DigitalCommons@UNMC
Home > Eppley Institute > Theses & Dissertations

Theses & Dissertations: Cancer Research
Theses/dissertations from 2024 2024.
Novel Spirocyclic Dimer (SpiD3) Displays Potent Preclinical Effects in Hematological Malignancies , Alexandria Eiken
Chemotherapy-Induced Modulation of Tumor Antigen Presentation , Alaina C. Larson
Understanding the role of MASTL in colon homeostasis and colitis-associated cancer development , Kristina Pravoverov
Dying Right: Supporting Anti-Cancer Therapy Through Immunogenic Cell Death , Elizabeth Schmitz
Therapeutic Effects of BET Protein Inhibition in B-cell Malignancies and Beyond , Audrey L. Smith
Targeting KSR1 to inhibit stemness and therapy resistance , Heidi M. Vieira
Identifying the Molecular Determinants of Lung Metastatic Adaptation in Prostate Cancer , Grace M. Waldron
Identification of Mitotic Phosphatases and Cyclin K as Novel Molecular Targets in Pancreatic Cancer , Yi Xiao
Theses/Dissertations from 2023 2023
Development of Combination Therapy Strategies to Treat Cancer Using Dihydroorotate Dehydrogenase Inhibitors , Nicholas Mullen
Overcoming Resistance Mechanisms to CDK4/6 Inhibitor Treatment Using CDK6-Selective PROTAC , Sarah Truong
Theses/Dissertations from 2022 2022
Omics Analysis in Cancer and Development , Emalie J. Clement
Investigating the Role of Splenic Macrophages in Pancreatic Cancer , Daisy V. Gonzalez
Polymeric Chloroquine in Metastatic Pancreatic Cancer Therapy , Rubayat Islam Khan
Evaluating Targets and Therapeutics for the Treatment of Pancreatic Cancer , Shelby M. Knoche
Characterization of 1,1-Diarylethylene FOXM1 Inhibitors Against High-Grade Serous Ovarian Carcinoma Cells , Cassie Liu
Novel Mechanisms of Protein Kinase C α Regulation and Function , Xinyue Li
SOX2 Dosage Governs Tumor Cell Identity and Proliferation , Ethan P. Metz
Post-Transcriptional Control of the Epithelial-to-Mesenchymal Transition (EMT) in Ras-Driven Colorectal Cancers , Chaitra Rao
Use of Machine Learning Algorithms and Highly Multiplexed Immunohistochemistry to Perform In-Depth Characterization of Primary Pancreatic Tumors and Metastatic Sites , Krysten Vance
Characterization of Metastatic Cutaneous Squamous Cell Carcinoma in the Immunosuppressed Patient , Megan E. Wackel
Visceral adipose tissue remodeling in pancreatic ductal adenocarcinoma cachexia: the role of activin A signaling , Pauline Xu
Phos-Tag-Based Screens Identify Novel Therapeutic Targets in Ovarian Cancer and Pancreatic Cancer , Renya Zeng
Theses/Dissertations from 2021 2021
Functional Characterization of Cancer-Associated DNA Polymerase ε Variants , Stephanie R. Barbari
Pancreatic Cancer: Novel Therapy, Research Tools, and Educational Outreach , Ayrianne J. Crawford
Apixaban to Prevent Thrombosis in Adult Patients Treated With Asparaginase , Krishna Gundabolu
Molecular Investigation into the Biologic and Prognostic Elements of Peripheral T-cell Lymphoma with Regulators of Tumor Microenvironment Signaling Explored in Model Systems , Tyler Herek
Utilizing Proteolysis-Targeting Chimeras to Target the Transcriptional Cyclin-Dependent Kinases 9 and 12 , Hannah King
Insights into Cutaneous Squamous Cell Carcinoma Pathogenesis and Metastasis Using a Bedside-to-Bench Approach , Marissa Lobl
Development of a MUC16-Targeted Near-Infrared Antibody Probe for Fluorescence-Guided Surgery of Pancreatic Cancer , Madeline T. Olson
FGFR4 glycosylation and processing in cholangiocarcinoma promote cancer signaling , Andrew J. Phillips
Theses/Dissertations from 2020 2020
Cooperativity of CCNE1 and FOXM1 in High-Grade Serous Ovarian Cancer , Lucy Elge
Characterizing the critical role of metabolic and redox homeostasis in colorectal cancer , Danielle Frodyma
Genomic and Transcriptomic Alterations in Metabolic Regulators and Implications for Anti-tumoral Immune Response , Ryan J. King
Dimers of Isatin Derived Spirocyclic NF-κB Inhibitor Exhibit Potent Anticancer Activity by Inducing UPR Mediated Apoptosis , Smit Kour
From Development to Therapy: A Panoramic Approach to Further Our Understanding of Cancer , Brittany Poelaert
The Cellular Origin and Molecular Drivers of Claudin-Low Mammary Cancer , Patrick D. Raedler
Mitochondrial Metabolism as a Therapeutic Target for Pancreatic Cancer , Simon Shin
Development of Fluorescent Hyaluronic Acid Nanoparticles for Intraoperative Tumor Detection , Nicholas E. Wojtynek
Theses/Dissertations from 2019 2019
The role of E3 ubiquitin ligase FBXO9 in normal and malignant hematopoiesis , R. Willow Hynes-Smith
BRCA1 & CTDP1 BRCT Domainomics in the DNA Damage Response , Kimiko L. Krieger
Targeted Inhibition of Histone Deacetyltransferases for Pancreatic Cancer Therapy , Richard Laschanzky
Human Leukocyte Antigen (HLA) Class I Molecule Components and Amyloid Precursor-Like Protein 2 (APLP2): Roles in Pancreatic Cancer Cell Migration , Bailee Sliker
Theses/Dissertations from 2018 2018
FOXM1 Expression and Contribution to Genomic Instability and Chemoresistance in High-Grade Serous Ovarian Cancer , Carter J. Barger
Overcoming TCF4-Driven BCR Signaling in Diffuse Large B-Cell Lymphoma , Keenan Hartert
Functional Role of Protein Kinase C Alpha in Endometrial Carcinogenesis , Alice Hsu
Functional Signature Ontology-Based Identification and Validation of Novel Therapeutic Targets and Natural Products for the Treatment of Cancer , Beth Neilsen
Elucidating the Roles of Lunatic Fringe in Pancreatic Ductal Adenocarcinoma , Prathamesh Patil
Theses/Dissertations from 2017 2017
Metabolic Reprogramming of Pancreatic Ductal Adenocarcinoma Cells in Response to Chronic Low pH Stress , Jaime Abrego
Understanding the Relationship between TGF-Beta and IGF-1R Signaling in Colorectal Cancer , Katie L. Bailey
The Role of EHD2 in Triple-Negative Breast Cancer Tumorigenesis and Progression , Timothy A. Bielecki
Perturbing anti-apoptotic proteins to develop novel cancer therapies , Jacob Contreras
Role of Ezrin in Colorectal Cancer Cell Survival Regulation , Premila Leiphrakpam
Evaluation of Aminopyrazole Analogs as Cyclin-Dependent Kinase Inhibitors for Colorectal Cancer Therapy , Caroline Robb
Identifying the Role of Janus Kinase 1 in Mammary Gland Development and Breast Cancer , Barbara Swenson
DNMT3A Haploinsufficiency Provokes Hematologic Malignancy of B-Lymphoid, T-Lymphoid, and Myeloid Lineage in Mice , Garland Michael Upchurch
Theses/Dissertations from 2016 2016
EHD1 As a Positive Regulator of Macrophage Colony-Stimulating Factor-1 Receptor , Luke R. Cypher
Inflammation- and Cancer-Associated Neurolymphatic Remodeling and Cachexia in Pancreatic Ductal Adenocarcinoma , Darci M. Fink
Role of CBL-family Ubiquitin Ligases as Critical Negative Regulators of T Cell Activation and Functions , Benjamin Goetz
Exploration into the Functional Impact of MUC1 on the Formation and Regulation of Transcriptional Complexes Containing AP-1 and p53 , Ryan L. Hanson
DNA Polymerase Zeta-Dependent Mutagenesis: Molecular Specificity, Extent of Error-Prone Synthesis, and the Role of dNTP Pools , Olga V. Kochenova
Defining the Role of Phosphorylation and Dephosphorylation in the Regulation of Gap Junction Proteins , Hanjun Li
Molecular Mechanisms Regulating MYC and PGC1β Expression in Colon Cancer , Jamie L. McCall
Pancreatic Cancer Invasion of the Lymphatic Vasculature and Contributions of the Tumor Microenvironment: Roles for E-selectin and CXCR4 , Maria M. Steele
Altered Levels of SOX2, and Its Associated Protein Musashi2, Disrupt Critical Cell Functions in Cancer and Embryonic Stem Cells , Erin L. Wuebben
Theses/Dissertations from 2015 2015
Characterization and target identification of non-toxic IKKβ inhibitors for anticancer therapy , Elizabeth Blowers
Effectors of Ras and KSR1 dependent colon tumorigenesis , Binita Das
Characterization of cancer-associated DNA polymerase delta variants , Tony M. Mertz
A Role for EHD Family Endocytic Regulators in Endothelial Biology , Alexandra E. J. Moffitt
Biochemical pathways regulating mammary epithelial cell homeostasis and differentiation , Chandrani Mukhopadhyay
EPACs: epigenetic regulators that affect cell survival in cancer. , Catherine Murari
Role of the C-terminus of the Catalytic Subunit of Translesion Synthesis Polymerase ζ (Zeta) in UV-induced Mutagensis , Hollie M. Siebler
LGR5 Activates TGFbeta Signaling and Suppresses Metastasis in Colon Cancer , Xiaolin Zhou
LGR5 Activates TGFβ Signaling and Suppresses Metastasis in Colon Cancer , Xiaolin Zhou
Theses/Dissertations from 2014 2014
Genetic dissection of the role of CBL-family ubiquitin ligases and their associated adapters in epidermal growth factor receptor endocytosis , Gulzar Ahmad
Strategies for the identification of chemical probes to study signaling pathways , Jamie Leigh Arnst
Defining the mechanism of signaling through the C-terminus of MUC1 , Roger B. Brown
Targeting telomerase in human pancreatic cancer cells , Katrina Burchett
The identification of KSR1-like molecules in ras-addicted colorectal cancer cells , Drew Gehring
Mechanisms of regulation of AID APOBEC deaminases activity and protection of the genome from promiscuous deamination , Artem Georgievich Lada
Characterization of the DNA-biding properties of human telomeric proteins , Amanda Lakamp-Hawley
Studies on MUC1, p120-catenin, Kaiso: coordinate role of mucins, cell adhesion molecules and cell cycle players in pancreatic cancer , Xiang Liu
Epac interaction with the TGFbeta PKA pathway to regulate cell survival in colon cancer , Meghan Lynn Mendick
Theses/Dissertations from 2013 2013
Deconvolution of the phosphorylation patterns of replication protein A by the DNA damage response to breaks , Kerry D. Brader
Modeling malignant breast cancer occurrence and survival in black and white women , Michael Gleason
The role of dna methyltransferases in myc-induced lymphomagenesis , Ryan A. Hlady
Design and development of inhibitors of CBL (TKB)-protein interactions , Eric A. Kumar
Pancreatic cancer-associated miRNAs : expression, regulation and function , Ashley M. Mohr
Mechanistic studies of mitochondrial outer membrane permeabilization (MOMP) , Xiaming Pang
Novel roles for JAK2/STAT5 signaling in mammary gland development, cancer, and immune dysregulation , Jeffrey Wayne Schmidt
Optimization of therapeutics against lethal pancreatic cancer , Joshua J. Souchek
Theses/Dissertations from 2012 2012
Immune-based novel diagnostic mechanisms for pancreatic cancer , Michael J. Baine
Sox2 associated proteins are essential for cell fate , Jesse Lee Cox
KSR2 regulates cellular proliferation, transformation, and metabolism , Mario R. Fernandez
Discovery of a novel signaling cross-talk between TPX2 and the aurora kinases during mitosis , Jyoti Iyer
Regulation of metabolism by KSR proteins , Paula Jean Klutho
The role of ERK 1/2 signaling in the dna damage-induced G2 , Ryan Kolb
Regulation of the Bcl-2 family network during apoptosis induced by different stimuli , Hernando Lopez
Studies on the role of cullin3 in mitosis , Saili Moghe
Characteristics of amyloid precursor-like protein 2 (APLP2) in pancreatic cancer and Ewing's sarcoma , Haley Louise Capek Peters
Structural and biophysical analysis of a human inosine triphosphate pyrophosphatase polymorphism , Peter David Simone
- Eppley Institute Website
- McGoogan Library
Advanced Search
- Notify me via email or RSS
- Collections
- Disciplines
Author Corner
Home | About | FAQ | My Account | Accessibility Statement
Privacy Copyright

- Why Choose Us
- Vision and Mission
- Hire Writers
- How it Works
List of 50+ Unique Cancer Dissertation Topics by Experts

Table of Content
Find Available Data
Look for a theoretical base for your topic, consult your mentor, participate in medical research surveys, take advantage of the available resources, ask questions that can be answered, read everything on the subject, check if the study was reviewed, check the results of your surveys, phases of new research, ask questions to your health care team.
Cancer is said to be one of the deadliest diseases and causes of death in the world. For a few years, it has received a lot of attention. Researchers and students are doing their studies and research to know more about the disease. Therefore, students often struggle with and look for one of the best cancer dissertation topics for writing their dissertation.
It is essential to choose a topic that not only benefits your research but also helps the reader or the public in general. So, if you are thinking of writing a dissertation on cancer, then it is best to know how to research to get the best cancer dissertation ideas.
How to Choose Cancer Dissertation Topics?
We know how difficult it is to choose a topic for a dissertation on cancer. Sometimes, more time is consumed while researching and choosing a topic than writing a dissertation. Therefore, nursing students often look for help from experts in such cases. However, those who choose to work on cancer research topics for their dissertation have to go through several things to find the right topic.
Luckily, we have just the right information to provide dissertation help and make it easy for you to select a perfect topic. All you have to do is go through the below-mentioned pointers.
Order on Whatsapp
The first and foremost thing to do while choosing a topic is to look for easily available data. It is essential to look for reliable information when writing a dissertation on a disease. So choose a topic for which you can easily find all the information, or the data can be available conveniently.
One of the most important things to take care of while choosing a research topic for your dissertation on cancer is to check if your idea has a solid theoretical base. Because if your topic doesn't include a relevant theoretical basis, it will look vague and unauthentic to your reader.
If you are sceptical about your decisions or topic selection. The best thing would be to seek nursing dissertation help from your professor or from experts. Your mentor can help you with planning to make it easy for you to choose a topic. They can also suggest the right resources or sources from where you can get enough information for your dissertation writing. So, never hesitate to seek help for unique cancer dissertation topics for your academic task.
Often, researchers take surveys to analyse data and get to a decision. Therefore, you can also do the same while choosing the right topic among various cancer dissertation ideas. When you participate in a medical research survey, you can get data analysis on various diseases of the same origin, like lung cancer, ovary cancer, breast cancer dissertation topics, and many more.
Cancer is a vast topic. So, to narrow it down among several cancer dissertation topics, you have to look for available resources and sources. If you do not have relevant and efficient resources to start your dissertation, there is no sense in choosing that topic. It is rather better if you seek help from dissertation writing services .
Your mentor can help you with planning to make it easy for you to choose cancer dissertation topics. They can also suggest the right resources or sources from where you can get enough information for your dissertation writing. Because if your topic doesn't include a relevant theoretical basis, it will look vague and unauthentic to your reader.
Well, reading all the details on the subject is essential before writing a dissertation on it. The reason is that if you don't, your knowledge will be incomplete, and it will get difficult for you to come up with an outcome or conclusion for your research. For example, you are selecting from breast cancer dissertation topics, but if you don't read everything about it. You will not be able to justify your arguments.
So, these ways can help you select the best cancer research topic for your dissertation. So, if you follow these tips, you can choose one of the best cancer dissertation topics for your academic task. But how will you ensure the authenticity and credibility of your research? Let's find out!
Explore Our FREE SAMPLES of Dissertations
How to Ensure the Reliability of Your Cancer Research? A Checklist
It is crucial to ensure that the things you have included in your dissertation or the research you have done on your cancer dissertation topics are reliable and relevant. Using correct and accurate data and information is vital when choosing a dissertation theme in the medical field or nursing dissertation topics . So, to help you with that, we have developed a short checklist that you have to tick off for complete authenticity.
Yes, it is essential to ensure that whatever sources or study you are taking reference from was peer-reviewed by the journal that has published them.
If you have used Surveys as your research method for choosing cancer dissertation topics. Go through your survey results and check how many people participated in the survey and how long it lasted.
If you are studying and choosing any new treatments, analysis, therapies, or symptoms as a part of your cancer dissertation ideas. Ensure you go through all the study phases or take regular updates about the new research.
This is one of the most important steps to confirm the credibility of your cancer dissertation topics. Always ask your healthcare team or committee to clarify your doubts on the chosen topic. It will help you get the correct answers for your research.
So, this was the quick checklist you need to tick off while working on a dissertation on cancer or any other nursing and medical field. We know it is a very time-consuming process, so we are here to help you. Below is the list of cancer dissertation topics for undergraduates and postgraduates to help narrow your field and topic selection.
Cancer Dissertation Topics for Undergraduates
1. Lung Cancer Pathophysiology
2. Breast Cancer: Literature Review
3. Cancer Insurance Evaluation
4. Case Brief on Colon Cancer and Colostomy
5. Environmentally and Lifestyle Linked Cancer
6. Skin Cancer Types, Cells of Origin
7. Wellness Programs for Colorectal Cancer
8. Addressing Risk Factors for Lung Cancer
9. Esophageal Cancer and Its Treatment
10. Breast Cancer: Research Review Paper
11. Genetic Alterations and Cancer
12. Breast Cancer: Threat to the Patients
13. Epidemiology of Breast Cancer in the UK
14. Does Marijuana Use and Misuse Cause Cancer?
15. Cancer Patients: The Effectiveness of Pain Diary
16. Health & Medicine: Breast Cancer in XIX Century
17. The Relationship Between Breast Cancer and Genes
18. Approach to Cancer Care: Diagnosing and Treatment
19. Passive Smoking and Pancreatic Cancer in Women
20. How to Lower your Cancer Risk. Nutrition Action Health Letter
21. Cancer Biology: Oncogenes and Tumor Suppressor Genes
22. Breast Cancer: Preventive Measures and Support Methods
23. Discuss the Latest Technologies Used for Cancer Treatments
24. Prostate Cancer Among Blacks in Maryland: Cost-Effectiveness Analysis
25. Post-operative Breast Cancer Patients With Depression: Annotated Bibliography
Struggling to Find Best Dissertation Topic?
Get a Unique Title & Dissertation Proposal Outline for FREE!
Cancer Dissertation Topics for Postgraduates
26. Deathography of Cancer
27. DNA Methylation in Cancer Therapy
28. Precision Therapy in Colorectal Cancer
29. Possible Trends in the Cause of Cancer
30. Skin Cancer: Examination and Prevention
31. Socioeconomic Factors of Oral Cancer
32. Omics Analysis in Cancer and Development
33. Cancer and Humor in Children: Approach to Research
34. The Role of Immunotherapy in Urothelial Cancer
35. Precision Medicine in Cancer Treatment
36. Methods For Screening For Cervical Cancer
37. Cancer Burden And Prevention Methods
38. Advances in Immunotherapy in Pediatric Solid Tumors
39. The Benefits Of Music Therapy For Breast Cancer Patients
40. Polymeric Chloroquine in Metastatic Pancreatic Cancer Therapy
41. Cancer Care Approaches: Diagnosis, Side Effects, and Treatment
42. Approaches to Illustrate Tumor Immune Microenvironment
43. Physical and Mental Care for Cancer Patients
44. Cancer Diagnostics, Staging and Complications
45. Pain Management Issues in Cancer Patients
46. Depression in Female Cancer Patients and Survivors
47. Cancer Pain Management and Education Programs
48. Cancer Metabolism: Diagnosis, Research, Effects
49. Type C Personality as a Risk Factor for Cancer
50. Nutritional "Cures" for Clients With Cancer
51. American Cancer Society: The Aspects of Melanoma
52. Virtual Colonoscopy to Screen for Colon Cancer
53. Immune Cell Metabolic Reprogramming in Cancer Development and Therapy
54. Interplay Between Tumor Immunology and Tumor Micro-environment
55. Application of Multi-omics Analysis in Thoracic Cancer Immunotherapy
So, these were some of the best cancer dissertation ideas for undergraduate and postgraduate nursing students. It will save you time spent on topic selection. Moreover, you can also find samples of dissertation on our Global Assignment Help website. It will help you understand how do you have to write a dissertation once the topic is decided. However, if you still feel the need of assistance, you can always turn to our experts for guidance.
Still Searching for a Perfect Cancer Dissertation Topic? Ask Us!
Are you still indecisive about your cancer research topic? Do you need help with it? Well, then what are we here for? Do not feel alone as we will guide you anywhere you get stuck. Our experts will not only help you with topic selection but with any part of your dissertation separately or completely. We can even suggest you more topics based on your personalised requirements.
You can even buy dissertation service from us, and we will ensure that you get full satisfaction. Moreover, if by any chance, you find any mistake in the document submitted by our experts. You should know that we offer 100% free revisions. All you have to do is contact our customer support staff and raise your query. They will help you get several suggestion for your cancer dissertation topics to completing it for you. Whatever you need, try seeking our help, today!
Also Read: List of 50+ Best Epidemiology Dissertation Topics
Let Us Help With Dissertation
Try Before You Buy !
Get Free PDF Link Directly to your WhatsApp !

Great!! Sumsa Free PDF Template has been delivered on your WhatsApp Number.
- Dissertation Conclusion Not A Discussion But Final Word
- Dissertation Abstract
- Dissertation Structure A Complete Guide
- Dissertation Table Of Contents
- Dissertation Chapters Guide
- Questionnaire For Dissertation
- Dissertation Appendix
- Dissertation Outline
- 7 Step Guide To Dissertation Proofreading And Editing
- 5 Best Fonts For Dissertation Writing
- Guide To Plan Your Dissertation
- Different Styles Of Dissertation Referencing
- Different Types Of Dissertation
- Professionals Social Work Dissertation Writing Tips
- Simple Tips To Write An Engaging Dissertation Introduction
- 7 Dissertation Writing Mistakes No One Ever Told You About Find Fixes Too
- How To Write A Dissertation
- How To Write A Dissertation Proposal
- How To Write A Dissertation Title Tips
- How To Write Dissertation Methodology
- What Happens If You Fail Your Dissertation
- How Long Should A Dissertation Be
- How To Choose The Best Dissertation Topic
- Writing A Dissertation In A Day
- Tips For Surviving Your Dissertation
Boost Grades & Leave Stress
Get A+ Within Your Budget!
Price Calculator
Offers & Benefits
Get upto 55% OFF on Your First Order !
Extra 25% OFF on Your First Order !
Use Our Seasonal Offers!
Coupon Code

FREE Features
- Topic Creation USD 4.04 FREE
- Outline USD 9.75 FREE
- Unlimited Revisions USD 21.6 FREE
- Editing/Proofreading USD 29.26 FREE
- Formatting USD 8.36 FREE
- Bibliography USD 7.66 FREE
Get all these features for
USD 84.3 FREE
RELATED BLOGS

Master the Art of Writing a Perfect Dissertation Proposal

Conclusion Essay: An Effective Handbook with Examples

Check Out the List of 15 Hardest Degrees in UK [2024]

A Captivating Guide On Essay Hook: Types, Steps & Examples

Learn the Key Differences Between Resume vs CV

List of 150+ Latest Narrative Essay Topics | 2024
Professional assignment writers.
Choose a writer for your task among hundreds of professionals
Please rotate your device
We don't support landscape mode yet. Please go back to portrait mode for the best experience
We use cookies to ensure that we give you the best experience on our website. If you continue to use this site we will assume that you are happy with it. Know more
Calculate the Price
Professional Academic Help at Pocket-Friendly Prices!
Estimated Price
Limited Time Offer
Exclusive Library Membership + FREE Wallet Balance
1 Month Access !
5000 Student Samples
10,000 Answers by Experts
Get $300 Now
- Open access
- Published: 26 November 2018
The 150 most important questions in cancer research and clinical oncology series: questions 94–101
Edited by Cancer Communications
Cancer Communications
Cancer Communications volume 38 , Article number: 69 ( 2018 ) Cite this article
23k Accesses
8 Citations
Metrics details
Since the beginning of 2017, Cancer Communications (former title: Chinese Journal of Cancer ) has published a series of important questions regarding cancer research and clinical oncology, to provide an enhanced stimulus for cancer research, and to accelerate collaborations between institutions and investigators. In this edition, the following 8 valuable questions are presented. Question 94. The origin of tumors: time for a new paradigm? Question 95. How can we accelerate the identification of biomarkers for the early detection of pancreatic ductal adenocarcinoma? Question 96. Can we improve the treatment outcomes of metastatic pancreatic ductal adenocarcinoma through precision medicine guided by a combination of the genetic and proteomic information of the tumor? Question 97. What are the parameters that determine a competent immune system that gives a complete response to cancers after immune induction? Question 98. Is high local concentration of metformin essential for its anti-cancer activity? Question 99. How can we monitor the emergence of cancer cells anywhere in the body through plasma testing? Question 100. Can phytochemicals be more specific and efficient at targeting P-glycoproteins to overcome multi-drug resistance in cancer cells? Question 101. Is cell migration a selectable trait in the natural evolution of carcinoma?
Until now, the battle against cancer is still ongoing, but there are also ongoing discoveries being made. Milestones in cancer research and treatments are being achieved every year; at a quicker pace, as compared to decades ago. Likewise, some cancers that were considered incurable are now partly curable, lives that could not be saved are now being saved, and for those with yet little options, they are now having best-supporting care. With an objective to promote worldwide cancer research and even accelerate inter-countries collaborations, since the beginning of 2017, Cancer Communications (former title: Chinese Journal of Cancer ) has launched a program of publishing 150 most important questions in cancer research and clinical oncology [ 1 ]. We are providing a platform for researchers to freely voice-out their novel ideas, and propositions to enhance the communications on how and where our focus should be placed [ 2 , 3 , 4 , 5 , 6 , 7 , 8 , 9 , 10 , 11 , 12 , 13 ]. In this edition, 8 valuable and inspiring questions, Question 94–101, from highly distinguished professionals from different parts of the world are presented. If you have any novel proposition(s) and Question(s), please feel free to contact Ms. Ji Ruan via email: [email protected].

Question 94: The origin of tumors: time for a new paradigm?
Background and implications.
“There is no worse blind man than the one who doesn’t want to see. There is no worse deaf man than the one who doesn’t want to hear. And there is no worse madman than the one who doesn’t want to understand.” —Ancient Proverb
In the past half-century, cancer biologists have focused on a dogma in which cancer was viewed as a proliferative disease due to mechanisms that activate genes (oncogenes) to promote cell proliferation or inactivate genes (tumor suppressor genes) to suppress tumor growth. In retrospect, these concepts were established based on functional selections, by using tissue culture (largely mouse NIH 3T3 cells) for the selection of transformed foci at the time when we knew virtually nothing about the human genome [ 14 ]. However, it is very difficult to use these genes individually or in combinations to transform primary human cells. Further, the simplified view of uncontrolled proliferation cannot explain the tumor as being a malignant organ or a teratoma, as observed by pathologists over centuries. Recently, the cancer genomic atlas project has revealed a wide variety of genetic alterations ranging from no mutation to multiple chromosomal deletions or fragmentations, which make the identification of cancer driver mutations very challenging in a background of such a massive genomic rearrangement. Paradoxically, this increase the evidences demonstrating that the oncogenic mutations are commonly found in many normal tissues, further challenging the dogma that genetic alteration is the primary driver of this disease.
Logically, the birth of a tumor should undergo an embryonic-like development at the beginning, similar to that of a human. However, the nature of such somatic-derived early embryo has been elusive. Recently, we provided evidence to show that polyploid giant cancer cells (PGCCs), which have been previously considered non-dividing, are actually capable of self-renewal, generating viable daughter cells via amitotic budding, splitting and burst, and capable of acquisition of embryonic-like stemness [ 15 , 16 , 17 ]. The mode of PGCC division is remarkably similar to that of blastomere, a first step in human embryogenesis following fertilization. The blastomere nucleus continuously divides 4–5 times without cytoplasmic division to generate 16–32 cells and then to form compaction/morulae before developing into a blastocyst [ 18 ]. Based on these data and similarity to the earliest stage of human embryogenesis, I propose a new theory that tumor initiation can be achieved via a dualistic origin, similar to the first step of human embryogenesis via the formation of blastomere-like cells, i.e. the activation of blastomere or blastomere-like cells which leads to the dedifferentiation of germ cells or somatic cells, respectively, which is then followed by the differentiation to generate their respective stem cells, and the differentiation arrest at a specific developmental hierarchy leading to tumor initiation [ 19 ]. The somatic-derived blastomere-like cancer stem cell follows its own mode of cell growth and division and is named as the giant cell cycle. This cycle includes four distinct but overlapping phases: the initiation, self-renewal, termination, and stability phases. The giant cell cycle can be tracked in vitro and in vivo due to their salient giant cell morphology (Fig. 1 ).
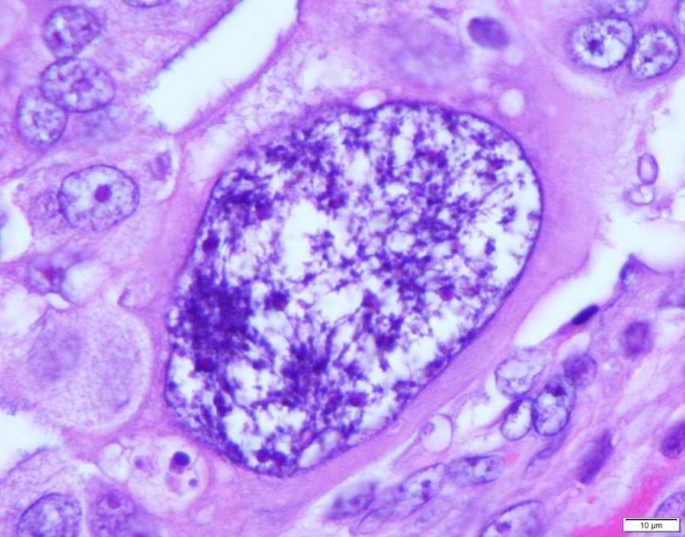
One mononucleated polyploid giant cancer cell (PGCC) in the background of regular size diploid cancer cells. The PGCC can be seen to be at least 100 times larger than that of regular cancer cells
This new theory challenges the traditional paradigm that cancer is a proliferative disease, and proposes that the initiation of cancer requires blastomere-like division that is similar to that of humans before achieving stable proliferation at specific developmental hierarchy in at least half of all human cancers. This question calls for all investigators in the cancer research community to investigate the role of PGCCs in the initiation, progression, resistance, and metastasis of cancer and to look for novel agents to block the different stages of the giant cell cycle.
The histopathology (phenotype) of cancers has been there all the time. It is just the theory of cancer origin proposed by scientists that changes from time to time. After all, trillions of dollars have been invested in fighting this disease by basing on its genetic origin in the past half-century, yet, little insight has been gained [ 14 ]. Here are two quotes from Einstein: “Insanity: doing the same thing over and over again expecting different results”, and “We cannot solve our problems with the same thinking we used when created them”.
In short, it is time to change our mindset and to start pursuing PGCCs, which we can observe under the microscope. But with very little understanding about these cells, it is time for a shift in paradigm.
Jinsong Liu.
Affiliation
Department of Pathology, The University of Texas MD Anderson Cancer Center, Houston, TX 77030-4095, USA.
Email address
Question 95: How can we accelerate the identification of biomarkers for the early detection of pancreatic ductal adenocarcinoma?
Pancreatic ductal adenocarcinoma (PDAC) is one of the most lethal cancers in the world with a dismal 5-year overall survival rate of less than 5%; which has not been significantly improved since the past decades. Although surgical resection is the only option for curative treatment of PDAC, only 15%–20% of patients with PDAC have the chance to undergo curative resection, leaving the rest with only palliative options in hope for increasing their quality of life; since they were already at unresectable and non-curative stages at their first diagnosis.
The lack of specific symptoms in the early-stage of PDAC is responsible for rendering an early diagnosis difficult. Therefore, more sensitive and specific screening methodologies for its early detection is urgently needed to improve its diagnosis, starting early treatments, and ameliorating prognoses. The diagnosis so far relies on imaging modalities such as abdominal ultrasound, computed tomography (CT), magnetic resonance imaging (MRI), endoscopic ultrasound (EUS), endoscopic retrograde cholangiopancreatography (ERCP), and positron emission tomography (PET). One may propose to screen for pancreatic cancer in high-risk populations, which is highly recommended, however screening intervention for all the people is not a wise choice; when considering the relatively low prevalence of PDAC, and the difficulty for diagnosing it in its early stage [ 20 ].
Therefore, alternative diagnostic tools for early detection of PDAC are highly expected. Among the biomarkers currently used in clinical practice, carbohydrate antigen 19–9 (CA19–9) is among the most useful one for supporting the diagnosis of PDAC, but it is neither sufficiently sensitive nor specific for its early detection. Yachida et al. reported in 2010 that the initiating mutation in the pancreas occurs approximately two decades before the PDAC to start growing in distant organs [ 21 ], which indicates a broad time of the window of opportunity for the early detection of PDAC. With the advancement in next-generation sequencing technology, the number of reported studies regarding novel potential molecular biomarkers in bodily fluids including the blood, feces, urine, saliva, and pancreatic juice for early detection of PDAC has been increasing. Such biomarkers may be susceptible to detect mutations at the genetic or epigenetic level, identifying important non-coding RNA (especially microRNA and long non-coding RNA), providing insights regarding the metabolic profiles, estimating the tumor level in liquid biopsies (circulating free DNA, circulating tumor cells and exosomes), and so on.
Another approach to identifying biomarkers for the early detection of pancreatic cancer is using animal models. In spontaneous animal models of pancreatic cancer, such as Kras-mutated mouse models, it is expected that by high throughput analyses of the genetic/epigenetic/proteomic alterations, some novel biomarkers might be able to be identified. For instance, Sharma et al. reported in 2017 that the detection of phosphatidylserine-positive exosomes enabled the diagnosis of early-stage malignancies in LSL-Kras G12D , Cdkn2a lox/lox : p48 Cre and LSL-Kras G12d/+ , LSL-Trp R172H/+ , and P48 Cre mice [ 22 ].
These analyses in clinical samples or animal models hold the clues for the early detection of PDAC, however, further studies are required to validate their diagnostic performance. What’s most important, will be the lining-up of these identified prospective biomarkers, to validate their sensitivities and specificities. This will determine their potential for widespread clinical applicability, and hopefully, accelerate the early diagnosis of PDAC.
Mikiya Takao 1,2 , Hirotaka Matsuo 2 , Junji Yamamoto 1 , and Nariyoshi Shinomiya 2 .
1 Department of Surgery, National Defense Medical College, 3-2 Namiki, Tokorozawa, Saitama 359-8513, Japan; 2 Department of Integrative Physiology and Bio-Nano Medicine, National Defense Medical College, 3-2 Namiki, Tokorozawa, Saitama 359-8513, Japan.
E-mail address
[email protected]; [email protected]; [email protected]; [email protected]
Question 96: Can we improve the treatment outcomes of metastatic pancreatic ductal adenocarcinoma through precision medicine guided by a combination of the genetic and proteomic information of the tumor?
Pancreatic ductal adenocarcinoma (PDAC) is one of the most malignant cancers, and nearly half of the patients had metastatic PDAC when they are initially diagnosed. When they are accompanied by metastatic tumors, unlike most solid cancer, PDAC cannot be cured with primary surgical resection alone [ 23 , 24 ]. Also, since PDAC has poor responses to conventional therapies, improvements in adjunctive treatment approach including chemo- and immuno-therapy are earnestly required. From this standpoint, recent results regarding the differences in the molecular evolution of pancreatic cancer subtypes provide a new insight into its therapeutic development [ 25 ], which may lead to the improvement of the prognosis of not only metastatic PDAC but also of locally advanced or recurrent PDAC.
In fact, new chemotherapeutic regimens such as the combination of gemcitabine with nab-paclitaxel and FOLFIRINOX have been reported to show improved prognosis despite a lack of examples of past successes in the treatment of patients with metastatic PDAC who had undergone R0 resection [ 26 ]. While many mutations including KRAS , CDKN2A , TP53, and SMAD4 are associated with pancreatic carcinogenesis, no effective molecular targeted drug has been introduced in the clinical setting so far. A recent report of a phase I/II study on refametinib, a MEK inhibitor, indicated that KRAS mutation status might affect the overall response rate, disease control rate, progression-free survival, and overall survival of PDAC in combination with gemcitabine [ 27 ].
While immunotherapy is expected to bring a great improvement in cancer treatment, until now, immune checkpoint inhibitors have achieved limited clinical benefit for patients with PDAC. This might be because PDAC creates a uniquely immunosuppressive tumor microenvironment, where tumor-associated immunosuppressive cells and accompanying desmoplastic stroma prevent the tumor cells from T cell infiltration. Recently reported studies have indicated that immunotherapy might be effective when combined with focal adhesion kinase (FAK) inhibitor [ 28 ] or IL-6 inhibitor [ 29 ], but more studies are required to validate their use in clinical practice.
As such, we believe that if the dynamic monitoring of drug sensitivity/resistance in the individual patients is coupled with precision treatment based on individualized genetics/epigenetics/proteomics alterations in the patients’ tumor, this could improve the treatment outcomes of PDAC.
Mikiya Takao 1,2 , Hirotaka Matsuo 2 , Junji Yamamoto 1 , and Nariyoshi Shinomiya 2.
Question 97: What are the parameters that determine a competent immune system that gives a complete response to cancers after immune induction?
Recently, cancer immunotherapy has shown great clinical benefit in multiple types of cancers [ 30 , 31 , 32 ]. It has provided new approaches for cancer treatment. However, it has been observed that only a fraction of patients respond to immunotherapy.
Much effort has been made to identify markers for immunotherapeutic response. Tumor mutation burden (TMB), mismatch repair (MMR) deficiency, PD-L1 expression, and tumor infiltration lymphocyte (TIL) have been found to be associated with an increased response rate in checkpoint blockade therapies. Unfortunately, a precise prediction is still challenging in this field. Moreover, when to stop the treatment of immunotherapy is an urgent question that remains to be elucidated.
In other words, there is no available approach to determine if a patient has generated a good immune response against the cancer after immunotherapy treatments. All of these indicate the complexity and challenges that reside for implementing novel man-induced cancer-effective immune response therapeutics. A variety of immune cells play collaborative roles at different stages to recognize antigens and eventually to generate an effective anti-cancer immune response. Given the high complexity of the immune system, a rational evaluation approach is needed to cover the whole process. Moreover, we need to perfect vaccine immunization and/or in vitro activation of T cells to augment the function of the immune system; particularly the formation of immune memory.
Edison Liu 1 , Penghui Zhou 2 , Jiang Li 2 .
1 The Jackson Laboratory, Bar Harbor, ME 04609, USA; 2 Sun Yat-sen University Cancer Center, Guangzhou, Guangdong 510060, P. R. China.
[email protected]; [email protected]; [email protected]
Question 98: Is high local concentration of metformin essential for its anti-cancer activity?
Metformin was approved as a first line of anti-diabetic drug since decades. Interestingly, the fact that clinical epidemiological studies have shown that metformin can reduce the risk of a variety of cancers stimulates considerable recognition to explore its anticancer activity.
Although the in vitro and in vivo experimental results have demonstrated that metformin can have some potential anti-tumor effects, more than 100 clinical trials did not achieve such desirable results [ 33 ]. We and others believe that the main problem resides in the prescribing doses used. For cancer treatment, a much higher dose may be needed for observing any anti-tumor activities, as compared to the doses prescribed for diabetics [ 34 , 35 , 36 ].
Further, if the traditional local/oral administration approach is favored, the prescribed metformin may not be at the required dose-concentration once it reaches the blood to have the effective anti-cancer activities. We, therefore, propose that intravesical instillation of metformin into the bladder lumen could be a promising way to treat for bladder cancer, at least. We have already obtained encouraging results both in vitro and in vivo experiments, including in an orthotopical bladder cancer model [ 36 , 37 ]. Now, we are waiting to observe its prospective clinical outcome.
Mei Peng 1 , Xiaoping Yang 2 .
1 Department of Pharmacy, Xiangya Hospital, Central South University. Changsha, Hunan 410083, P. R. China; 2 Key Laboratory of Study and Discovery of Small Targeted Molecules of Hunan Province, Department of Pharmacy, School of Medicine, Hunan Normal University, Changsha, Hunan 410013, P. R. China.
[email protected]; [email protected]
Question 99: How can we monitor the emergence of cancer cells anywhere in the body through plasma testing?
The early detection of cancer is still a relentless worldwide challenge. The sensitivity and specificity of traditional blood tumor markers and imaging technologies are still to be greatly improved. Hence, novel approaches for the early detection of cancer are urgently needed.
The emergence of liquid biopsy technologies opens a new driveway for solving such issues. According to the definition of the National Cancer Institute of the United States, a liquid biopsy is a test done on a sample of blood to look for tumorigenic cancer cells or pieces of tumor cells’ DNA that are circulating in the blood [ 38 ]. This definition implies two main types of the current liquid biopsy: one that detects circulating tumor cells and the other that detects non-cellular material in the blood, including tumor DNA, RNA, and exosomes.
Circulating tumor cells (CTCs) are referred to as tumor cells that have been shed from the primary tumor location and have found their way to the peripheral blood. CTCs were first described in 1869 by an Australian pathologist, Thomas Ashworth, in a patient with metastatic cancer [ 39 ]. The importance of CTCs in modern cancer research began in the mid-1990s with the demonstration that CTCs exist early in the course of the disease.
It is estimated that there are about 1–10 CTCs per mL in whole blood of patients with metastatic cancer, even fewer in patients with early-stage cancer [ 40 ]. For comparison, 1 mL of blood contains a few million white blood cells and a billion erythrocytes. The identification of CTCs, being in such low frequency, requires some special tumoral markers (e.g., EpCAM and cytokeratins) to capture and isolate them. Unfortunately, the common markers for recognizing the majority of CTCs are not effective enough for clinical application [ 41 ]. Although accumulated evidences have shown that the presence of CTCs is a strong negative prognostic factor in the patients with metastatic breast, lung and colorectal cancers, detecting CTCs might not be an ideal branch to hold on for the hope of early cancer detection [ 42 , 43 , 44 , 45 ].
Circulating tumor DNA (ctDNA) is tumor-derived fragmented DNA in the circulatory system, which is mainly derived from the tumor cell death through necrosis and/or apoptosis [ 46 ]. Given its origin, ctDNA inherently carries cancer-specific genetic and epigenetic aberrations, which can be used as a surrogate source of tumor DNA for cancer diagnosis and prognostic prediction. Ideally, as a noninvasive tumor early screening tool, a liquid biopsy test should be able to detect many types of cancers and provide the information of tumor origin for further specific clinical management. In fact, the somatic mutations of ctDNA in different types of tumor are highly variable, even in the different individuals with the same type of tumor [ 47 ]. Additionally, most tumors do not possess driver mutations, with some notable exceptions, which make the somatic mutations of ctDNA not suitable for early detection of the tumor.
Increased methylation of the promoter regions of tumor suppressor genes is an early event in many types of tumor, suggesting that altered ctDNA methylation patterns could be one of the first detectable neoplastic changes associated with tumorigenesis [ 48 ]. ctDNA methylation profiling provides several advantages over somatic mutation analysis for cancer detection including higher clinical sensitivity and dynamic range, multiple detectable methylation target regions, and multiple altered CpG sites within each targeted genomic region. Further, each methylation marker is present in both cancer tissue and ctDNA, whereas only a fraction of mutations present in cancer tissue could be detected in ctDNA.
In 2017, there were two inspiring studies that revealed the values of using ctDNA methylation analysis for cancer early diagnosis [ 49 , 50 ]. After partitioning the human genome into blocks of tightly coupled CpG methylation sites, namely methylation haplotype blocks (MHBs), Guo and colleagues performed tissue-specific methylation analyses at the MHBs level to accurately determine the tissue origin of the cancer using ctDNA from their enrolled patients [ 49 ]. In another study, Xu and colleagues identified a hepatocellular carcinoma (HCC) enriched methylation marker panel by comparing the HCC tissue and blood leukocytes from normal individuals and showed that methylation profiles of HCC tumor DNA and matched plasma ctDNA were highly correlated. In this study, after quantitative measurement of the methylation level of candidate markers in ctDNA from a large cohort of 1098 HCC patients and 835 normal controls, ten methylation markers were selected to construct a diagnostic prediction model. The proposed model demonstrated a high diagnostic specificity and sensitivity, and was highly correlated with tumor burden, treatment response, and tumor stage [ 50 ].
With the rapid development of highly sensitive detection methods, especially the technologies of massively parallel sequencing or next-generation sequencing (NGS)-based assays and digital PCR (dPCR), we strongly believe that the identification of a broader “pan-cancer” methylation panel applied for ctDNA analyses, probably in combination with detections of somatic mutation and tumor-derived exosomes, would allow more effective screening for common cancers in the near future.
Edison Liu 1 , Hui-Yan Luo 2 .
[email protected]; [email protected]
Question 100: Can phytochemicals be more specific and efficient at targeting P-glycoproteins to overcome multi-drug resistance in cancer cells?
Though several anticancer agents are approved to treat different types of cancers, their full potentials have been limited due to the occurrence of drug resistance. Resistance to anticancer drugs develops by a variety of mechanisms, one of which is increased drug efflux by transporters. The ATP-binding cassette (ABC) family drug efflux transporter P-glycoprotein (P-gp or multi-drug resistance protein 1 [MDRP1]) has been extensively studied and is known to play a major role in the development of multi-drug resistance (MDR) to chemotherapy [ 51 ]. In brief, overexpressed P-gp efflux out a wide variety of anticancer agents (e.g.: vinca alkaloids, doxorubicin, paclitaxel, etc.), leading to a lower concentration of these drugs inside cancer cells, thereby resulting in MDR. Over the past three decades, researchers have developed several synthetic P-gp inhibitors to block the efflux of anticancer drugs and have tested them in clinical trials, in combination with chemotherapeutic drugs. But none were found to be suitable enough in overcoming MDR and to be released for marketing, mainly due to the side effects associated with cross-reactivity towards other ABC transporters (BCRP and MRP-1) and the inhibition of CYP450 drug metabolizing enzymes [ 52 , 53 ].
On the other hand, a number of phytochemicals have been reported to have P-gp inhibitory activity. Moreover, detailed structure–activity studies on these phytochemicals have delineated the functional groups essential for P-gp inhibition [ 53 , 54 ]. Currently, one of the phytochemicals, tetrandrine (CBT-1 ® ; NSC-77037), is being used in a Phase I clinical trial ( http://www.ClinicalTrials.gov ; NCT03002805) in combination with doxorubicin for the treatment of metastatic sarcoma. Before developing phytochemicals or their derivatives as P-gp inhibitors, they need to be investigated thoroughly for their cross-reactivity towards other ABC transporters and CYP450 inhibition, in order to avoid toxicities similar to the older generation P-gp inhibitors that have failed in clinical trials.
Therefore, the selectivity for P-gp over other drug transporters and drug metabolizing enzymes should be considered as important criterias for the development of phytochemicals and their derivatives for overcoming MDR.
Mohane Selvaraj Coumar and Safiulla Basha Syed.
Centre for Bioinformatics, School of Life Sciences, Pondicherry University, Kalapet, Puducherry 605014, India.
[email protected]; [email protected]
Question 101: Is cell migration a selectable trait in the natural evolution of carcinoma?
The propensity of solid tumor malignancy to metastasize remains the main cause of cancer-related death, an extraordinary unmet clinical need, and an unanswered question in basic cancer research. While dissemination has been traditionally viewed as a late process in the progression of malignant tumors, amount of evidence indicates that it can occur early in the natural history of cancer, frequently when the primary lesion is still barely detectable.
A prerequisite for cancer dissemination is the acquisition of migratory/invasive properties. However, whether, and if so, how the migratory phenotype is selected for during the natural evolution of cancer and what advantage, if any, it may provide to the growing malignant cells remains an open issue. The answers to these questions are relevant not only for our understating of cancer biology but also for the strategies we adopt in an attempt of curbing this disease. Frequently, indeed, particularly in pharmaceutical settings, targeting migration has been considered much like trying “to shut the stable door after the horse has bolted” and no serious efforts in pursuing this aim has been done.
We argue, instead, that migration might be an intrinsic cancer trait that much like proliferation or increased survival confers to the growing tumor masses with striking selective advantages. The most compelling evidence in support for this contention stems from studies using mathematical modeling of cancer evolution. Surprisingly, these works highlighted the notion that cell migration is an intrinsic, selectable property of malignant cells, so intimately intertwined with more obvious evolutionarily-driven cancer traits to directly impact not only on the potential of malignant cells to disseminate but also on their growth dynamics, and ultimately provide a selective evolutionary advantage. Whether in real life this holds true remains to be assessed, nevertheless, work of this kind defines a framework where the acquisition of migration can be understood in a term of not just as a way to spread, but also to trigger the emergence of malignant clones with favorable genetic or epigenetic traits.
Alternatively, migratory phenotypes might emerge as a response to unfavorable conditions, including the mechanically challenging environment which tumors, and particularly epithelial-derived carcinoma, invariably experience. Becoming motile, however, may not per se being fixed as phenotypic advantageous traits unless it is accompanied or is causing the emergence of specific traits, including drug resistance, self-renewal, and survival. This might be the case, for example, during the process of epithelial-to-mesenchymal transition (EMT), which is emerging as an overarching mechanism for dissemination. EMT, indeed, may transiently equip individual cancer cells not only with migratory/invasive capacity but also with increased resistance to drug treatment, stemness potential at the expanse of fast proliferation.
Thus, within this framework targeting pro-migratory genes, proteins and processes may become a therapeutically valid alternative or a complementary strategy not only to control carcinoma dissemination but also its progression and development.
Giorgio Scita.
IFOM, The FIRC Institute of Molecular Oncology, Via Adamello 16, 20139 Milan, Italy; Department of Oncology and Hemato-Oncology (DIPO), School of Medicine, University of Milan, Via Festa del Perdono 7, 20122, Italy.
Qian CN, Zhang W, Xu RH. Defeating cancer: the 150 most important questions in cancer research and clinical oncology. Chin J Cancer. 2016;35(1):104. https://doi.org/10.1186/s40880-016-0165-4 .
Article PubMed PubMed Central Google Scholar
Wee JT, Poh SS. The most important questions in cancer research and clinical oncology: question 1. Could the vertical transmission of human papilloma virus (HPV) infection account for the cause, characteristics, and epidemiology of HPV-positive oropharyngeal carcinoma, non-smoking East Asian female lung adenocarcinoma, and/or East Asian triple-negative breast carcinoma? Chin J Cancer. 2017;36(1):13. https://doi.org/10.1186/s40880-016-0168-1 .
Venniyoor A. The most important questions in cancer research and clinical oncology—Question 2–5. Obesity-related cancers: more questions than answers. Chin J Cancer. 2017;36(1):18. https://doi.org/10.1186/s40880-017-0185-8 .
Chinese Journal of C. The 150 most important questions in cancer research and clinical oncology series: questions 6–14: Edited by Chinese Journal of Cancer. Chin J Cancer. 2017;36(1):33. https://doi.org/10.1186/s40880-017-0200-0 .
Article Google Scholar
Chinese Journal of C. The 150 most important questions in cancer research and clinical oncology series: questions 15–24: Edited by Chinese Journal of Cancer. Chin J Cancer. 2017;36(1):39. https://doi.org/10.1186/s40880-017-0205-8 .
Chinese Journal of C. The 150 most important questions in cancer research and clinical oncology series: questions 25–30: Edited by Chinese Journal of Cancer. Chin J Cancer. 2017;36(1):42. https://doi.org/10.1186/s40880-017-0210-y .
Chinese Journal of C. The 150 most important questions in cancer research and clinical oncology series: questions 31–39: Edited by Chinese Journal of Cancer. Chin J Cancer. 2017;36(1):48. https://doi.org/10.1186/s40880-017-0215-6 .
Chinese Journal of C. The 150 most important questions in cancer research and clinical oncology series: questions 40–49. Chin J Cancer. 2017;36(1):55. https://doi.org/10.1186/s40880-017-0222-7 .
Chinese Journal of C. The 150 most important questions in cancer research and clinical oncology series: questions 50–56. Chin J Cancer. 2017;36(1):69. https://doi.org/10.1186/s40880-017-0236-1 .
Chinese Journal of C. The 150 most important questions in cancer research and clinical oncology series: questions 57–66: Edited by Chinese Journal of Cancer. Chin J Cancer. 2017;36(1):79. https://doi.org/10.1186/s40880-017-0249-9 .
Chinese Journal of C. The 150 most important questions in cancer research and clinical oncology series: questions 67–75: Edited by Chinese Journal of Cancer. Chin J Cancer. 2017;36(1):86. https://doi.org/10.1186/s40880-017-0254-z .
Editorial Office of Chinese Journal of C. The 150 most important questions in cancer research and clinical oncology series: questions 76–85: Edited by Chinese Journal of Cancer. Chin J Cancer. 2017;36(1):91. https://doi.org/10.1186/s40880-017-0259-7 .
Chinese Journal of C. The 150 most important questions in cancer research and clinical oncology series: questions 86–93: Edited by Chinese Journal of Cancer. Chin J Cancer. 2018;37(1):1. https://doi.org/10.1186/s40880-018-0266-3 .
Weinberg RA. Coming full circle-from endless complexity to simplicity and back again. Cell. 2014;157(1):267–71. https://doi.org/10.1016/j.cell.2014.03.004 .
Article CAS PubMed Google Scholar
Niu N, Mercado-Uribe I, Liu J. Dedifferentiation into blastomere-like cancer stem cells via formation of polyploid giant cancer cells. Oncogene. 2017;36(34):4887–900. https://doi.org/10.1038/onc.2017.72 .
Article CAS PubMed PubMed Central Google Scholar
Niu N, Zhang J, Zhang N, Mercado-Uribe I, Tao F, Han Z, et al. Linking genomic reorganization to tumor initiation via the giant cell cycle. Oncogenesis. 2016;5(12):e281. https://doi.org/10.1038/oncsis.2016.75 .
Zhang S, Mercado-Uribe I, Xing Z, Sun B, Kuang J, Liu J. Generation of cancer stem-like cells through the formation of polyploid giant cancer cells. Oncogene. 2014;33(1):116–28. https://doi.org/10.1038/onc.2013.96 .
Hemberger M, Dean W, Reik W. Epigenetic dynamics of stem cells and cell lineage commitment: digging Waddington’s canal. Nat Rev Mol Cell Biol. 2009;10(8):526–37. https://doi.org/10.1038/nrm2727 .
Liu J. The dualistic origin of human tumors. Semin Cancer Biol. 2018. https://doi.org/10.1016/j.semcancer.2018.07.004 .
Zhou B, Xu JW, Cheng YG, Gao JY, Hu SY, Wang L, et al. Early detection of pancreatic cancer: where are we now and where are we going? Int J Cancer. 2017;141(2):231–41. https://doi.org/10.1002/ijc.30670 .
Yachida S, Jones S, Bozic I, Antal T, Leary R, Fu B, et al. Distant metastasis occurs late during the genetic evolution of pancreatic cancer. Nature. 2010;467(7319):1114–7. https://doi.org/10.1038/nature09515 .
Sharma R, Huang X, Brekken RA, Schroit AJ. Detection of phosphatidylserine-positive exosomes for the diagnosis of early-stage malignancies. Br J Cancer. 2017;117(4):545–52. https://doi.org/10.1038/bjc.2017.183 .
Ryan DP, Hong TS, Bardeesy N. Pancreatic adenocarcinoma. N Engl J Med. 2014;371(11):1039–49. https://doi.org/10.1056/NEJMra1404198 .
Takada T, Yasuda H, Amano H, Yoshida M, Uchida T. Simultaneous hepatic resection with pancreato-duodenectomy for metastatic pancreatic head carcinoma: does it improve survival? Hepatogastroenterology. 1997;44(14):567–73.
CAS PubMed Google Scholar
Bailey P, Chang DK, Nones K, Johns AL, Patch AM, Gingras MC, et al. Genomic analyses identify molecular subtypes of pancreatic cancer. Nature. 2016;531(7592):47–52. https://doi.org/10.1038/nature16965 .
Frigerio I, Regi P, Giardino A, Scopelliti F, Girelli R, Bassi C, et al. Downstaging in stage IV pancreatic cancer: a new population eligible for surgery? Ann Surg Oncol. 2017;24(8):2397–403. https://doi.org/10.1245/s10434-017-5885-4 .
Article PubMed Google Scholar
Van Laethem JL, Riess H, Jassem J, Haas M, Martens UM, Weekes C, et al. Phase I/II study of refametinib (BAY 86-9766) in combination with gemcitabine in advanced pancreatic cancer. Target Oncol. 2017;12(1):97–109. https://doi.org/10.1007/s11523-016-0469-y .
Jiang H, Hegde S, Knolhoff BL, Zhu Y, Herndon JM, Meyer MA, et al. Targeting focal adhesion kinase renders pancreatic cancers responsive to checkpoint immunotherapy. Nat Med. 2016;22(8):851–60. https://doi.org/10.1038/nm.4123 .
Mace TA, Shakya R, Pitarresi JR, Swanson B, McQuinn CW, Loftus S, et al. IL-6 and PD-L1 antibody blockade combination therapy reduces tumour progression in murine models of pancreatic cancer. Gut. 2018;67(2):320–32. https://doi.org/10.1136/gutjnl-2016-311585 .
Immunotherapy Beats Chemo for Bladder Cancer. Cancer Discov. 2017;7(5):OF8. https://doi.org/10.1158/2159-8290.cd-nb2017-035 .
Dummer R, Ascierto PA, Gogas HJ, Arance A, Mandala M, Liszkay G, et al. Encorafenib plus binimetinib versus vemurafenib or encorafenib in patients with BRAF-mutant melanoma (COLUMBUS): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol. 2018;19(5):603–15. https://doi.org/10.1016/S1470-2045(18)30142-6 .
Garassino MC, Cho BC, Kim JH, Mazieres J, Vansteenkiste J, Lena H, et al. Durvalumab as third-line or later treatment for advanced non-small-cell lung cancer (ATLANTIC): an open-label, single-arm, phase 2 study. Lancet Oncol. 2018;19(4):521–36. https://doi.org/10.1016/S1470-2045(18)30144-X .
U.S. National Library of Medicine. ClinicalTrials.gov.
Liu Z, Yokoyama NN, Blair CA, Li X, Avizonis D, Wu XR, et al. High sensitivity of an Ha-RAS transgenic model of superficial bladder cancer to metformin is associated with approximately 240-fold higher drug concentration in urine than serum. Mol Cancer Ther. 2016;15(3):430–8. https://doi.org/10.1158/1535-7163.MCT-15-0714-T .
Menendez JA, Quirantes-Pine R, Rodriguez-Gallego E, Cufi S, Corominas-Faja B, Cuyas E, et al. Oncobiguanides: paracelsus’ law and nonconventional routes for administering diabetobiguanides for cancer treatment. Oncotarget. 2014;5(9):2344–8. https://doi.org/10.18632/oncotarget.1965 .
Peng M, Su Q, Zeng Q, Li L, Liu Z, Xue L, et al. High efficacy of intravesical treatment of metformin on bladder cancer in preclinical model. Oncotarget. 2016;7(8):9102–17. https://doi.org/10.18632/oncotarget.6933 .
Peng M, Huang Y, Tao T, Peng CY, Su Q, Xu W, et al. Metformin and gefitinib cooperate to inhibit bladder cancer growth via both AMPK and EGFR pathways joining at Akt and Erk. Sci Rep. 2016;6:28611. https://doi.org/10.1038/srep28611 .
Definition of liquid biopsy. In: NCI Dictionary of Cancer Terms. National Cancer Institute. https://www.cancer.gov/publications/dictionaries/cancer-terms/def/liquid-biopsy .
Ta A. A case of cancer in which cells similar to those in the tumors were seen in the blood after death. Aust Med J. 1869;14:146–9.
Google Scholar
Miller MC, Doyle GV, Terstappen LW. Significance of circulating tumor cells detected by the cell search system in patients with metastatic breast colorectal and prostate cancer. J Oncol. 2010;2010:617421. https://doi.org/10.1155/2010/617421 .
Nagrath S, Sequist LV, Maheswaran S, Bell DW, Irimia D, Ulkus L, et al. Isolation of rare circulating tumour cells in cancer patients by microchip technology. Nature. 2007;450(7173):1235–9. https://doi.org/10.1038/nature06385 .
Bidard FC, Proudhon C, Pierga JY. Circulating tumor cells in breast cancer. Mol Oncol. 2016;10(3):418–30. https://doi.org/10.1016/j.molonc.2016.01.001 .
Cohen SJ, Punt CJ, Iannotti N, Saidman BH, Sabbath KD, Gabrail NY, et al. Relationship of circulating tumor cells to tumor response, progression-free survival, and overall survival in patients with metastatic colorectal cancer. J Clin Oncol. 2008;26(19):3213–21. https://doi.org/10.1200/JCO.2007.15.8923 .
Ignatiadis M, Dawson SJ. Circulating tumor cells and circulating tumor DNA for precision medicine: dream or reality? Ann Oncol. 2014;25(12):2304–13. https://doi.org/10.1093/annonc/mdu480 .
Krebs MG, Sloane R, Priest L, Lancashire L, Hou JM, Greystoke A, et al. Evaluation and prognostic significance of circulating tumor cells in patients with non-small-cell lung cancer. J Clin Oncol. 2011;29(12):1556–63. https://doi.org/10.1200/JCO.2010.28.7045 .
Diaz LA Jr, Bardelli A. Liquid biopsies: genotyping circulating tumor DNA. J Clin Oncol. 2014;32(6):579–86. https://doi.org/10.1200/JCO.2012.45.2011 .
Kandoth C, McLellan MD, Vandin F, Ye K, Niu B, Lu C, et al. Mutational landscape and significance across 12 major cancer types. Nature. 2013;502(7471):333–9. https://doi.org/10.1038/nature12634 .
Esteller M. Epigenetics in cancer. N Engl J Med. 2008;358(11):1148–59. https://doi.org/10.1056/NEJMra072067 .
Guo S, Diep D, Plongthongkum N, Fung HL, Zhang K, Zhang K. Identification of methylation haplotype blocks aids in deconvolution of heterogeneous tissue samples and tumor tissue-of-origin mapping from plasma DNA. Nat Genet. 2017;49(4):635–42. https://doi.org/10.1038/ng.3805 .
Xu RH, Wei W, Krawczyk M, Wang W, Luo H, Flagg K, et al. Circulating tumour DNA methylation markers for diagnosis and prognosis of hepatocellular carcinoma. Nat Mater. 2017;16(11):1155–61. https://doi.org/10.1038/nmat4997 .
Robey RW, Pluchino KM, Hall MD, Fojo AT, Bates SE, Gottesman MM. Revisiting the role of ABC transporters in multidrug-resistant cancer. Nat Rev Cancer. 2018. https://doi.org/10.1038/s41568-018-0005-8 .
Chung FS, Santiago JS, Jesus MF, Trinidad CV, See MF. Disrupting P-glycoprotein function in clinical settings: what can we learn from the fundamental aspects of this transporter? Am J Cancer Res. 2016;6(8):1583–98.
CAS PubMed PubMed Central Google Scholar
Syed SB, Coumar MS. P-Glycoprotein mediated multidrug resistance reversal by phytochemicals: a review of SAR & future perspective for drug design. Curr Top Med Chem. 2016;16(22):2484–508.
Abdallah HM, Al-Abd AM, El-Dine RS, El-Halawany AM. P-Glycoprotein inhibitors of natural origin as potential tumor chemo-sensitizers: a review. J Adv Res. 2015;6(1):45–62. https://doi.org/10.1016/j.jare.2014.11.008 .
Download references
Author information
Authors and affiliations, rights and permissions.
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License ( http://creativecommons.org/licenses/by/4.0/ ), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated.
Reprints and permissions
About this article
Cite this article.
Cancer Communications. The 150 most important questions in cancer research and clinical oncology series: questions 94–101. Cancer Commun 38 , 69 (2018). https://doi.org/10.1186/s40880-018-0341-9
Download citation
Received : 13 November 2018
Accepted : 19 November 2018
Published : 26 November 2018
DOI : https://doi.org/10.1186/s40880-018-0341-9
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Tumor origin
- Polyploid giant cancer cell
- Pancreatic ductal adenocarcinoma
- Liquid biopsy
- Spontaneous animal model
- Chemotherapy
- Immunotherapy
- Precision treatment
- Vaccine immunization
- Circulating tumor cell
- Circulating tumor DNA
- CpG methylation
- Methylation haplotype block
- Phytochemicals
- P-Glycoprotein
- Multi-drug resistance
- P-Glycoprotein inhibitor
- Epithelial-to-mesenchymal transition
- Pro-migratory gene
ISSN: 2523-3548
- General enquiries: [email protected]
Cochrane Breast Cancer
Top 10 breast cancer topics needing a cochrane systematic review.

Deciding which research topics to focus on in medicine and health depends on many factors. These factors can include the currency of a topic, feedback from people providing or receiving care, and the priorities of funders.
In late 2019, the Cochrane Breast Cancer Group (part of Cochrane’s Cancer Network) conducted a formal priority-setting exercise to help decide which review topics were most needed in the Cochrane Library. The Group did this by circulating a survey listing 25 new or existing review topics to a diverse group of individuals who are part of the international breast cancer community. The survey asked individuals to rank their top 10 topics from the list. Read details about the aims and methods used for this priority-setting exercise, which adhered to the standards outlined in Cochrane’s priority setting guidance note .
What were the top 10 review topics?
Read about the ranking of the 25 new or existing review topics .
What is next?
Support to author teams For the top 10 topics, the Cochrane Breast Cancer Group will prioritise these topics during the editorial and peer-review process.
For all breast cancer review topics registered with Cochrane, the Cochrane Breast Cancer Group continues to work on these topics with author teams as these remain important topics. There will be no noticeable change in the support provided to author teams.
Future topics The Cochrane Breast Cancer Group is open to receiving new topic ideas. If you have suggestions for new topics that are not currently covered in the Cochrane Library, please send your idea to [email protected] .
Repeating this priority-setting exercise The priority-setting exercise may be repeated every 3 years, depending on resources.
Who responded to the survey?
The survey was circulated to over 800 individuals. Of the 199 people who responded, 90 people (45%) provided complete responses. The respondents were doctors (59%), researchers (18%) and people who had received treatment or currently receiving treatment for breast cancer (14%). Most respondents were from the UK, followed by the USA, Argentina, and India.
How did we calculate the ranking for each review topic?
The average ranking was calculated for each topic. This method is commonly used to determine ranking scores from surveys. This approach considers the number of counts for each ranking on a topic, the weighting of each rank (where a ranking of 1 gets the most weight) and the total number of counts.
[Cover image: foliage of the Yew tree. Taxanes, a class of chemotherapy drugs, were originally derived from the Yew tree]

UKnowledge > College of Medicine > Toxicology and Cancer Biology > Theses & Dissertations
Theses and Dissertations--Toxicology and Cancer Biology
Theses/dissertations from 2024 2024.
UNDERSTANDING THE MECHANISM OF FERROPTOSIS SUSCEPTIBILITY VARIATION IN COLORECTAL CANCER , Aziza Alshahrani
Elucidation of Mismatch Repair Regulation by ABL1: Advantages/Disadvantages of Tyrosine Kinase Inhibitor Treatment , Hannah Daniels
RPS6KB1 IS A CRITICAL TARGET FOR OVERCOMING TUMOR LINEAGE PLASTICITY AND THERAPY RESISTANCE , Saptadwipa Ganguly
PORCUPINE’S ROLE IN THE ENHANCEMENT OF ENZALUTAMIDE EFFICACY IN DRUG RESISTANT PROSTATE CANCER , Katelyn Jones
ACQUIRED TREATMENT RESISTANCE IN PROSTATE CANCER VIA THE PRODUCTION OF RADIATION DERIVED EXTRACELLULAR VESICLES CONTAINING MITOCHONDRIAL PROTEINS , Caitlin Miller
Delineating Contributions of Genotype and Lineage to Lung Cancer Therapy Response , Kassandra Jo Naughton
THE CRITICAL ROLE OF NAC1 IN TRIPLE-NEGATIVE BREAST CANCER STEMNESS AND IMMUNOSUPPRESSION , chrispus ngule
THERAPEUTIC APPROACHES AND NOVEL MECHANSIMS IN CANCER PROGRESSION , Kendall Simpson
Theses/Dissertations from 2023 2023
ELUCIDATING THE FUNCTIONAL IMPORTANCE OF PEROXIREDOXIN IV IN PROSTATE CANCER AND ITS SECRETION MECHANISM , Na Ding
Targeting EZH2 to Improve Outcomes of Lung Squamous Cell Carcinoma , Tanner DuCote
UNDERSTANDING AND TARGETING THE TPH1-SEROTONIN-HTR3A AXIS IN SMALL CELL LUNG CANCER , Yanning Hao
CONSERVED NOVEL INTERACTIONS BETWEEN POST-REPLICATIVE REPAIR AND MISMATCH REPAIR PROTEINS HAVE DIFFERENTIAL EFFECTS ON DNA REPAIR PATHWAYS , Anna K. Miller
UNDERSTANDING THE ROLE OF PEROXIREDOXIN IV IN COLORECTAL CANCER DEVELOPMENT , Pratik Thapa
BEYOND MITOSIS, PLK1-MEDIATED PHOSPHORYLATION RE-WIRES CANCER METABOLISM AND PROMOTES CANCER PROGRESSION , Qiongsi Zhang
Theses/Dissertations from 2022 2022
ELUCIDATING THE ROLE OF POLYCOMB REPRESSIVE COMPLEX 2 IN LUNG STEM CELL FATE AND LUNG DISEASE , Aria Byrd
SEX DIMORPHISM IN HEMATOPOIESIS AND BONE MARROW NICHE , xiaojing cui
EXTRACELLULAR VESICLES AND CANCER THERAPY: AN INSIGHT INTO THE ROLE OF OXIDATIVE STRESS , Jenni Ho
OVERCOMING RESISTANCE TO SG-ARIS IN CASTRATION-RESISTANT PROSTATE CANCER , Chaohao Li
Theses/Dissertations from 2021 2021
THE TUMOR SUPPRESSOR PAR-4 REGULATES HYPERTROPHIC OBESITY , Nathalia Araujo
Epigenetic States Regulate Tumor Aggressiveness and Response to Targeted Therapies in Lung Adenocarcinoma , Fan Chen
DELINEATING THE ROLE OF FATTY ACID METABOLISM TO IMPROVE THERAPEUTIC STRATEGIES FOR COLORECTAL CANCER , James Drury
DEVELOPMENT OF TOOLS FOR ATOM-LEVEL INTERPRETATION OF STABLE ISOTOPE-RESOLVED METABOLOMICS DATASETS , Huan Jin
MECHANISMS OF CADMIUM-INDUCED AND EPIDERMAL GROWTH FACTOR RECEPTOR MUTATION-DRIVEN LUNG TUMORIGENESIS , Hsuan-Pei Lin
SCIENCE-BASED REGULATION OF PHARMACOLOGICAL SUBSTANCES IN COMPETITION HORSES , Jacob Machin
Advanced Search
- Notify me via email or RSS
Browse by Author
- Collections
- Disciplines
Author Corner
- Submit Research
New Title Here
Below. --> connect.
- Law Library
- Special Collections
- Copyright Resource Center
- Graduate School
- Scholars@UK

- We’d like your feedback
Home | About | FAQ | My Account | Accessibility Statement
Privacy Copyright
University of Kentucky ®
An Equal Opportunity University Accreditation Directory Email Privacy Policy Accessibility Disclosures
An official website of the United States government
Official websites use .gov A .gov website belongs to an official government organization in the United States.
Secure .gov websites use HTTPS A lock ( Lock Locked padlock icon ) or https:// means you've safely connected to the .gov website. Share sensitive information only on official, secure websites.
- Publications
- Account settings
- Advanced Search
- Journal List
The 150 most important questions in cancer research and clinical oncology series: questions 86–93
Edited by Chinese Journal of Cancer
- Author information
- Article notes
- Copyright and License information
Received 2017 Dec 18; Accepted 2018 Jan 5; Collection date 2018.
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License ( http://creativecommons.org/licenses/by/4.0/ ), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated.
Since the beginning of 2017, Chinese Journal of Cancer has published a series of important questions in cancer research and clinical oncology, which spark diverse thoughts, interesting communications, and potential collaborations among researchers all over the world. In this article, 8 more questions are presented as follows. Question 86. In which circumstances is good supportive care associated with a survival advantage in patients with cancer? Question 87. Can we develop animal models to mimic immunotherapy response of cancer patients? Question 88. What are the mechanisms underlying hepatitis B virus-associated non-hepatocellular cancers? Question 89. Can we more precisely target tumor metabolism by identifying individual patients who would benefit from the treatment? Question 90. What type of cranial irradiation-based prophylactic therapy combination can dramatically improve the survival of patients with extensive small-cell lung cancer? Question 91. How can postoperative radiotherapy prolong overall survival of the patients with resected pIIIA-N2 non-small cell lung cancer? Question 92. What are the key molecular events that drive oral leukoplakia or erythroplakia into oral cancer? Question 93. How could we track the chemotherapeutics-driven evolution of tumor genome in non-small cell lung cancer for more effective treatment?
Keywords: Supportive care, Animal model, Mimic immunotherapy, Hepatitis B virus-associated cancer, Non-hepatocellular cancer, Tumor metabolism, Prophylactic therapy, Postoperative radiotherapy, Survival, Molecular event, Oral cancer, Tumor genome
To accelerate our endeavors to overcome cancer, Chinese Journal of Cancer has launched a program of publishing 150 most important questions in cancer research and clinical oncology [ 1 ]. Since the beginning of 2017, Chinese Journal of Cancer has published a series of important questions in cancer research and clinical oncology [ 2 – 12 ], which spark diverse thoughts, interesting communications, and potential collaborations among researchers all over the world. In this article, Questions 86–93 are selected and presented. This program of collecting and publishing the key questions is still ongoing. Please send your thoughtful questions to Ms. Ji Ruan via email: [email protected].
Question 86: In which circumstances is good supportive care associated with a survival advantage in patients with cancer?
Background and implications.
It is well documented that good supportive care throughout the treatment and survival phases of cancer as well as palliative care towards the end of life improve the quality of life of the patients [ 13 ]. In some circumstances, good supportive care may also prolong survival. Quintin et al. [ 14 ] performed a global analysis of data from multiple trials and showed that quality of life and presenting symptoms were prognostic factors for survival of patients with cancer in addition to other clinical characteristics. For example, febrile neutropenia following chemotherapy is a life-threatening adverse effect and can be mitigated by giving the chemotherapy with granulocyte colony stimulating factor (G-CSF). It is well documented that mortality from infection is reduced by G-CSF [ 15 ]; however, it is not clear that this may be translated into an overall survival advantage. Prophylactic use of antiemetics increases the tolerance of chemotherapy, allowing full dose to be given and courses of chemotherapy to be completed, which has been shown to prolong survival [ 16 ]. Good symptom control with chemotherapy may also prolong survival. In a randomized study, second-line chemotherapy was given with or without early palliative care to patients with non-small cell lung cancer, and the results showed that those receiving the palliative care in addition to their chemotherapy had significantly longer survival than those receiving chemotherapy only (11.6 vs. 8.9 months, P = 0.02) [ 17 ]. Further, it is intriguing that psychosocial support may prolong survival. A weekly psychosocial support group and self-hypnosis for pain was added to anticancer therapy for breast cancer patients in a randomized trial and resulted in prolonged survival as compared with those who only received anticancer therapy [ 18 ]. The relationship between social networks and social support has been equivocal although a large breast cancer study showed an increase in both all-cause mortality and breast cancer mortality in women who are socially isolated [ 19 , 20 ]. Certainly, the narratives of exceptional survivors of incurable cancer ascribed some of their outcomes to family support [ 21 ].
Clearly, in some circumstances, the addition of good supportive care which addresses cancer-associated symptoms and adverse effects of treatment can be added to anticancer treatment to prolong survival. More researches are needed to better define when this occurs.
Affiliation and email
Sansom Institute for Health Research, University of South Australia, Adelaide, South Australia 5001, Australia.
Question 87: Can we develop animal models to mimic immunotherapy response of cancer patients?
Efforts on immuno-oncology (I/O) research to fight cancer are in exponential phase of growth due to recent breakthrough in the development of immune checkpoint inhibitors and unprecedented rate of regulatory approval to shorten the otherwise lengthy bench to bedside process. The prevalent models include syngeneic, genetically engineered, and partially humanized mouse models each with its advantages and limitations. The lack of precise animal models that would be capable of mimicking human immune microenvironment is one of the major challenges for proper preclinical evaluation of I/O therapies and identifying patients most likely to be benefited from specific I/O strategies.
The ideal animal models should also possess effective biomarkers for monitoring the immune functions of the host as well as therapeutic effects of I/O. In current clinical practice, the remarkable progress in the development of immune checkpoint inhibitors went solo without parallel advancement of definitive patient selection tool. The cost, toxicities, and the time delay for the 40%–60% of patients not benefiting from immunotherapy makes it imperative to identify valid prognostic biomarkers [e.g., programmed death-ligand 1 (PD-L1) expression, mismatch repair (MMR) deficiency, cluster of differentiation 8 (CD8) T cell infiltrates, tumor mutation burden] that could predict patient response and facilitate differentiation of durable response versus transient response. Given the dynamic nature of the immune response and the complexity of immune/tumor interaction, development of biomarkers for immunotherapies is highly challenging. Presence of tumor-specific antigens, expression of immunosuppressive molecules [PD-L1, indoleamine 2,3-dioxygenase (IDO), and so on] by tumor cells, and mutation load and landscape all contribute to the response of tumor cells to I/O therapies. While most of the biomarker-searching efforts had focused on tumor characteristics, the role of host immune system is equally important. The effectiveness of a given immunotherapeutic approach depends on a pre-existing immune state of a patient.
In summary, development of clinically relevant animal models possessing discerning prognostic markers is critical to fulfill the promise of immunotherapy as a paradigm-shifting strategy to fight the most aggressive and intractable cancers.
Qian Shi and Meng Qiao.
Affiliation and emails
Crown Biosciences Inc., Taicang, Jiangsu Province, 215400, P. R. China.
[email protected]; [email protected].
Question 88: What are the mechanisms underlying hepatitis B virus-associated non-hepatocellular cancers?
Hepatitis B virus (HBV) infection is a strong risk factor for the development of hepatocellular carcinoma. Epidemiological studies have also shown that HBV infection may increase the incidence of several types of non-hepatocellular cancers, including gastric adenocarcinoma, pancreatic ductal carcinoma, and non-Hodgkin lymphoma (NHL). Clinical studies further suggested that some of these HBV-associated non-hepatocellular cancers, for instance a subtype of NHL, diffuse large B cell lymphoma, exhibit a more aggressive disease course with poor prognosis, independent of its pathological subtype. However, what are the mechanisms underlying these associations and whether the viral infection is indeed disease-causing or rather a contributing co-factor remain unclear. Two major hypotheses, direct viral infection of the corresponding cell types and chronic viral antigen stimulations, have been proposed. In both scenarios, infection may result in dysregulation of host cellular processes and increased genome instability, and in the case of direct infection, like in hepatocellular carcinoma, integration of viral DNA into the host genome may lead to activation of selective oncogenes. More detailed morphological and molecular studies, including characterization of the genome of these HBV-associated non-hepatocellular cancers and the repertories of infiltrating immune cells, may provide further clues to this question. It will also be of interest to determine if there is an association between genotype (strain of HBV) and phenotype (type of cancer). Finally, in areas/countries with a high prevalence of infection and initiated the mandatory HBV vaccine program decades ago, theoretically, the incidence of these non-hepatocellular cancers should decrease with time. Of note, this may be complicated by the increased contribution of other risk factors, especially life style-related factors. Chronic HBV infection is endemic in some parts of Asia, Africa, and South America and remains to be a public health burden in these areas. Further understanding the molecular mechanisms underlying the HBV-associated cancers will help us to develop novel or more precise therapies for the affected patients.
Yao Liu and Qiang Pan-Hammarström.
Division of Clinical Immunology, Department of Laboratory Medicine, Karolinska University Hospital, Huddinge, Stockholm SE 141 86, Sweden.
[email protected]; [email protected].
Question 89: Can we more precisely target tumor metabolism by identifying individual patients who would benefit from the treatment?
Background and implication.
During the process of tumorigenesis, tumor cells must face two challenges: first, obtaining the nutrients needed for the rapid growth; and second, evading the surveillance and attack from the host immune system. Tumor cell’s unique metabolic program can be used to meet these challenges. Glycolysis is the major metabolic process used by malignant tumors, even when oxygen supply is adequate, which is termed as “the Warburg effect”. Glycolysis decreases the pH value of the tumor microenvironment (TME); therefore, tumor cells can inhibit the activities of antigen-presenting cells (APCs) and cytotoxic T lymphocytes (CTLs) by controlling the acidity of TME, eventually leading to tumor cell immune escape. A second group of metabolism-related modification directly targets the major histocompatibility complex-I (MHC-I) and related molecules and hence sensitizes cancer cells to the cytolytic actions of the anti-tumor adaptive immune response.
Recent findings from in vitro and in vivo studies have shown that targeting tumor and immune cell metabolism hold the promising possibilities toward clinical therapeutics for treating cancer [ 22 , 23 ]. However, clinical benefit has only been observed in a small number of patients [ 24 – 28 ]. Most patients still do not respond to these new therapies, and nearly all patients with certain types of cancer (i.e., pancreatic and colorectal cancers) do not respond. The reason is probably because tumor metabolism may vary over the course of tumor development, or some hidden tumor metabolic products modulate signaling pathways important for immune cell activation. A new hypothesis has been proposed that tumor cells can change their metabolism by waves of gene regulation to adjust to their different needs [ 29 ]. Some of these waves are originated by deregulated expression of oncogenes, which have already been linked to metabolic remodeling. On the other hand, different parts of solid tumor sometimes possess different epigenetic characteristics and may be derived from distinct cancer stem cell populations. Therefore, the most serious challenge in reshaping the tumor-specific metabolism and immune profiles in TME is to understand the metabolic heterogeneity which is extremely complicated depending not only on tumor and immune cell types but also on tumor stages and subset of patient population.
Nevertheless, the success associated with these new approaches has opened new investigations addressing several questions: How much metabolism pathways represent true vulnerabilities for tumor development and immunosuppression in different types and stages of cancer? Are there other factors that may be blocking, even temporarily, which is critical for tumor control? How different subsets of tumor cell populations respond to metabolic intervention? Can we identify ahead of time the patients who would benefit from metabolic targeted therapy?
Notably, tumor and immune cells share similar metabolic needs and reprogramming during proliferation to support their increased biosynthetic and energy demands [ 30 , 31 ] and often compete for the same nutrients. Therefore, deprivation of nutrients in TME must be cautiously explored to eliminate potential negative impacts on the anti-tumor immunity. Understanding the underlying mechanisms of metabolic interplay between tumor and immune cells will provide new precise directions to manipulate the tumor metabolism for better treatment outcome.
Jianyang Wang and Zhouguang Hui.
Department of Radiation Oncology (JW and ZH), Department of VIP Medical Services (ZH), National Cancer Center/Cancer Hospital, Chinese Academy of Medical Sciences & Peking Union Medical College, Beijing 100021, China.
[email protected]; [email protected].
Question 90: What type of cranial irradiation-based prophylactic therapy combination can dramatically improve the survival of patients with extensive small-cell lung cancer?
Brain metastasis is a common reason of treatment failure in small-cell lung cancer (SCLC), particularly in extensive disease which represents approximately two-thirds of newly diagnosed SCLCs. Recent studies have found that thoracic radiotherapy (TRT) can increase the 2-year overall survival (OS) rate of patients with extensive SCLC after chemotherapy [ 32 – 34 ]. However, it remains controversial that whether prophylactic cranial irradiation (PCI) can prolong OS [ 35 – 38 ]. The combination of TRT and PCI may boost the chances of survival, but there will be not much predictable OS benefit even if more prospective studies with large sample sizes are conducted. After first-line chemotherapy, the comprehensive treatment based on TRT and PCI, such as combining with new anti-metastatic drugs, will make great strides toward OS improvement. The application of more accurately targeted therapy is now available and promising. Maintenance treatment with sunitinib can prolong progression-free survival (PFS) in extensive SCLC [ 39 ]. Recent studies on new drugs targeting the signaling pathways (e.g., Notch signaling) related to neuroendocrine differentiation, DNA reparation, and immune checkpoint are ongoing. The Notch signaling pathway influences multiple processes in normal cell morphogenesis, including the differentiation of multipotent progenitor cells (neuron differentiation), cell apoptosis, and cell proliferation. Rovalpituzumab tesirine (Rova-T) targeting the Notch signaling pathway showed promising results in a phase I trial [ 40 ]. Poly ADP-ribose polymerase (PARP) is DNA repairase and is critical in DNA damage repair. By inhibiting PARP, proliferation of malignant cells can be suppressed. Veliparib, a PARP inhibitor, has yielded antitumor activity in SCLC [ 41 ]. Researches on immunotherapy primarily focus on cytotoxic T-lymphocyte-associated antigen-4 (CTLA-4) and programmed cell death protein 1 (PD-1)/programmed death ligand 1 (PD-L1) inhibitors. Nivolumab alone and in combination with ipilimumab resulted in encouraging response rates (RR) in a phase I/II trial in the relapsed tumor setting [ 42 ]. The development of anti-metastasis agents is clearly critical for further improving the survival benefits of the patients with extensive SCLC. In addition, advanced irradiation technique is expected to be adopted in future clinical trails to decrease irradiation-induced injury in hippocampus for protecting cognitive function [ 43 ].
Lei Deng and Zhouguang Hui.
Department of Radiation Oncology (LD and ZH), Department of VIP Medical Services (ZH), National Cancer Center/Cancer Hospital, Chinese Academy of Medical Sciences & Peking Union Medical College, Beijing 100021, China.
[email protected]; [email protected].
Question 91: How can postoperative radiotherapy prolong overall survival of the patients with resected pIIIA-N2 non-small cell lung cancer?
For patients with resected pIIIA-N2 non-small cell lung cancer (NSCLC), the main reason of treatment failure is locoregional and/or distant relapse. Adjuvant chemotherapy can prolong overall survival to some extent. However, the role of postoperative radiotherapy is not well defined.
A meta-analysis study on postoperative radiotherapy published in 1998 concluded that postoperative radiotherapy did not prolong the survival, even in patients with stage III and pN2 NSCLC, which may due to the toxicities with suboptimal, outdated irradiation equipment and techniques [ 44 ]. Improvements in conformal radiotherapy techniques have led to a resurgence of interest in studying the effect of postoperative radiotherapy on pIIIA-N2 NSCLC. Several retrospective, large-size, case–control studies have shown that postoperative radiotherapy using three dimensional conformal radiotherapy (3D-CRT) or intensity modulated radiation therapy (IMRT) techniques can prolong overall survival [ 45 ]. However, the benefit still needs to be confirmed by randomized clinical trials (RCTs). Up to now, there are three such phase III RCTs. CALGB 9734, the earliest one, failed because of slow accrual [ 46 ]. LUNGART, the ongoing one, began in 2007 and aims to enroll 700 patients by its conclusion in 2022. The other ongoing phase III multi-center RCT ( NCT00880971 ), conducted by our institute, has accrued 400 patients over planned 500 patients. However, due to the heterogeneity of pIIIA-N2 NSCLC, only certain subgroups of patients may benefit from postoperative radiotherapy. Selecting suitable candidates or the populations at high risk who may benefit from postoperative radiotherapy is the next and profound task.
It is expected that by combining with targeted therapy and/or immunotherapy, the therapeutic effects of postoperative radiotherapy can be enhanced. For patients with completely resected NSCLC with epidermal growth factor receptor (EGFR) activating mutation, two recently reported RCTs have showed that adjuvant EGFR tyrosine kinase inhibitors (TKIs) significantly prolonged disease-free survival as compared with adjuvant chemotherapy [ 47 , 48 ]. Therefore, for pIIIA-N2 NSCLC patients with EGFR-activating mutation receiving EGFR TKIs, the value of postoperative radiotherapy should be further evaluated. Theoretically, any new agent that can inhibit metastasis could enhance the efficacy of postoperative radiotherapy, and more efforts are warranted in this direction.
Yu Men and Zhouguang Hui.
Department of VIP Medical Services, National Cancer Center/Cancer Hospital, Chinese Academy of Medical Sciences & Peking Union Medical College, Beijing 100021, China.
[email protected]; [email protected].
Question 92: What are the key molecular events that drive oral leukoplakia or erythroplakia into oral cancer?
The natural history of cancer is poorly understood. The main reason is that in the vast majority of the cases, malignant tumors are diagnosed after becoming clinically perceptible. The paradox is that, for patients dying from cancer, the time from diagnosis to death is often much shorter than the long period preceding diagnosis. Most of our knowledge is based on the analysis of established malignant tumors in comparison with histologically normal tissue, and the use of naturally occurring or genetically engineered animal models that may not recapitulate the natural history of human cancer. Initiation is thought to be the first step of the multistep model of cancer development, followed by promotion and progression. However, the stepwise and sequential progression model is being challenged by some clinical observations. One of the best examples is the natural history of oral leukoplakia or erythroplakia, the most frequent, potentially malignant lesions of the oral cavity. They can remain for many years without changing, can regress spontaneously or after cessation of tobacco smoking, alcohol drinking, or smokeless tobacco, and can transform to invasive squamous cell carcinoma (SCC) at the same site or at distance from the potentially malignant lesion. The reported rate of malignant transformation has been low in community-based studies in developing countries (0.06% per year) and higher in observational studies in western countries involving patients followed in hospital-based academic centers (1%–5% per year) [ 49 ].
We believe that the longitudinal and spatial dynamics of early-stage tumorigenesis in the oral cavity through comprehensive evaluation of cellular and molecular changes in the epithelial and stromal cells represent a unique setting to get more insight into the natural history of carcinomas. The disease is prevalent in different parts of the world and associated with various environmental agents: in western countries, it frequently affects patients with smoking and alcohol drinking history in the form of oral leukoplakia, whereas in Southeast Asia it frequently affects patients consuming areca nut, betel leaf, and quid who preferentially develop erythroplakia. Of note, oral potentially malignant lesions and SCC negative for human papillomavirus affecting patients with no smoking or alcohol drinking history, although representing a minority of all patients, have an increasing incidence over the past decades for unknown reasons. The oral cavity is easily accessible, and it is considered to be a molecular mirror of molecular alterations induced by smoking in the upper and lower aerodigestive tract. Prospectively validated in situ biomarkers of risk (e.g., loss of heterozygocity at prespecified chromosomal sites) can be used to define cohorts of patients with potentially malignant lesions at high risk of developing oral cancer. These elements represent a strong rationale for intensive exploration in this unique setting. It has the potential to foster international collaborations toward the better understanding of the biology of early-stage tumorigenesis, and provide an opportunity to develop personalized prevention strategies that will benefit patients far beyond the decreased incidence of oral cancer.
Pierre Saintigny.
Univ Lyon, Université Claude Bernard Lyon 1, INSERM 1052, CNRS 5286, Centre Léon Bérard, Centre de recherche en cancérologie de Lyon; Department of Translational Research and Innovation, and Department of Medical Oncology, Centre Léon Bérard, Lyon, 69008, France.
Question 93: How could we track the chemotherapeutics-driven evolution of tumor genome in non-small cell lung cancer for more effective treatment?
Currently, effective drug treatments for the patients with non-small cell lung cancer (NSCLC) comprise mainly of standard platinum-based cytotoxic treatment, targeted therapies including inhibitors for epidermal growth factor receptor (EGFR) and anaplastic lymphoma kinase (ALK), and immunotherapy. However, treatment resistance will inevitably occur in most patients after a certain period of time. This is believed to be partially caused by the heterogeneity in tumor genome. Cancer is a genomic disease, and cancer genome constantly undergoes changes under selective pressure from anticancer drug treatment. This alteration is also named tumor evolution, which partially explains acquired drug resistance. For example, some acquired secondary mutations, e.g., EGFR C797S, have been detected in the patient who initially harbors EGFR T790M mutation when resistance against first-line EGFR inhibitor occurs. Therefore, there is a need to dynamically monitor tumor clonal evolution in NSCLC patients. Methods for monitoring tumor evolution include multiregional sequencing and liquid biopsies. In multiregional sequencing, tumor masses from several regions are sequenced in parallel through next-generation sequencing. In liquid biopsies, a serial of circulating molecules or cells in the blood including circulating tumor DNAs (ctDNAs) and circulating tumor cells (CTCs) could reflect the information of tumor genome. These methods could represent the whole tumor genomic landscape and reflect tumor heterogeneity. In addition to this, longitudinal or serial monitoring tumor genome through liquid biopsies or multiregional sequencing could keep track of the tumor genome in both time and space. Of course, it remains a technical challenge in collecting biopsy samples from multiple time points in the same patient. Advances in imaging-guided transthoracic biopsy of lung lesions are the hope for delivering personalized treatments in response to the evolving tumor genome for dramatically improving treatment outcomes in NSCLC patients.
Yi Xiong and Zhi-Xing Lu.
Xiangya school of medicine, Central South University, Changsha, Hunan 410008, P. R. China.
[email protected]; [email protected].
- 1. Qian CN, Zhang W, Xu RH. Defeating cancer: the 150 most important questions in cancer research and clinical oncology. Chin J Cancer. 2016;35:104. doi: 10.1186/s40880-016-0165-4. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 2. Wee JT, Poh SS. The most important questions in cancer research and clinical oncology. Question 1. Could the vertical transmission of human papilloma virus (HPV) infection account for the cause, characteristics, and epidemiology of HPV-positive oropharyngeal carcinoma, non-smoking East Asian female lung adenocarcinoma, and/or East Asian triple-negative breast carcinoma? Chin J Cancer. 2017;36:13. doi: 10.1186/s40880-016-0168-1. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 3. Venniyoor A. The most important questions in cancer research and clinical oncology: question 2–5. Obesity-related cancers: more questions than answers. Chin J Cancer. 2017;36:18. doi: 10.1186/s40880-017-0185-8. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 4. Chinese Journal of Cancer The 150 most important questions in cancer research and clinical oncology series: questions 6–14. Chin J Cancer. 2017;36:33. doi: 10.1186/s40880-017-0200-0. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 5. Chinese Journal of Cancer The 150 most important questions in cancer research and clinical oncology series: questions 15–24. Chin J Cancer. 2017;36:39. doi: 10.1186/s40880-017-0205-8. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 6. Chinese Journal of Cancer The 150 most important questions in cancer research and clinical oncology series: questions 25–30. Chin J Cancer. 2017;36:42. doi: 10.1186/s40880-017-0210-y. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 7. Chinese Journal of Cancer The 150 most important questions in cancer research and clinical oncology series: questions 31–39. Chin J Cancer. 2017;36:48. doi: 10.1186/s40880-017-0215-6. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 8. Chinese Journal of Cancer The 150 most important questions in cancer research and clinical oncology series: questions 40–49. Chin J Cancer. 2017;36:55. doi: 10.1186/s40880-017-0222-7. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 9. Chinese Journal of Cancer The 150 most important questions in cancer research and clinical oncology series: questions 50–56. Chin J Cancer. 2017;36:69. doi: 10.1186/s40880-017-0236-1. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 10. Chinese Journal of Cancer The 150 most important questions in cancer research and clinical oncology series: questions 57–66. Chin J Cancer. 2017;36:79. doi: 10.1186/s40880-017-0249-9. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 11. Chinese Journal of Cancer The 150 most important questions in cancer research and clinical oncology series: questions 67–75. Chin J Cancer. 2017;36:86. doi: 10.1186/s40880-017-0254-z. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 12. Chinese Journal of Cancer The 150 most important questions in cancer research and clinical oncology series: questions 76–85. Chin J Cancer. 2017;36:91. doi: 10.1186/s40880-017-0259-7. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 13. Rosenbaum E, Gautier H, Fobair P, Neri E, Festa B, Hawn M, Andrews A, Hirschberger N, Selim S, Spiegal D. Cancer supportive care, improving the quality of life for cancer patients. A program evaluation report. Support Care Cancer. 2004;12:293–301. doi: 10.1007/s00520-004-0599-0. [ DOI ] [ PubMed ] [ Google Scholar ]
- 14. Quintin C, Martinelli F, Coeans C, et al. A global analysis of multitrial data investigating quality of life and symptoms as prognostic factors for survival in different tumour sites. Cancer. 2014;120:302–311. doi: 10.1002/cncr.28382. [ DOI ] [ PubMed ] [ Google Scholar ]
- 15. Kuderer NM, Dale DC, Crawford J, Lyman GH. Impact of primary prophylaxis with granulocyte colony-stimulating factor on febrile neutropenia and mortality in adult cancer patients receiving chemotherapy: a systematic review. J Clin Oncol. 2007;25:3158–3167. doi: 10.1200/JCO.2006.08.8823. [ DOI ] [ PubMed ] [ Google Scholar ]
- 16. Chan VTC, Yeo W. Antiemetic therapy options for chemotherapy-induced nausea and vomiting in breast cancer patients. Breast Cancer Targets Ther. 2011;3:151–160. doi: 10.2147/BCTT.S12955. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 17. Temel JS, Greer JA, et al. Early palliative care for patients with metastatic non-small cell lung cancer. New Engl J Med. 2010;363:733–742. doi: 10.1056/NEJMoa1000678. [ DOI ] [ PubMed ] [ Google Scholar ]
- 18. Spiegel D, Bloom JR, Kraemer HC, Gottheil E. Effect of psychosocial treatment on survival of patients with metastatic breast cancer. Lancet. 1989;2:1447. doi: 10.1016/S0140-6736(89)92051-5. [ DOI ] [ PubMed ] [ Google Scholar ]
- 19. Eli K, Nishimoto R, Mediansky L, Mantell J, Hamovitch M. Social relations, social support and survival among patients with cancer. J Psychosom Res. 1992;36:531–541. doi: 10.1016/0022-3999(92)90038-4. [ DOI ] [ PubMed ] [ Google Scholar ]
- 20. Kroenke CH, Kubznsky LD, Schernhammer ES, Holmes LD, Kawachi I. Social networks, social support, and survival after breast cancer diagnosis. J Clin Oncol. 2008;24:1010–1111. doi: 10.1200/JCO.2005.04.2846. [ DOI ] [ PubMed ] [ Google Scholar ]
- 21. Frenkel M, Gross S, Popper Giveon A, Sapire K, Hermoni D. Living outliers: experiences, insights and narratives of exceptional survivors of incurable cancer. Future Oncol. 2015;11:1741–1749. doi: 10.2217/fon.15.58. [ DOI ] [ PubMed ] [ Google Scholar ]
- 22. Luciani F, et al. Effect of proton pump inhibitor pretreatment on resistance of solid tumors to cytotoxic drugs. J Natl Cancer Inst. 2004;96(22):1702–1713. doi: 10.1093/jnci/djh305. [ DOI ] [ PubMed ] [ Google Scholar ]
- 23. Zhang Y, et al. Involvement of metformin and AMPK in the radioresponse and prognosis of luminal versus basal-like breast cancer treated with radiotherapy. Oncotarget. 2014;5(24):12936–12949. doi: 10.18632/oncotarget.2683. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 24. Boroughs LK, DeBerardinis RJ. Metabolic pathways promoting cancer cell survival and growth. Nat Cell Biol. 2015;17(4):351–359. doi: 10.1038/ncb3124. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 25. Biswas SK. Metabolic reprogramming of immune cells in cancer progression. Immunity. 2015;43(3):435–449. doi: 10.1016/j.immuni.2015.09.001. [ DOI ] [ PubMed ] [ Google Scholar ]
- 26. Xing Y, et al. Metabolic reprogramming of the tumour microenvironment. FEBS J. 2015;282(20):3892–3898. doi: 10.1111/febs.13402. [ DOI ] [ PubMed ] [ Google Scholar ]
- 27. Wang BY, et al. Intermittent high dose proton pump inhibitor enhances the antitumor effects of chemotherapy in metastatic breast cancer. J Exp Clin Cancer Res. 2015;34:85. doi: 10.1186/s13046-015-0194-x. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 28. Psutka SP, et al. The association between metformin use and oncologic outcomes among surgically treated diabetic patients with localized renal cell carcinoma. Urol Oncol. 2015;33(2):67. doi: 10.1016/j.urolonc.2014.07.008. [ DOI ] [ PubMed ] [ Google Scholar ]
- 29. Smolkova K, et al. Waves of gene regulation suppress and then restore oxidative phosphorylation in cancer cells. Int J Biochem Cell Biol. 2011;43(7):950–968. doi: 10.1016/j.biocel.2010.05.003. [ DOI ] [ PubMed ] [ Google Scholar ]
- 30. Macintyre AN, Rathmell JC. Activated lymphocytes as a metabolic model for carcinogenesis. Cancer Metab. 2013;1(1):5. doi: 10.1186/2049-3002-1-5. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 31. Marelli-Berg FM, Fu H, Mauro C. Molecular mechanisms of metabolic reprogramming in proliferating cells: implications for T-cell-mediated immunity. Immunology. 2012;136(4):363–369. doi: 10.1111/j.1365-2567.2012.03583.x. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 32. Jeremic B, Shibamoto Y, Nikolic N, et al. Role of radiation therapy in the combined-modality treatment of patients with extensive disease small-cell lung cancer: a randomized study. J Clin Oncol. 1999;17(7):2092–2099. doi: 10.1200/JCO.1999.17.7.2092. [ DOI ] [ PubMed ] [ Google Scholar ]
- 33. Zhu H, Zhou Z, Wang Y, et al. Thoracic radiation therapy improves the overall survival of patients with extensive-stage small cell lung cancer with distant metastasis. Cancer. 2011;117(23):5423–5431. doi: 10.1002/cncr.26206. [ DOI ] [ PubMed ] [ Google Scholar ]
- 34. Slotman BJ, van Tinteren H, Praag JO, et al. Use of thoracic radiotherapy for extensive stage small-cell lung cancer: a phase 3 randomised controlled trial. Lancet. 2015;385(9962):36–42. doi: 10.1016/S0140-6736(14)61085-0. [ DOI ] [ PubMed ] [ Google Scholar ]
- 35. Auperin A, Arriagada R, Pignon JP, et al. Prophylactic cranial irradiation for patients with small-cell lung cancer in complete remission. Prophylactic cranial irradiation overview collaborative Group. N Engl J Med. 1999;341:476–484. doi: 10.1056/NEJM199908123410703. [ DOI ] [ PubMed ] [ Google Scholar ]
- 36. Meert AP, Paesmans M, Berghmans T, et al. Prophylactic cranial irradiation in small cell lung cancer: a systematic review of the literature with meta-analysis. BMC Cancer. 2001;1:5. doi: 10.1186/1471-2407-1-5. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 37. Slotman B, Faivre-Finn C, Kramer G, et al. Prophylactic cranial irradiation in extensive small-cell lung cancer. N Engl J Med. 2007;357:664–672. doi: 10.1056/NEJMoa071780. [ DOI ] [ PubMed ] [ Google Scholar ]
- 38. Takahashi T, Yamanaka T, Seto T, et al. Prophylactic cranial irradiation versus observation in patients with extensive-disease small-cell lung cancer: a multicentre, randomised, open-label, phase 3 trial. Lancet Oncol. 2017;18(5):663–671. doi: 10.1016/S1470-2045(17)30230-9. [ DOI ] [ PubMed ] [ Google Scholar ]
- 39. Ready NE, PangHH GuL, et al. Chemotherapy with or without maintenance sunitinib for untreated extensive-stage small-cell lung cancer: a randomized, double-blind, placebo-controlled phase II study-CALGB 30504 (alliance) J Clin Oncol. 2015;33(15):1660–1665. doi: 10.1200/JCO.2014.57.3105. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 40. Rudin CM, Pietanza MC, Bauer TM, et al. Rovalpituzumab tesirine, a DLL3-targeted antibody-drug conjugate, in recurrent small-cell lung cancer: a first-in-human, first-in-class, open-label, phase 1 study. Lancet Oncol. 2017;18(1):42–51. doi: 10.1016/S1470-2045(16)30565-4. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 41. Byers LA, Krug LM, Waqar SN, et al. Improved small cell lung cancer (SCLC) response rates with veliparib and temozolomide: results from a Phase II trial. J Thorac Oncol. 2017;12(Suppl. 1):S406–S407. doi: 10.1016/j.jtho.2016.11.466. [ DOI ] [ Google Scholar ]
- 42. Antonia SJ, López-Martin JA, Bendell J, et al. Nivolumab alone and nivolumab plus ipilimumab in recurrent small-cell lung cancer (CheckMate 032): a multicentre, open-label, phase 1/2 trial. Lancet Oncol. 2016;17(7):883–895. doi: 10.1016/S1470-2045(16)30098-5. [ DOI ] [ PubMed ] [ Google Scholar ]
- 43. Gondi V, Hermann BP, Mehta MP, et al. Hippocampal dosimetry predicts neurocognitive function impairment after fractionated stereotactic radiotherapy for benign or low-grade adult brain tumors. Int J Radiat Oncol Biol Phys. 2013;85(2):348–354. doi: 10.1016/j.ijrobp.2012.11.031. [ DOI ] [ PubMed ] [ Google Scholar ]
- 44. PORT Meta-analysis Trialists Group Postoperative radiotherapy in non-small-cell lung cancer: systematic review and meta-analysis of individual patient data from nine randomised controlled trials. Lancet (London, England) 1998;352:257–263. doi: 10.1016/S0140-6736(98)06341-7. [ DOI ] [ PubMed ] [ Google Scholar ]
- 45. Patel SH, Ma Y, Wernicke AG, Nori D, Chao KS, Parashar B. Evidence supporting contemporary post-operative radiation therapy (PORT) using linear accelerators in N2 lung cancer. Lung Cancer (Amsterdam, Netherlands) 2014;84:156–160. doi: 10.1016/j.lungcan.2014.02.016. [ DOI ] [ PubMed ] [ Google Scholar ]
- 46. Perry MC, Kohman LJ, Bonner JA, Gu L, Wang X, Vokes EE, et al. A phase III study of surgical resection and paclitaxel/carboplatin chemotherapy with or without adjuvant radiation therapy for resected stage III non-small-cell lung cancer: cancer and leukemia group B 9734. Clin Lung Cancer. 2007;8:268–272. doi: 10.3816/CLC.2007.n.005. [ DOI ] [ PubMed ] [ Google Scholar ]
- 47. Wu Y, Zhong W, Wang Q, Xu S, Mao W, Wu L, et al. Gefitinib (G) versus vinorelbine + cisplatin (VP) as adjuvant treatment in stage II–IIIA (N1–N2) non-small-cell lung cancer (NSCLC) with EGFR-activating mutation (ADJUVANT): a randomized, phase III trial (CTONG 1104) J Clin Oncol. 2017;35:8500. [ Google Scholar ]
- 48. Yue D, Xu S, Wang Q, Li X, Shen Y, Zhao H, et al. Efficacy and safety of erlotinib vs vinorelbine/cisplatin as adjuvant therapy for stage IIIA EGFR mutant NSCLC patients. In: IASLC 18th world conference on lung cancer. 2017. p. 134.
- 49. Patel SC, Carpenter WR, Tyree S, Couch ME, Weissler M, Hackman T, et al. Increasing incidence of oral tongue squamous cell carcinoma in young white women, age 18 to 44 years. J Clin Oncol. 2011;29(11):1488–1494. doi: 10.1200/JCO.2010.31.7883. [ DOI ] [ PubMed ] [ Google Scholar ]
- View on publisher site
- PDF (919.7 KB)
- Collections
Similar articles
Cited by other articles, links to ncbi databases.
- Download .nbib .nbib
- Format: AMA APA MLA NLM
Add to Collections

IMAGES
VIDEO
COMMENTS
Studies on MUC1, p120-catenin, Kaiso: coordinate role of mucins, cell adhesion molecules and cell cycle players in pancreatic cancer, Xiang Liu. PDF. Epac interaction with the TGFbeta PKA pathway to regulate cell survival in colon cancer, Meghan Lynn Mendick.
Table of Content. How to Choose Cancer Dissertation Topics? Find Available Data. Look for a Theoretical Base for Your Topic. Consult Your Mentor. Participate in Medical Research Surveys. Take Advantage of the Available Resources. Ask Questions That Can be Answered. Read Everything on the Subject.
Changing how the world understands and treats cancer. Our scientists pursue every aspect of cancer research—from exploring the biology of genes and cells, to developing immune-based treatments, uncovering the causes of metastasis, and more.
Explore 20 impactful oncology dissertation topics for 2023. From cancer research to treatment strategies, choose a topic that aligns with your passion and contribute to advancements in the field of oncology.
Since the beginning of 2017, Cancer Communications (former title: Chinese Journal of Cancer) has published a series of important questions regarding cancer research and clinical oncology, to provide an enhanced stimulus for cancer research, and to accelerate collaborations between institutions and investigators.
Long Noncoding RNAs in Cancer. Highlights research in carcinogenesis and tumor progression, bridging the gap between basic research and clinical applications to advance diagnosis, personalized therapeutics and management strateg...
Top 10 breast cancer topics needing a Cochrane systematic review. Deciding which research topics to focus on in medicine and health depends on many factors. These factors can include the currency of a topic, feedback from people providing or receiving care, and the priorities of funders.
SCIENCE-BASED REGULATION OF PHARMACOLOGICAL SUBSTANCES IN COMPETITION HORSES, Jacob Machin. Master's theses and doctoral dissertations from the University of Kentucky Department of Toxicology and Cancer Biology are available here.
To accelerate our endeavors to overcome cancer, Chinese Journal of Cancer has launched a program of publishing 150 most important questions in cancer research and clinical oncology .
Results. We identified 14 distinct topics in cancer literature. The results revealed the uptrend topics - including cell signaling (T-2), immunotherapy (T-3), clinical trials (T-5), disparities and epidemiology (T-7), cancer practice and policy (T-8), outcome research (T-9), and molecular therapeutics (T-10). - and downtrend topics such as cell ...