Fig. 1 ) that classified each tentative clozapine treatment period into one of three possible categories:
Clozapine treatment period with identified start-date – in which we could have high certainty both that clozapine was administered and that the inferred start-date was a reliable one.
Clozapine treatment period with undetermined start-date – in which we had enough data to verify, with high level of certainty, that the patient did indeed receive clozapine, but a reliable start-date could not be established. For these treatment periods, there was no valid start-date, but rather a first known date of the treatment. These treatments could have been started only a few days before the first known date or, alternatively, years before it.
Unsubstantiated – In which there were insufficient data to ascertain that clozapine was given.

Clozapine treatment periods categorization per blood tests pattern, baseline type blood tests and pharmacy records
Of note, "pre-status" tentative treatment periods that were discovered and defined by blood tests records (i.e., treatment periods that were not created originally from start and end signals) were addressed during the categorization process in the same manner as status-based treatment periods.
Refining start-dates and end-dates
After the initial classification of treatment periods to these three categories, we implemented further rules to refine the start and end-date of each treatment period, using the treatment period's classification. These refinement rules were created to improve the accuracy of the start-date and the end-date, to overcome clerical errors in the early days of ZTAS, and to improve categorisation. The refinement rules are elaborated in the supplementary material (S2).
Merging clozapine treatment periods
After each treatment period was assigned a category and the start-date and end-date were refined, more information on treatment periods could be inferred using the adjacent periods. We examined the already-existing datasets, alongside the gaps between each period, in order to identify treatment periods that were wrongly identified by the algorithm as two separate periods. We have used clinical heuristics to merge periods, for example:
A clozapine treatment with an identified start-date cannot be a continuation of a previous period, unless it was truncated due to technical error, mandating the gap between the periods to be extremely short, and the first period to be relatively short.
Very short gaps between treatment periods (< 7 days) that do not entail even a short weekly pattern in the following treatment period (sometimes referred as "interrupted protocol"), are unlikely a new period and more often represent an error of commission or technical glitch. A common example would be a patient travelling abroad for more than 30 days and forgetting to send blood results. An "interrupted" status would then be added. Upon the patient's return, if clozapine was administered continuously, the status will change again to "on-treatment", representing the same treatment period, assigned for merging. If the patient stopped clozapine while abroad, the algorithm would recognize the start of a new weekly pattern of blood tests, thus categorizing the new treatment period as one with an identified start-date that would not be merged.
The complete set of rules by which treatment periods were merged are outlined in the supplementary material (S3).
Excluding clozapine treatment periods
Following the merging stage, unsubstantiated treatment periods that were not merged were excluded, and were disregarded in further analyses (except validation). Due to the various indications used, those periods were suspected to be "empty" treatment, meaning that no clozapine was given. These periods remained in the database, unlike the omitted periods, for two reasons:
They might be a focus of interest – for example, concerning the reasons that prevented the administration of clozapine.
Though suspected to be "empty", this impression relied mainly on the continuing lack of corroborative evidence. However, it was assumed that despite the absence of data, some were false negative treatments, i.e. clozapine was given.
Validation of results
After forming the new merged table of all clozapine treatment periods, at least 10% of the treatment periods were randomly selected and manually compared to their full text HER records by an experienced psychiatrist (AS). The accuracy of the start-date, end-date and the classification of the treatment periods were manually verified.
According to the ZTAS database, 2,056 SLaM patients were registered with ZTAS, 41 of whom had never had a blood test or an assigned status. ZTAS recorded 210,173 blood tests and 10,923 statuses for the remaining 2,015 SLaM patients. Patients had a mean of 103 blood tests (SD 76.6, range 1–341), 5 statuses (SD 4.8, range 1–41), and 108 pharmacy dispensaries (SD 98.3, range 1–571).
Figure 2 shows that 3,191 tentative treatment periods were first identified based on the start- and end-signals. An additional 1,241 tentative periods were identified after analysing the blood test data and pharmacy dispensary data. 693 tentative treatments were omitted; 30 (0.9%) were omitted because the period ended within 7 days, and 663 (53.4%) because they were "pre-status" periods with three or fewer blood tests. 510 tentative treatment periods were merged with adjacent periods based on the criteria outlined in Table S3. Glossary of main definitions is listed in Table 1 .

Results of categorization process, per each algorithmic step
After merging, re-categorizing, and refining, there were 3,212 treatment periods. Of these, 425 (13.2%) remained unsubstantiated treatment periods due to insufficient data to confirm that clozapine was given, and therefore were excluded.
In total, the algorithm identified 2,787 clozapine treatment periods: 1,902 (68.2%) with an identified start-date and 885 (31.8%) with an indeterminate start-date.
The 2,787 treatments belonged to 1,916 patients. The mean number of treatment periods per patient was 1.45 (median 1, interquartile range 1). 1,346 (70.0%) patients had only one treatment period, 373 (19.5%) had two, and 197 (10.3%) had 3–8 periods. 65.6% of the patients were male, 45.3% were of White ethnicity, and 41.7% were of Black ethnicity. The mean age at the point of the first known clozapine treatment was 39.0 (SD 12.1). Demographic characteristics of patients and treatment periods are displayed in Table 2 .
The final result of the algorithm was 2,787 clozapine treatment periods, which belonged to one of two categories: treatment periods with identified start dates and treatment periods with undetermined start-dates. Similar to the two types of start-points, the endpoints can also be categorised into two types: treatment periods with identified end-dates and treatment periods with undetermined end-dates. Treatments with identified end-dates are those ending with a clear end signal, for example, a "discontinued" status or the end of blood monitoring. Treatments with undetermined end-dates result from unavailable information due to patients being transferred outside of SLaM or treatments that remained ongoing at the end of the study window (October 2019). The proportions of treatments with each start-point and endpoint type are elaborated in Table 3 .
Validations
The validation process results of the algorithm reliability showed high level of accuracy, both in treatment periods' classification as well as in the determination of the periods' start and end-dates (Table 4 ).
This study describes the development process and implementation of an algorithm designed to identify clozapine treatment dates which can be used by researchers when conducting clozapine observational studies. By combining clinical experience with informatics expertise, we were able to create a complex algorithm relying on multiple datasets, each of which had severe limitations as a standalone source of data, but when judiciously combined, yielded highly accurate results.
The final database, which consisted of 2,787 clozapine treatment periods, can serve as an important resource for clozapine studies exploring its efficacy, safety, adherence, and other research area, which may aid to increase clozapine utilization and to prevent redundant clozapine cessation. The validation and verification process yielded very good results, showing that the carefully, specifically designed automated algorithm was successful in spotting "false" treatment periods, and was able to yield good accuracy in determining the start and stop-dates of each period.
It is common for real-world databases to suffer from missing, redundant, and falsely entered information. Errors are bound to occur, especially when the users contributing to this database are both numerous and heterogeneous in professional background (clinical, administrative, etc.). Prolonged development and implementation processes may further contribute to erroneous entries, as time-based changes yield non-uniform records. The algorithm presented in this study attempts to use both clinical insight as well as data-analysis procedures to overcome as many of these errors as possible.
The authors present this study as an example of what can be achieved through the multidisciplinary process of the algorithm creation, consisting of a continuous joint discussion between informaticians and clinicians. While the latter had brought their clinical expertise along with insight into the reality of clinical practice, the informaticians could translate those insights into the structure of the database and relay the numerous problems back to the clinicians for further exploration and feedback. Both the coding itself and the clinical deliberations were conducted collaboratively throughout the process.
Limitations
The main limitation of the methodology stems from missing data. The start-date was indeterminable for over 30% of the treatments. Despite the interplay between the three datasets and the encouraging validation results, missing data was present in all datasets, leading to misclassification or inaccurate dating. As dates might have shifted and valid treatment periods could have been excluded (or not recorded), epidemiological data should be interpreted with caution.
An additional limitation is the possible truncation of treatment periods. It was the authors’ intention to avoid over-merging (joining two separate treatment periods into one), thus risking over-truncation. The authors felt that future studies that might rely on this database, would preferentially accept possible redundant truncation and missing data, as opposed to falsely assumed data. A simple example is patients who were transferred to other hospital Trusts for periods of time between periods of SLaM care, so a break was recorded in the treatment period, often without available documentation whether clozapine treatment continued seamlessly, or was halted and then renewed. Another prominent example is treatment periods with undetermined start-date, which are recorded shortly after a previous clozapine treatment. One option to consider would be that the truncation is a mere technical fault, and that the two periods are actually a continuum. A second option would be that the subsequent treatment is a new clozapine period, for which the algorithm failed to identify a start-date. When the gap exceeded 2–3 months, a third explanation of a clozapine initiation in a different trust is also possible. To avoid redundant merging, it was decided not to merge these periods with the previous period. Many heuristics were examined in order to differentiate and decipher those instances, but none were proven to be sufficiently reliable. Even though the start-date can sometimes be ascertained over a relatively narrow timeframe (as it must occur after the previous end-date), it was decided to leave those labelled as "undetermined start-date" to mark the uncertainty arising. In several cases, for example, it was found that the previous treatment period had ended somewhat earlier than the attributed end-date, making the in-between-periods gap important enough to address these two treatment periods as separate. For example, a patient that was lost to follow-up and stopped taking clozapine might present to the emergency department, and a registration of "interrupted" status would then be recorded, along with the same day record of "new treatment" due to admission to a psychiatric ward.
Contrary to possible over-truncation of treatment periods, some treatment periods may contain several cycles of titration and re-titration. During validation, several incidents in which monthly blood tests returned to weekly pattern were preserved, suggesting clozapine cessation and re-titration. Sampling clinical notes in those dates showed that some of these cases are indeed interruptions or even "micro-interruptions" (while other may be a response to an abnormal blood test, does escalation, etc.). Future improvements of the algorithms can be devised to detect pattern changes, and better outline these conjoined treatment periods.
The validation process showed that 15% of the excluded treatments were false negative, meaning that clozapine was in fact administered in those periods. Though not many, these windows may still contain valuable information. The treatment periods mislabelled and mistakenly excluded were either too short for blood-pattern recognition or were commenced during the early years of the existence of ZTAS and CRIS early, when data was often not recorded properly.
It should be noted that while the database generated by the algorithm can be applied to various aspects of clozapine research, it is not necessarily representative of all clozapine users. The database includes all patients prescribed clozapine within SLaM, which may differ demographically from populations in other regions of the UK or worldwide. However, the significant over-representation of male patients in our sample has been observed in the UK with similar proportions and in other parts of the world, albeit to a lesser extent [ 23 , 24 ].We believe the implications of our study are twofold. The first, more concrete outcome is the creation of a robust clinical database that can facilitate further observational studies on clozapine. The second, albeit currently less tangible, result is the potential to adapt the heuristics and methodology of our algorithm for use in other large psychiatric services to produce additional clozapine databases. However, achieving this goal necessitates significant adaptations due to regulatory differences between countries [ 25 ], such as variations in blood monitoring frequencies and protocols, as well as differences in database structures, the availability of additional reliable datasets (such as dosage information), or the absence of datasets utilized in our methodology. A future area of interest is the development of an algorithm to identify clozapine-induced adverse effects, particularly in relation to clozapine doses. The development of such a tool can have diverse benefits for ensuring patient safety.
This paper describes a highly tailored algorithm developed through close collaboration between clinicians and data scientists. The combined expertise in clinical practices, particularly regarding the medication of interest, along with proficiency in data acquisition and analysis, facilitated the creation of an extensive database comprising clozapine treatment periods. Consequently, this paper presents two applicable products. Firstly, it introduces the described validated clozapine treatment database. Secondly, it presents a validated methodology for compiling clozapine treatment databases, which can be adapted to other large routine clinical databases, in the UK or globally, with necessary modifications to accommodate varying dispensing and blood monitoring regulations. These databases, as SLaM's clozapine database, may serve as a useful tool for researchers through two approaches. Firstly, it may serve as a platform for large dataset queries, for instance when exploring comparisons with other antipsychotics. Secondly, it may serve as a portal to specific sub-populations, which are often challenging to investigate, enabling the study of rare phenomena or clozapine-specific events. future endeavours should aim to include more detailed data, such as dosage information and adverse events.
Availability of data and materials
The data used in this study is available in the CRIS system, as well as the database created by this study.
However, CRIS data is available to researchers at SLaM only, due to patients' confidentiality. Access to it require authorization from SLaM BRC (Biomedical Research Center).
Kane JM, et al. Clinical guidance on the identification and management of treatment-resistant schizophrenia. J Clin Psychiatry. 2019;80:18com12123.
Article PubMed Google Scholar
Crilly J. The history of clozapine and its emergence in the US market: a review and analysis. Hist Psychiatry. 2007;18:39–60.
Mortimer AM. Antipsychotic treatment in schizophrenia: Atypical options and NICE guidance. Eur Psychiatry. 2003;18:209–19.
Siskind D, Mccartney L, Goldschlager R, Kisely S. Clozapine v. first- and second-generation antipsychotics in treatment-refractory schizophrenia: systematic review and meta-analysis. Br J Psychiatry. 2016;209:385–92.
Siskind D, Siskind V, Kisely S. Clozapine response rates among people with treatment-resistant schizophrenia: data from a systematic review and meta-analysis. Can J Psychiatry. 2017;62:772–7.
Article PubMed PubMed Central Google Scholar
McEvoy JP, et al. Effectiveness of clozapine versus olanzapine, quetiapine, and risperidone in patients with chronic schizophrenia who did not respond to prior atypical antipsychotic treatment. Am J Psychiatry. 2006;163:600–10.
Kane J, Honigfeld G, Singer J, Meltzer H. Clozapine for the treatment-resistant schizophrenic. A double-blind comparison with chlorpromazine. Arch Gen Psychiatry. 1988;45:789–96.
Article CAS PubMed Google Scholar
Vermeulen JM, et al. Clozapine and long-term mortality risk in patients with schizophrenia: a systematic review and meta-analysis of studies lasting 1.1–12.5 Years. Schizophr Bull. 2019;45:315–29.
Hayes RD, et al. The effect of clozapine on premature mortality: an assessment of clinical monitoring and other potential confounders. Schizophr Bull. 2015;41:644.
Jakobsen MI, Schaug JP, Nielsen J, Simonsen E. Antipsychotic prescribing practices for outpatients with schizophrenia and reasons for non-clozapine treatment - Data from a Danish quality assessment audit. Nord J Psychiatry. 2023;77:481–90.
Legge SE, et al. Reasons for discontinuing clozapine: a cohort study of patients commencing treatment. Schizophr Res. 2016;174:113–9.
de Bartolomeis A, et al. Clozapine’s multiple cellular mechanisms: What do we know after more than fifty years? A systematic review and critical assessment of translational mechanisms relevant for innovative strategies in treatment-resistant schizophrenia. Pharmacol Ther. 2022;236:108236.
Samanaite R, et al. Biological predictors of clozapine response: a systematic review. Front Psychiatry. 2018;9:327.
Mijovic A, MacCabe JH. Clozapine-induced agranulocytosis. Ann Hematol. 2020;99:2477–82.
Article CAS PubMed PubMed Central Google Scholar
Segev A, et al. Clozapine-induced myocarditis: electronic health register analysis of incidence, timing, clinical markers and diagnostic accuracy. Br J Psychiatry. 2021;219:644–51.
Kelly DL, Freudenreich O, Sayer MA, Love RC. Addressing barriers to clozapine underutilization: a national effort. Psychiatr Serv. 2018;69:224–7.
Leucht S, et al. Antipsychotic drugs versus placebo for relapse prevention in schizophrenia: a systematic review and meta-analysis. Lancet. 2012;379:2063–71.
Stewart R, et al. The South London and Maudsley NHS Foundation Trust Biomedical Research Centre (SLAM BRC) case register: development and descriptive data. BMC Psychiatry. 2009;9:51.
Fernandes AC, et al. Development and evaluation of a de-identification procedure for a case register sourced from mental health electronic records. BMC Med Inform Decis Mak. 2013;13:71.
ZTAS, Zaponex Treatment Access System. https://www.ztas.co.uk/ . Accessed 1 Aug 2024.
Perera G, et al. Cohort profile of the South London and Maudsley NHS Foundation Trust Biomedical Research Centre (SLaM BRC) Case Register: current status and recent enhancement of an Electronic Mental Health Record-derived data resource. BMJ Open. 2016;6:e008721.
Taylor DM, Barnes TRE, Young AH. The Maudsley Prescribing Guidelines in Psychiatry. 14th ed. West Sussex: Wiley-Blackwell; 2021.
Wellesley Wesley E, et al. Gender disparities in clozapine prescription in a cohort of treatment-resistant schizophrenia in the South London and Maudsley case register. Schizophr Res. 2021;232:68–76.
Stroup TS, Gerhard T, Crystal S, Huang C, Olfson M. Geographic and clinical variation in Clozapine use in the United States. Psychiatr Serv. 2014;65:186–92.
Oloyede E, et al. Clozapine haematological monitoring for neutropenia: a global perspective. Epidemiol Psychiatr Sci. 2022;31:e83.
South London and Maudsley Biomedical Research Centre Case Register - Health Research Authority. https://www.hra.nhs.uk/planning-and-improving-research/application-summaries/research-summaries/south-london-and-maudsley-biomedical-research-centre-case-register-3/ . Accessed 1 Aug 2024.
Download references
Acknowledgements
This paper represents independent research part funded by the NIHR Maudsley Biomedical Research Centre at South London and Maudsley NHS Foundation Trust and King’s College London. The views expressed are those of the author(s) and not necessarily those of the NIHR or the Department of Health and Social Care. RS is part-funded by: i) the NIHR Maudsley Biomedical Research Centre at the South London and Maudsley NHS Foundation Trust and King’s College London; ii) the National Institute for Health Research (NIHR) Applied Research Collaboration South London (NIHR ARC South London) at King’s College Hospital NHS Foundation Trust; iii) UKRI – Medical Research Council through the DATAMIND HDR UK Mental Health Data Hub (MRC reference: MR/W014386); iv) the UK Prevention Research Partnership (Violence, Health and Society; MR-VO49879/1), an initiative funded by UK Research and Innovation Councils, the Department of Health and Social Care (England) and the UK devolved administrations, and leading health research charities. JHM receives funding from the National Institute for Health Research (NIHR) NIHR131157, NIHR150308, NIHR131175, and the Biomedical Research Centre at South London and Maudsley NHS Foundation Trust and King’s College London.
Author information
Aviv Segev and Risha Govind contributed equally to this work.
Authors and Affiliations
NIHR Biomedical Research Centre, South London and Maudsley NHS Foundation Trust, London, UK
Aviv Segev, Risha Govind, Amelia Jewell, Laura Mangiaterra, Stefano Bonora, Robert Stewart & Matthew Broadbent
Department of Psychosis Studies, Institute of Psychiatry, Psychology and Neuroscience, King’s College London, London, UK
Aviv Segev, Hamilton Morrin, Laura Mangiaterra, Stefano Bonora & James H. MacCabe
Faculty of Medicine, Tel Aviv University, Tel Aviv, Israel
Shalvata Mental Center, Hod Hasharon, Israel
Institute of Psychiatry, Psychology and Neuroscience, King’s College London, London, UK
Risha Govind, Ehtesham Iqbal & Robert Stewart
Pharmacy Department, South London and Maudsley NHS Foundation Trust, London, UK
Ebenezer Oloyede
Department of Psychiatry, University of Oxford, Oxford, UK
Maudsley Training Programme, South London and Maudsley NHS Foundation Trust, London, UK
Hamilton Morrin
Birmingham and Solihull Mental Health Foundation Trust, Birmingham, UK
Rowena Jones
Institute for Mental Health, University of Birmingham, Birmingham, UK
Atkinson Morley Regional Neurosciences Centre, St. George’s Hospital, London, UK
Laura Mangiaterra
Department of Health Sciences, Università Degli Studi Di Milano, Milan, Italy
Stefano Bonora
National Psychosis Unit, South London and Maudsley NHS Trust, Beckenham, Kent, UK
James H. MacCabe
You can also search for this author in PubMed Google Scholar
Contributions
AS, RG and JM initiated and designed the study. AS, RG co-developed the algorithm. EO and JM assisted in devising the clinical heuristics. EI assisted in devising the algorithms. AJ designed and implemented the founding databases. AS, HM, RJ, LM and SB validated the results of the algorithm. RS, MB and JM has supervised and consulted the database creation. AS, RG, EO and HM drafted the manuscript. AJ, RS, MB and JM critically revised the manuscript. All authors read and approved the final manuscript.
Corresponding author
Correspondence to Aviv Segev .
Ethics declarations
Ethics approval and consent to participate.
The CRIS data, including the linked data used in this manuscript, has received research ethics approval for secondary analyses (Oxford REC C, reference 18/SC/0372). The CRIS Oversight Committee ensures that research conducted using health records is ethical and legal, and service users can opt-out of their CRIS data being used for research [ 26 ].
Consent for publication
Not relevant to the current manuscript.
Competing interests
RS declares research support received within the last 3 years from Janssen, GSK and Takeda. JHM has received investigator-initiated research funding from H Lundbeck. He has received research funding for clinical trials from H Lundbeck and Karuna Therapeutics. He has participated in advisory boards for H Lundbeck, LB Pharma, Newron Pharmaceuticals and Teva UK Ltd.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Supplementary material 1., rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Cite this article.
Segev, A., Govind, R., Oloyede, E. et al. Developing a validated methodology for identifying clozapine treatment periods in electronic health records. BMC Psychiatry 24 , 584 (2024). https://doi.org/10.1186/s12888-024-06022-5
Download citation
Received : 18 May 2024
Accepted : 14 August 2024
Published : 27 August 2024
DOI : https://doi.org/10.1186/s12888-024-06022-5
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Schizophrenia
BMC Psychiatry
ISSN: 1471-244X
- General enquiries: [email protected]

Interactives: Case Studies (January 2021)
Two interactive case studies are presented in the January 2021 issue of Pharmacy Times®.
RJ is a 68-year-old man with type 2 diabetes (T2D) who calls the pharmacy with concerns about hyperglycemia. His fasting blood glucose level increased to more than 200 mg/dL from an average of 133 mg/dL over the past week. Prior to this week, RJ’s blood sugar levels were greatly improving ever since he lost 8 lb. He noticed his blood sugar levels rising a week ago, after he started tamsulosin 0.4 mg daily, which was prescribed by his primary care physician (PCP) to help with his urinary symptoms. No other medications were started or discontinued during that time. RJ says that his PCP had never heard that this medication causes hyperglycemia but suggested that he call the pharmacy team to further inquire.
How should the pharmacist respond to RJ?
The pharmacist on duty at a local community pharmacy is working with AF, a pharmacy intern in his second professional year of pharmacy school. AF tells the pharmacist that he just finished reading the DAPA-CKD trial1 for his upcoming journal club presentation. He read the results and noted that the number needed to treat (NNT) for the primary outcome (eg, sustained decline in estimated glomerular filtration rate of at least 50%, end-stage kidney disease, or death from renal of cardiovascular cases) was 19. AF is unsure what this means and how it was calculated, as he missed his drug literature and evaluation class last week. The pharmacist reviews the results section of the paper and notes that over a median of 2.4 years, the primary outcome occurred in 197 of 2152 participants (9.2%) in the dapaglifloizin group and 312 of 2152 participants (14.5%) in the placebo group [HR, 0.61 (0.51-0.72); P < .001]. 1 AF asks the pharmacist to explain this concept to him.
What should the pharmacist tell him?
CASE 1: Hyperglycemia is not a listed adverse effect in the package insert for tamsulosin. There are, however, documented case reports suggesting that the 2 may be linked. 1 These reports note that hyperglycemia was induced 1 to 2 days after the initiation of tamsulosin in patients with T2D. Discontinuation led to full resolution of symptoms. It has been suggested that α1 receptors can contribute to glucose uptake through non-insulin—dependent pathways. 2 By blocking these peripheral receptors, glucose uptake is hampered. RJ should be screened to ensure that no other identifiable causes, such as illness, infection, or stress, can be contributing to his hyperglycemia. His PCP can recommend that RJ hold the dose of his tamsulosin for a few days to see whether his glucose levels return to normal.
- Borgsteede S, Bruggeman R, Hoefnagel R,M Huiskes, van PuijenbroekE. Tamsulosin and hyperglycaemia in patients with diabetes. Neth J Med . 2010;68(3):141-143.
- Boyda HN, ProcyshynRM, Pang CCY, Barr AM. Peripheral adrenoceptors: the impetus behind glucose dysregulation and insulin resistance. J Neuroendocrinol . 2013;25(3):217-228. doi:10.1111/jne.12002
CASE 2: The pharmacist can teach AF that the NNT is the number of patients who need to be treated with a specified intervention/drug for 1 patient to benefit from it. So in this case, 19 patients need to be treated with dapagliflozin over 2.4 years for 1 patient to benefit. The NNT is the inverse of the absolute risk reduction (ARR) and is calculated as NNT = 100/ARR (%).
The ARR is the absolute difference in outcome rates between the control group (14.5%) and the treatment group (9.2%). In this case, the ARR is 5.3%. Therefore, the NNT is 100/5.3 =18.867. Because NNTs are normally rounded up to the nearest whole number, the NNT for this trial would be 19.
- Heerspink HJL, Stefánsson BV, Correa-Rotter R, et al; DAPA-CKD Trial Committees and Investigators. Dapagliflozin in patients with chronic kidney disease. N Engl J Med. 2020;383(15):1436-1446. doi:10.1056/NEJMoa2024816
STEFANIE C. NIGRO, PHARMD, BCACP, CDE , is an assistant professor/clinical pharmacist at Massachusetts College of Pharmacy and Health Sciences in Boston.

Navigating Bispecific Antibodies for Multiple Myeloma: Best Practices and Clinical Implementation

Oral Iron Supplementation Remains Gold Standard in Anemia Care

Navigating the Complexities of NSCLC Treatment: The Clinical and Economic Impact of Care

Technicians Prepare for Growing Roles in Immunizations
Condition Watch, August 2024: Dermatology

Apothecary Shoppe Focuses on In-Depth Counseling, Compounding Services
2 Commerce Drive Cranbury, NJ 08512
609-716-7777


IMAGES
VIDEO
COMMENTS
In our first case pulmonary embolism (PE) occurred within 6months of pulmonary tuberculosis diagnosis.Patient took ATT for 6 months and after being diagnosed as a case of PE, low-molecular-weight heparin was prescribed for 3 days and after that he was given Tab. warfarin (5mg) OD.
Clinical Pharmacy Guide: Cancer Drug Treatment Assessment and Review 5th Edition . Example Case Studies . The following case studies demonstrate a systematic approach to the clinical review and assessment of cancer drug orders using Appendix A: Clinical Cancer Drug Order Review Checklist. Summaries of all BC Cancer treatment protocols
As a qualifying pharmacist, case studies bring together the threads of study over the past four years. This includes your study of subjects such as: Pharmacology. Pharmaceutical chemistry. Pharmaceutics. Clinical pharmacy practice. In practice, pharmacists are expected to draw on this knowledge and clinically apply it where necessary.
Explore the latest in clinical pharmacy and pharmacology, including topics in drug safety, development, pharmacogenetics, and pharmacoeconomics. ... This case control study evaluates potential signals for suicidal and self-injurious adverse drug reactions associated with semaglutide and liraglutide. ... Get unlimited access and a printable PDF ...
pharmacists. The case ars e mean tt o b studiee idn sequence; concept and laborators y test evaluations presente ind on case e wil ble used i n subsequent cases AH norma. l laboratory test values b wile thosl e use atd the Lo Angeles s County/U.S.C. Medical Center; values may diffe a othert r hospitals o dependinn th methoe dg used.
Drugs like antispasmodics, tricyclic antidepressants, 5-HT3 antagonists and probiotics are proved effective in this condition. Objective: The survey aimed to find out Awareness about Irritable Bowel Syndrome (IBS) among the pharmacy graduates of 3 rd to 5th professional Pharmacy.
This revised and extended second edition focuses on current and emerging topics in drug development, their molecular mechanisms of action as well as regulatory issues. In addition, in-depth insights into clinical drug research and trial methodology are presented on the basis of concrete case studies. This updated book makes a valuable ...
Dr. Goicoechea teaches pharmacy students on the Cardiology Service of the Los Angeles County/University of Southern California Medical Center, 1200 North State Street, Los Angeles 90033. 382 fexcept for intermittent soft S 3 heart sound, bilateral basilar râles, slightly enlarged heart, 1 + ankle edema.
viii Pharmacy Case Studies PCS prelims 14/1/09 12:52 pm Page viii. Preface ... Clinical Pharmacy and Clinical Pharmacokinetics and has held positions inCommunity and Hospital Pharmacy. She has published widely in the evalu-ation of clinical pharmacy services and education. She currently holds a non-
Interactive Case Studies (August 2020) How would you respond to these patients? CASE 1: NS is a 55-year-old man who recently visited his primary-care provider (PCP) for a follow up. His medical history includes hypothyroidism, hypertension, and type 2 diabetes (T2D). He takes levothyroxine 75 mcg daily, lisinopril 20 mg daily, and metformin 1 ...
Case Studies (January 2018) Case 1. TJ is a 60-year-old 75-kg man who presents to the emergency department after experiencing a fall, sudden loss of balance, confusion, and slurred speech. For the past 2 months, he has been taking warfarin for a deep vein thrombosis in his right leg; his current international normalized ratio (INR) level is ...
regarding Topics in Evidence-Based Pharmacy Practice. Features include review articles that discusses clinical trials, studies, and guidelines that impacts clinical decisions makings and recommendations. Editorials allow professionals to share their ideas and opinions to the changing world of pharmacy.
Case 006-Hypertension Case # 5. Case 007-Dyslipidemia Case # 1. Case 008-Dyslipidemia Case # 2. Case 009-Stable Ischemic Heart Disease. Case 010-Acute Coronary Syndromes. Case 011-Cardiovascular Respiratory Decompensation. Case 012-Chronic Heart Failure Case # 1. Case 013-Chronic Heart Failure Case # 2.
The incidence of a clinical drug interactions 3-5% in pts taking 3 or fewer medication, but 20% in pts taking 10-20 drugs. 20% of hospital admissions result from an adverse drug event or a Drug-Drug Interaction. 60-80% of computerized DDI alerts are overridden. To prevent a SERIOUS drug interaction, you would have to review over 2,700 alerts.
Pharmacy TimesApril 2021. Volume 89. Issue 04. Pages: 59. Interactive case studies from April 2021. Case 1. EP is a patient with epilepsy. He has been taking phenytoin at a therapeutic dose for 4 years. EP was recently prescribed valproic acid because he has had breakthrough seizures over the past few months.
findings (articles and summit highlights), two case studies met the inclusion criteria. The first case study involved the University of Minnesota Medical Center, a multi-campus academic medical center. This facility used technology such as pagers for decentralized technicians, computerized provider order entry, and wireless computers on wheels. The
PDF | On Sep 16, 2020, Zaheer-Ud-Din Babar published Pharmacy Practice Research Case Studies | Find, read and cite all the research you need on ResearchGate
Clinical Pharmacy: Case Studies: Case Number 21 Ischemic Heart Disease - Angina Pectoris. Margaret M. McCarron, MD, and Frank J. Goicoechea View all authors and affiliations. ... PDF/ePub View PDF/ePub. Similar articles: Restricted access. Clinical Pharmacy: Case Studies: Case Number 25 Gentamicin Therapy.
The aim of this work was to design, deliver, and evaluate group case studies focused on the integration of analytical and medicinal chemistry with pharmacy practice. A patient-centered beta-blocker workshop was developed and delivered to year two MPharm and BSc students. Students were tasked with reviewing patient clinical data along with analytical spectra presented as patient case studies ...
Clinical pharmacists can provide administrative and clinical duties, including non-medical prescribing, advice on polypharmacy and medicines optimisation. The aim of this study was to investigate the distribution of clinical pharmacists in general practice across England, and explore the relationship between the distribution and regional ...
Background Clozapine is the only recommended antipsychotic medication for individuals diagnosed with treatment-resistant schizophrenia. Unfortunately, its wider use is hindered by several possible adverse effects, some of which are rare but potentially life threatening. As such, there is a growing interest in studying clozapine use and safety in routinely collected healthcare data. However ...
Volume 89. Issue 1. Two interactive case studies are presented in the January 2021 issue of Pharmacy Times®. CASE 1. RJ is a 68-year-old man with type 2 diabetes (T2D) who calls the pharmacy with concerns about hyperglycemia. His fasting blood glucose level increased to more than 200 mg/dL from an average of 133 mg/dL over the past week.
Case study four. The practice-based pharmacists undertakes a variety of roles and brings many benefits to the practice: Pharmacist-led clinics: NSAID reduction clinics; persuading patients to move back to simple analgesia, i.e. paracetamol. Benzodiazepine withdrawal clinics; dose reduction schedules with GP prescribing support.