- Open access
- Published: 19 May 2023

The efficacy and effectiveness of COVID-19 vaccines around the world: a mini-review and meta-analysis
- Marzieh Soheili 1 ,
- Sorour Khateri 2 ,
- Farhad Moradpour 3 ,
- Pardis Mohammadzedeh 4 ,
- Mostafa Zareie 4 ,
- Seyede Maryam Mahdavi Mortazavi 5 ,
- Sima Manifar 6 ,
- Hamed Gilzad Kohan 7 &
- Yousef Moradi 3
Annals of Clinical Microbiology and Antimicrobials volume 22 , Article number: 42 ( 2023 ) Cite this article
15k Accesses
18 Citations
181 Altmetric
Metrics details
This meta-analysis evaluated the Efficacy and Effectiveness of several COVID-19 vaccines, including AstraZeneca, Pfizer, Moderna, Bharat, and Johnson & Johnson, to better estimate their immunogenicity, benefits, or side effects.
Studies reporting the Efficacy and Effectiveness of COVID-19 vaccines from November 2020 to April 2022 were included. The pooled Effectiveness/Efficacy with a 95% confidence interval (95% CI) with Metaprop order was calculated. The results were presented in forest plots. Predefined subgroup analyses and sensitivity analyses were also performed.
A total of twenty articles were included in this meta-analysis. After the first dose of the vaccine, the total effectiveness of all COVID-19 vaccines in our study was 71% (95% CI 0.65, 0.78). The total effectiveness of vaccines after the second dose was 91% (95% CI 0.88, 0.94)). The total efficacy of vaccines after the first and second doses was 81% (95% CI 0.70, 0.91) and 71% (95% CI 0.62, 0.79), respectively. The effectiveness of the Moderna vaccine after the first and second dose was the highest among other studied vaccines ((74% (95% CI, 0.65, 0.83) and 93% (95% CI, 0.89, 0.97), respectively). The highest first dose overall effectiveness of the studied vaccines was against the Gamma variant (74% (95% CI, 0.73, 0.75)), and the highest effectiveness after the second dose was observed against the Beta variant (96% (95% CI, 0.96, 0.96)). The Efficacy for AstraZeneca and Pfizer vaccines after the first dose was 78% (95% CI, 0.62, 0.95) and 84% (95% CI, 0.77, 0.92), respectively. The second dose Efficacy for AstraZeneca, Pfizer, and Bharat was 67% (95% CI, 0.54, 0.80), 93% (95% CI, 0.85, 1.00), and 71% (95% CI, 0.61, 0.82), respectively. The overall efficacy of first and second dose vaccination against the Alfa variant was 84% (95% CI, 0.84, 0.84) and 77% (95% CI, 0.57, 0.97), respectively, the highest among other variants.
mRNA-based vaccines against COVID-19 showed the highest total efficacy and effectiveness than other vaccines. In general, administering the second dose produced a more reliable response and higher effectiveness than a single dose.
Introduction
The coronavirus disease 2019 (COVID-19) is an acute respiratory infection caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). This β-coronavirus is an enveloped, non-segmented positive-sense RNA virus, which primarily spreads through the respiratory tract [ 1 , 2 , 3 ]. COVID-19 infection is often associated with systemic inflammation and inflammatory biomarkers such as IL-6, IL-10, and TNF-α) increase in the patients [ 4 , 5 , 6 ]. Cough, fever, and shortness of breath are the dominant symptoms of COVID-19 infection. Additionally, fatigue, increased sputum production, sore throat, headache, and gastrointestinal symptoms might be observed [ 6 , 7 , 8 ]. Elderly patients with underlying disorders such as hypertension, chronic obstructive pulmonary disease, diabetes, and cardiovascular complications are more prone to develop acute respiratory distress syndrome. Other severe symptoms include septic shock, metabolic acidosis, and coagulation dysfunction, which might lead to death [ 9 , 10 ]. Various medications have already been tested for treating COVID-19 patients. However, the evidence to support the beneficial effects of these drugs is often controversial [ 11 , 12 , 13 ]. Molnupiravir is the first oral antiviral drug that has recently shown a significant benefit in reducing hospitalization or death in COVID-19 patients [ 14 ].
According to the World Health Organization (WHO) report, from the emergence of COVID-19 in December 2019 to November 2021, more than 250,000,000 confirmed cases of COVID-19 have been reported, and more than five million deaths have been attributed to the disease globally [ 15 ]. Since the COVID-19 pandemic, several studies have started to develop safe and efficacious vaccines. Numerous clinical trials have been conducted to evaluate the efficacy and safety of experimental vaccines [ 16 , 17 , 18 ]. WHO reported as of November 8, 2021, more than seven billion vaccine doses have been administered worldwide [ 15 ]. Additionally, as per the WHO report, until November 9, 2021, 130 vaccine candidates were under clinical development, and 156 candidates were in the pre-clinical development phase. Different types of COVID-19 vaccines have been developed worldwide, including protein subunit, recombinant, viral vector, RNA- and DNA-based, and sub-unit vaccines [ 19 ].
Up to now, several COVID-19 vaccines have been authorized or approved for use. WHO issued an emergency use authorization for the Pfizer COVID-19 vaccine On December 31, 2020 (BNT162b2). Next, on February 15, 2021, the Astra-Zeneca/Oxford COVID-19 vaccine (manufactured by the Serum Institute of India and SKBio) received emergency use approval, followed by Ad26.COV2.S (developed by Janssen (Johnson & Johnson)) on March 12, 2021, and Moderna vaccine on April 30, 2021 [ 20 ]. Pfizer COVID-19 vaccine is a lipid nanoparticle formulation that contains a nucleoside-modified RNA against the S protein of the SARS-CoV-2 virus [ 21 ]. Moderna is a lipid nanoparticle–encapsulated nucleoside-modified messenger RNA vaccine encoding prefusion stabilized full-length spike protein of SARS-CoV-2 (24). The Oxford/AstraZeneca COVID-19 vaccine (ChAdOx1 nCoV-19 vaccine, AZD1222) contains a replication-deficient chimpanzee adenoviral vector ChAdOx1, delivering the SARS-CoV-2 structural surface glycoprotein antigen (spike protein; nCoV-19) gene (22, 23). Janssen is a non-replicating, recombinant human adenovirus type 26, containing a full-length SARS-CoV-2 S protein [ 22 ]. Bharat (CovaxinTM) is an inactivated-virus vaccine developed in Vero cells combined with Alhydroxiquim-II (Algel-IMDG), chemosorbed imidazoquinoline onto aluminum hydroxide gel. This complex is an adjuvant to boost immune response for longer-lasting immunity [ 23 ].
Careful planning for the COVID-19 vaccination program requires comprehensive review studies to evaluate the efficacy and safety of the vaccines. This study aims to conduct a meta-analysis to assess the Effectiveness and Efficacy of COVID-19 vaccines, including AstraZeneca, Pfizer, Moderna, Bharat, and Johnson & Johnson. Well-designed meta-analysis studies will provide a more accurate overview to evaluate Efficacy and safety outcomes compared to individual studies and contribute to a better understanding of the use of the vaccine in different populations.
Materials and methods
The present systematic review and meta-analysis were conducted according to Preferred reporting items for systematic reviews and meta-analysis (PRISMA) guidelines for reviewing analytical observational studies [ 24 ].
Search strategy and screening
International databases were searched to find all original published articles, including Medline (PubMed), Web of Science, Embase (Elsevier), Cochrane Library, Scopus, Ovid, and CINHAL, to retrieve all articles evaluating and reporting the efficacy and side effects of all COVID-19 vaccine (Pfizer–BioNTech, Oxford–AstraZeneca, Moderna, Janssen, CoronaVac, Covaxin, Novavax and Convidecia) in fully vaccinated and partially vaccinated people. The studies which have compared these items with non-vaccinated individuals were also included. In addition to searching the mentioned databases, gray literature was searched by reviewing articles in the first ten pages of Google scholar. A manual search was performed by reviewing references from related studies. This search was conducted with language limitations from November 2020 to September 2022. The search protocol was developed based on four primary roots involving “vaccination,“ “COVID-19,“ “Side effect,“ and “Efficacy.“ All related components to these keywords were “vaccinated”, “non-vaccinated”, “partial vaccinated”, “fully vaccinated”, “Pfizer–BioNTech”, “Oxford–AstraZeneca”, “Sinopharm BIBP”, “Moderna”, “Janssen”, “CoronaVac”, “Covaxin”, “Novavax”, “Convidecia”, “symptoms”, “signs” (“fever”, “cough”, “malaise”, “dyspnea”, “myalgia”, “sore throat”, and “diarrhea”), “thrombosis”, “emboli”, “thromboembolism”, “thromboembolic”, which were added to the searched queries based on scientific Mesh terms, EMTREE, and Thesaurus. Reference Manager bibliographic software was applied to manage searched citations. Duplicate entries were searched by considering the papers’ title, year of publication, authors, and specifications of types of sources. In case of questionable records, the texts were compared. After reviewing the primary search results, each article was double-checked by title and available abstract, and some of the articles were omitted based on the selection criteria. The evaluation of the considered papers was based on the inclusion and exclusion criteria by the two researchers separately (SM, MS). After the screening, (YM) selected the articles by evaluating their full texts.
Eligibility criteria
We included all observational and interventional studies that assessed the Efficacy/Effectiveness and side effects of all types of COVID-19 vaccines (Pfizer–BioNTech, Oxford–AstraZeneca, Sinopharm BIBP, Moderna, Janssen, CoronaVac, Covaxin, Novavax and Convidecia) in fully vaccinated and partially vaccinated people. The studies comparing these items with non-vaccinated individuals were also included. We excluded duplicate citations, non-peer-reviewed articles in which the abstract and full text were unavailable, and other languages.
Data extraction
After screening according to the three assessment steps for titles, abstracts, and full texts, the full text of each selected article was extracted for detailed analysis. The data were retrieved using a checklist recording author, publication year, type of study, mean age, sample size, number of positive tests, Effectiveness/Efficacy after one dose, Effectiveness/Efficacy after the second dose, and number of confirmed COVID cases, hospitalization, and death. From systematic search to final data extraction, all processes were followed independently by two research experts (PM, FM). After the screening, the data extraction was finally approved by (YM).
Risk of bias
The qualitative evaluation of studies was done according to the Newcastle-Ottawa Quality Assessment Scale (NOS) [ 25 ] by two of the authors (FM, YM). This scale is designed to evaluate the qualitative properties of observational studies (random clinical trials, case-control, retrospective, cohort, and cross-sectional studies). NOS examined each study through six items in three groups: selection, comparability, and exposure. Stars were given to each item, and the maximum score was 9. If the scores assigned to the published articles differed, the external discussion method would be used [ 26 , 27 ].
The Jadad checklist was used by two separate authors (PM and FM) to explore potential risks of bias in interventional studies. These scales include items to assess the adequacy of random sequence generation, allocation concealment, blinding, the detection of incomplete outcome data, selective outcome reporting, and other potential sources of bias [ 28 ].
Statistical analysis
The random-effects model was used to calculate the pooled Effectiveness/Efficacy with a 95% confidence interval (95% CI) with Metaprop order. Calculating the cumulative relative risk (RR) with the 95% confidence interval and the meta set command was used considering the relative risk’s logarithm and logarithm standard deviation. Statistical analysis was performed using STATA 16.0 (Stata Corp, College Station, TX, USA), and statistical significance was considered at P-Value < 0.05. Heterogeneity among studies was evaluated by applying the I square value and reported as a percentage (%) to show the extent of variation between studies. A forest plot was used for presenting the meta-analysis results schematically. Egger’s test and funnel plot were applied to evaluate the publication bias. In addition, a subgroup analysis was done to identify different sources of heterogeneity.
Results and discussion
Characteristics of included studies and the participants.
A total of 2622 publications were screened for evaluating two items about COVID-19 vaccines: (I) Efficacy and (II) Effectiveness. These two items were assessed according to the virus variant (Alpha, Beta, Delta, and Gamma) and the type of vaccine (AstraZeneca, Pfizer, Moderna, Janssen, and Bharat). Data on other vaccines were not included due to inadequate published data. Of these publications, 20 studies met the systematic reviews’ inclusion criteria (non-randomized and randomized) and were included in our meta-analysis (Fig. 1 ).
Identification of studies via databases and registers
One study was the cohort, four were randomized clinical trials (RCT), and fifteen were case-control. Clinical trials have evaluated vaccines’ efficacy, and observational studies such as cohorts and case controls have assessed their effectiveness. All selected papers were written in English. A total of 1,246,266 cases were included in this study that had received the COVID-19 vaccines. All vaccines were injected intramuscularly (IM). The participants were > 12 years old. The characteristics of included studies have been summarized in Table 1 .
The overall effectiveness of COVID-19 vaccines
After the first dose of the vaccine, the overall effectiveness of all COVID-19 vaccines was estimated to be 71% (95% CI 0.65, 0.78) (Fig. 2 ).
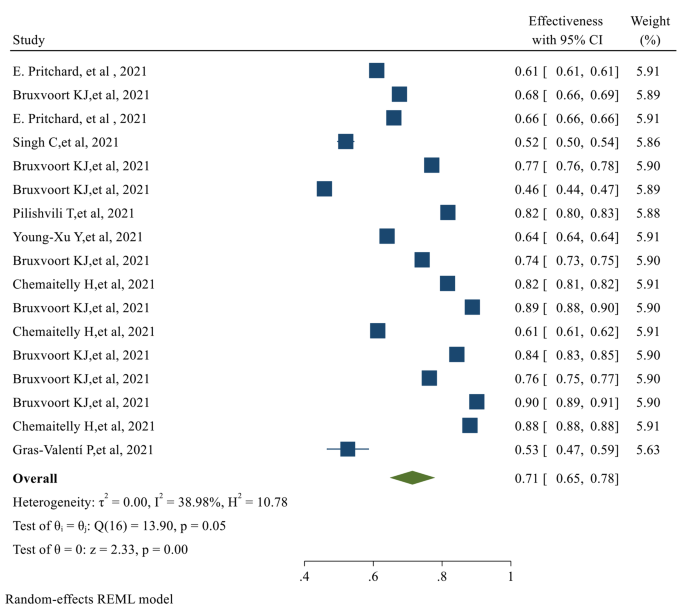
The overall Effectiveness of studied COVID-19 vaccines after the first dose
The overall Effectiveness of vaccines after the second dose was 91% (95% CI 0.88, 0.94), with a significant P-value ( p-value < 0.05 ) (Fig. 3 )
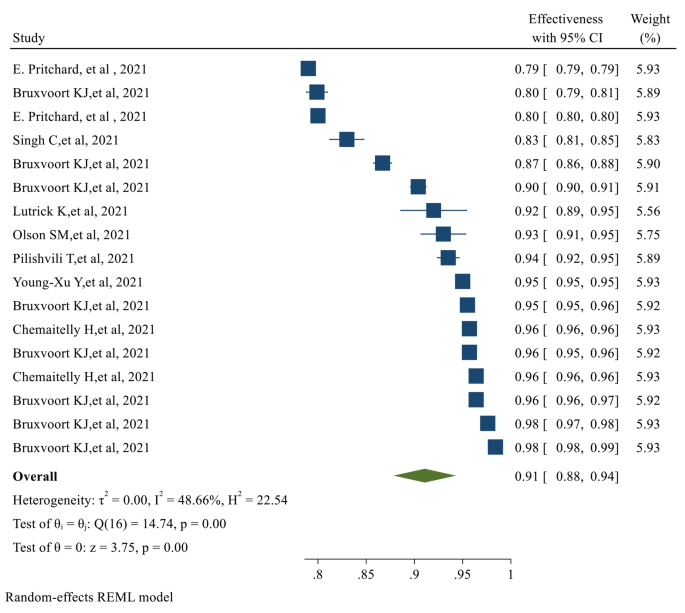
The overall Effectiveness of studied COVID-19 vaccines after the second dose. The overall Efficacy of COVID-19 vaccines
The overall Efficacy of the first dose of the vaccines evaluated in our study was 81% (95% CI 0.70, 0.91) (Fig. 4 )
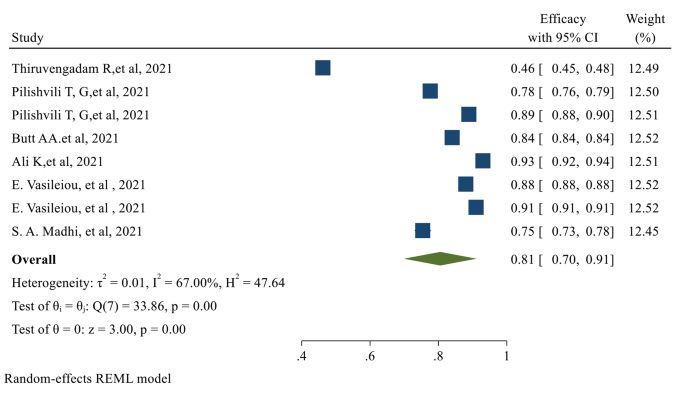
The overall Efficacy of the first dose of the studied vaccines
After the second dose of vaccination, the overall Efficacy of vaccines was 71% (95% CI 0.62, 0.79) with a significant P-value (Fig. 5 )
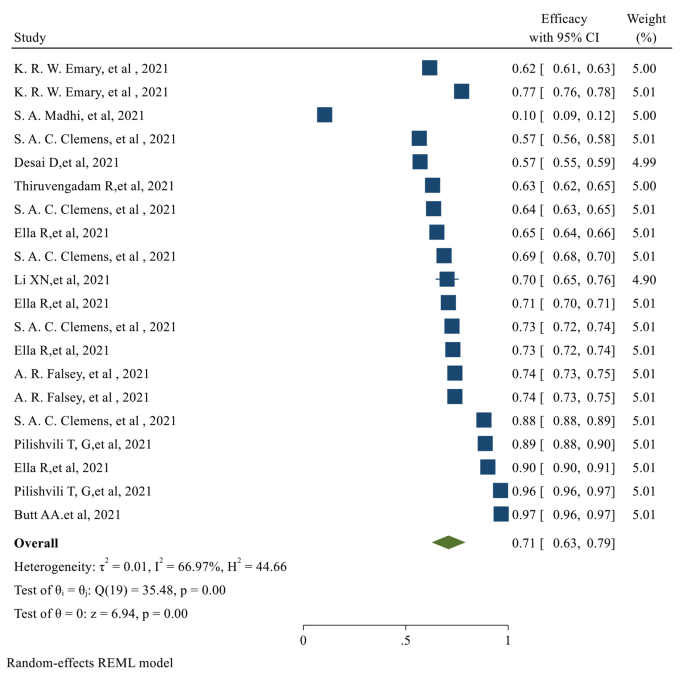
The overall Efficacy of the studied vaccines after the second dose
The individual efficacy of COVID-19 vaccines
The efficacy after the first dose was evaluated only in 8 of the selected studies, which assessed the efficacy of the AstraZeneca and Pfizer vaccines. No data was published on the efficacy after the first dose for Moderna, Johnson & Johnson, and Bharat. After the first dose of AstraZeneca and Pfizer vaccines, the pooled efficacy was 78% (95% CI 0.062, 0.95) and 84% (95% CI 0.77, 0.92), respectively. Of the selected publications, eighteen studies reported the efficacy after the second dose of vaccinations. The published data for the second dose Efficacy was only available for AstraZeneca, Pfizer, and Bharat vaccines. The second dose pooled Efficacy for AstraZeneca, Pfizer, and Bharat was 67% (95% CI 0.54, 0.80), 93% (95% CI 0.85, 1.00), and 71% (95% CI 0.61, 0.82) respectively (Table 2 ).
The individual effectiveness of COVID-19 vaccines
The first dose Effectiveness of the vaccines was evaluated in seventeen studies. For Moderna, AstraZeneca, and Pfizer, the pooled effectiveness after the first dose was 74% (95% CI 0.065, 0.83), 69% (95% CI 0.55, 0.82), and 67% (95% CI 0.51, 0.83) respectively. It was observed that the Effectiveness of Moderna after the first dose was higher than other types of vaccines. The second dose Effectiveness of the vaccines was reported in 17 studies. The pooled effectiveness after the second dose of Moderna, AstraZeneca, and Pfizer vaccines was 93% (95% CI 0.89, 0.97), 89% (0.80, 0.97), and 90% (95% CI 0.83, 0.96) respectively; Moderna had higher effectiveness after the second dose, among other studied vaccines (Table 2 ).
Efficacy of the vaccines against the virus variants
The overall first and second-dose vaccination Efficacy against different COVID-19 variants is listed in Table 2 . The first dose of overall vaccine Efficacy against the Alpha variant was 84%, which was higher than other variants (95% CI 0.84, 0.84). The overall efficacy of the first dose vaccination against the delta variant was only 46% (95% CI 0.45, 0.48), which was the lowest. Similarly, the highest second dose Efficacy was observed against the Alpha variant, which was 77% (95% CI 0.57, 0.97). The overall efficacy of the second dose against the Delta and Beta variants was 64% (95% CI 0.58, 0.69) and 10% (95% CI 0.09, 0.12), respectively.
Effectiveness of the vaccines against the virus variants
The overall first and second-dose vaccination Effectiveness against different COVID-19 variants is reported in Table 2 . The first dose Effectiveness of vaccination against the Gamma variant was 74% (95% CI 0.73, 0.75) which was more than other variants. However, the overall first dose Effectiveness was 82% (95% CI 0.81, 0.82). After the second dose, the highest effectiveness was against the Beta variant (96% (95% CI 0.96, 0.96)). The overall effectiveness after the second vaccination dose was 96% (95% CI 0.096, 0.96) (Table 2 ).
The risk of confirmed COVID infection after vaccination (risk ratio)
Two categories of the selected studies assessed the risk ratio of COVID after vaccination: observational and experimental. Only the pooled risk ratio of AstraZeneca was evaluated in the experimental studies, which was 50% (95% CI 0.35, 0.71). In the observational studies, AstraZeneca and Moderna had the lowest pooled risk ratios, which were 18% (95% CI 0.04, 0.84) and 19% (95% CI 0.17, 0.22), respectively. Bharat had the highest pooled risk ratio (82% (95% CI 0.75, 0.89) (Table 3 ); however, the number of studies on the Bharat vaccine was fewer than other types of vaccines. Based on the reported experimental studies for the vaccine variants, the Beta variant had the highest (79% (95% CI 0.43, 1.44)), and the Gamma variant had the lowest risk ratio (31% (95% CI 0.18, 0.54)). In the observational studies, Delta had the highest (52% (95% CI 0.27, 1.01), and Gamma had the lowest risk ratio (2% (95% CI 0.02, 0.02)) (Table 3 ).
Since the emergence of COVID-19, the effort to develop effective vaccines against the infection has been started. Due to the highly contagious nature of the virus, vaccination has been considered a significant measure in the fight against COVID-19. World Health Organization (WHO) allows countries to issue emergency use authorizations for COVID-19 vaccines in line with their national regulations and legislation. Domestic emergency use authorizations are issued at the countries’ discretion and are not subject to WHO approval. Up to now, several vaccines have been developed and marketed to limit the spread of COVID-19 infection. As of January 12, 2022, several COVID 19 vaccines have been given Emergency Use Listing (EUL), including those developed by Pfizer/BioNTech, AstraZeneca, Johnson & Johnson, Moderna, Sinopharm, Sinovac, Bharat Biotech, etc. [ 29 ].
Despite the significant role of COVID-19 vaccination in confining the infection, vaccines’ Efficacy and Effectiveness have not yet been comprehensively discussed. The present study meticulously looked into the Efficacy and Effectiveness of several vaccines.
Our analysis revealed that the overall effectiveness of the studied vaccines after the first dose is significantly less than their effectiveness after the second dose. The first dose’s effectiveness was evaluated in 17 studies. After the first dose, Moderna, AstraZeneca, and Pfizer’s Effectiveness was 74%, 69%, and 67%, respectively. The Effectiveness of Moderna after the first dose was higher than other types of studied vaccines. Second dose Effectiveness was evaluated in 17 studies. After the second dose of Moderna, AstraZeneca, and Pfizer vaccination, the effectiveness was 93%, 89%, and 90, respectively. Moderna provided higher effectiveness after the second dose among other studied vaccines. Therefore, administering the second dose should produce a more reliable response and higher effectiveness than a single dose.
Surprisingly, the overall efficacy of the first dose was significantly more than the second dose; 81% (95% CI 0.70, 0.91) for the first dose compared to 71% (95% CI 0.62, 0.79) for the second dose. This can be explained by the fact that the efficacy after the first dose was evaluated only in 8 studies that assessed only AstraZeneca and Pfizer vaccines. No data was available regarding the efficacy after the first dose of Moderna, Bharat, and Johnson & Johnson vaccines. We observed that the first dose Efficacy of the Pfizer vaccine is significantly more than the AstraZeneca vaccine. The Efficacy for AstraZeneca and Pfizer after the first dose vaccination was 78% and 84%, respectively. Concerning the second dose Efficacy, the published data were available only for AstraZeneca, Pfizer, and Bharat. In Total, eighteen studies evaluated the efficacy of these vaccines after the second dose. The Efficacy for AstraZeneca, Pfizer, and Bharat was 67%, 93%, and 71%, respectively.
We also investigated the Efficacy and Effectiveness of the first and second-dose vaccination against the COVID-19 virus variants. The overall efficacy of vaccination against the Alfa variant after the first dose was 84%, which was more than other variants. The highest efficacy after the second dose vaccination was also observed for the Alpha variant (77%). The first dose’s effectiveness against the Gamma variant was the highest (74%). Although, the overall first dose effectiveness was 82%. The highest second dose Effectiveness was against the Beta variant (96%), and the overall effectiveness after the second vaccination dose was 96% against all variants.
Up to now, there are other meta-analyses published on the efficacy and effectiveness of the COVID-19 vaccines. For example, in the meta-analysis reported by Pormohammad et., al, the efficacy of mRNA-based and adenovirus-vectored COVID-19 vaccines in phase II/III randomized clinical trial has been reported as 94.6% (95% CI 0.936–0.954) and 80.2% (95% CI 0.56–0.93), respectively. Additionally, the mRNA-based vaccines showed the highest reported side effects except for diarrhea and arthralgia [ 30 ]. However, the research had not reported the efficacy against different variants of the COVID-19 virus. Moreover, the Efficacy and Effectiveness of individual vaccines have not been mentioned; the vaccine Efficacy has been reported based on the vaccine classes. Another meta-analysis reported that the effectiveness of the Pfizer-BioNTech and Moderna vaccines was 91.2% and 98.1%, respectively, while the effectiveness of the CoronaVac vaccine was 65.7% in fully vaccinated individuals [ 31 ]. However, this study has not reported the effectiveness of the vaccines against COVID-19 variants or their efficacy.
Additionally, A previously reported network meta-analysis of various COVID-19 vaccines found Moderna was the most effective vaccine against COVID-19 infection, with an efficacy rate of 88%, followed by Sinopharm and Bharat. The least effective vaccines were Coronavac, Curevac, and AstraZeneca. The mRNA-based vaccines were superior in preventing infection and symptomatic infection, while the inactivated vaccines were most effective in preventing severe COVID-19 infection. Concerning safety, Sinopharm had the highest safety profile in local side effects, while ZF2001 had the highest safety in unsolicited side effects. Inactivated vaccines had the best safety profile in local and systemic side effects, while mRNA-based vaccines had the poorest safety profile. Thromboembolic events were reported after J&J, AstraZeneca, Pfizer, and Moderna vaccine administration. However, no confirmed vaccine-Induced Thrombotic Thrombocytopenia (VITT) cases were reported after mRNA vaccines [ 32 ].
It is necessary to mention that some vaccines’ overall or variant-specific Effectiveness and Efficacy are unavailable after the first or second dose. Moreover, the timing of the second dosing of the vaccines is not elicited in some trials, which may have led to the lower observed overall efficacy after the second dose. Additionally, some reports had noticeable bias by not including enough samples or not considering a broad enough geographical, economic, and age diversity.
We searched various databases and websites to include the maximum number of relevant publications to prevent database bias; after performing Egger’s regression test, we did not find significant publication bias. However, publication bias and heterogeneity for some pooled results must be considered when interpreting the outcomes.
Despite the valuable information provided by this meta-analysis, the study has some limitations to consider, such as the time frame of the studies (November 2020 to April 2022), the exclusion of unpublished data or ongoing investigations, the subjectivity of study selection criteria, and the limited number of vaccines evaluated. Additionally, the study did not consider differences in vaccine distribution among countries or provide data on the vaccines’ effectiveness against severe disease, hospitalization, or death. Despite its limitations, the meta-analysis highlights the need to continue monitoring the vaccines’ effectiveness.
In conclusion, Moderna, an mRNA-based vaccine, showed the highest total effectiveness after the first dose. Although the Pfizer vaccine showed a higher Efficacy after the first and second doses than AstraZeneca and Bharat, our conclusion has some limitations due to the lack of any published study regarding the Moderna and Johnson & Johnson vaccines’ efficacy. First-dose vaccination generally showed the highest overall effectiveness against the Gamma variant. Second dose vaccination showed a 96% overall Effectiveness against all variants. The efficacy of vaccination against the Alfa variant after the first dose was more than other variants. The highest efficacy after the second vaccination dose was also observed for the Alpha variant. Due to the timeline of the studies, all the vaccines are missing longer-term Efficacy and Effectiveness evaluations. This meta-analysis incorporated all relevant studies for summarizing and analyzing the Effectiveness and Efficacy of several vaccines for COVID-19. The results of this study support the overall Efficacy and Effectiveness of all studied COVID-19 vaccines and support the ongoing global public health effort for vaccination against COVID-19.
Data Availability
The data extracted for analyses are available by the corresponding author upon reasonable requests.
Abbreviations
Coronavirus Disease 2019
Severe Acute Respiratory Syndrome Coronavirus 2
World Health Organization
She J, et al. 2019 novel coronavirus of pneumonia in Wuhan, China: emerging attack and management strategies. Clin Transl Med. 2020;9(1):19.
Article PubMed PubMed Central Google Scholar
Lu R, et al. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet. 2020;395(10224):565–74.
Article CAS PubMed PubMed Central Google Scholar
Zhu N, et al. A novel coronavirus from patients with Pneumonia in China, 2019. N Engl J Med. 2020;382(8):727–33.
Gilzad-Kohan H, Jamali F. Anti-inflammatory Properties of drugs used to Control COVID-19 and their Effects on the renin-angiotensin system and angiotensin-converting Enzyme-2. J Pharm Pharm Sci. 2020;23:259–77.
Article PubMed Google Scholar
Chen G, et al. Clinical and immunological features of severe and moderate coronavirus disease 2019. J Clin Invest. 2020;130(5):2620–9.
Guan WJ, et al. Clinical characteristics of Coronavirus Disease 2019 in China. N Engl J Med. 2020;382(18):1708–20.
Article CAS PubMed Google Scholar
Wang D, et al. Clinical characteristics of 138 hospitalized patients with 2019 Novel Coronavirus-Infected pneumonia in Wuhan, China. JAMA. 2020;323(11):1061–9.
Balasubramani J, Anbalagan M. Research productivity on COVID-19 in Dimension database: an analytical study. J Emerg Manag. 2021;18(7):63–9.
Chen T, et al. Clinical characteristics of 113 deceased patients with coronavirus disease 2019: retrospective study. BMJ. 2020;368:m1091.
Zhou F, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054–62.
Islam MI. Current drugs with potential for treatment of COVID-19: a Literature Review. J Pharm Pharm Sci. 2020;23(1):58–64.
Article Google Scholar
Çalık Başaran N, et al. Outcome of noncritical COVID-19 patients with early hospitalization and early antiviral treatment outside the ICU. Turk J Med Sci. 2021;51(2):411–20.
Tsang HF, et al. An update on COVID-19 pandemic: the epidemiology, pathogenesis, prevention and treatment strategies. Expert Rev Anti Infect Ther. 2021;19(7):877–88.
Singh AK, et al. Molnupiravir in COVID-19: a systematic review of literaturef. Diabetes Metab Syndr. 2021;15(6):102329.
WHO., WHO Coronavirus (COVID-19) Dashboard. 2021.
Checcucci E et al. The vaccine journey for COVID-19: a comprehensive systematic review of current clinical trials in humans. Panminerva Med, 2020.
Rego GNA et al. Current clinical trials protocols and the global effort for immunization against SARS-CoV-2. Vaccines (Basel), 2020. 8(3).
Smit M, et al. Prophylaxis for COVID-19: a systematic review. Clin Microbiol Infect. 2021;27(4):532–7.
WHO., The COVID-19 vaccine tracker. 2021.
Francis AI, et al. Review of COVID-19 vaccine subtypes, efficacy and geographical distributions. Postgrad Med J. 2022;98(1159):389–94.
Polack FP, et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. New England Journal of Medicine; 2020.
Sadoff J, et al. Safety and efficacy of single-dose Ad26. COV2. S vaccine against Covid-19. N Engl J Med. 2021;384(23):2187–201.
Kumar VM, et al. Strategy for COVID-19 vaccination in India: the country with the second highest population and number of cases. npj Vaccines. 2021;6(1):1–7.
Moher D, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009;151(4):264–9.
Wells G et al. The Newcastle-Ottawa Quality Assessment Scale (NOS) for assessing the quality of non-randomized studies in meta-analyses. Clin Epidemiol [Internet], 2017: p. 1–2.
Von Elm E, et al. The strengthening the reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Ann Intern Med. 2007;147(8):573–7.
Von Elm E, et al. The strengthening the reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. J Clin Epidemiol. 2008;61(4):344–9.
Moher D, et al. Assessing the quality of randomized controlled trials: an annotated bibliography of scales and checklists. Control Clin Trials. 1995;16(1):62–73.
WHO. Coronavirus disease (COVID-19): Vaccines. 2022; Available from: https://www.who.int/news-room/questions-and-answers/item/coronavirus-disease-(covid-19)-vaccines .
Pormohammad A et al. Efficacy and safety of COVID-19 vaccines: a systematic review and Meta-analysis of Randomized clinical trials. Vaccines (Basel), 2021. 9(5).
Zheng C, et al. Real-world effectiveness of COVID-19 vaccines: a literature review and meta-analysis. Int J Infect Dis. 2022;114:252–60.
Toubasi AA, et al. Efficacy and safety of COVID-19 vaccines: a network meta-analysis. J Evid Based Med. 2022;15(3):245–62.
Download references
Acknowledgements
Not applicable.
Author information
Authors and affiliations.
Department of Pharmaceutical and Administrative Sciences, College of Pharmacy, Western New England University, 1215 Wilbraham Road, Springfield, MA, 01119, USA
Marzieh Soheili
Department of Physical Medicine and Rehabilitation, School of Medicine, Hamedan University of Medical Sciences, Hamedan, Iran
Sorour Khateri
Social Determinants of Health Research Center, Research Institute for Health Development, Kurdistan University of Medical Sciences, Sanandaj, Iran
Farhad Moradpour & Yousef Moradi
Department of Epidemiology and Biostatistics, School of Medicine, Kurdistan University of Medical Sciences, Sanandaj, Iran
Pardis Mohammadzedeh & Mostafa Zareie
Pediatric Gastroenterology Fellowship, Department of Pediatrics, School of Medicine, Namazi teaching Hospital, Shiraz University of Medical Sciences, Shiraz, Iran
Seyede Maryam Mahdavi Mortazavi
Massachusetts College of Pharmacy and Health Sciences (MCPHS), 179 Longwood Avenue, Boston, MA, 02115, USA
Sima Manifar
Hamed Gilzad Kohan
You can also search for this author in PubMed Google Scholar
Contributions
Study concept and design: YM. Acquisition, analysis, and interpretation of data: YM, MS, HGK, FM, PM, SK, MZ, SM, and SMMM. Drafting of the manuscript: YM, HGK, MS. Critical revision of the manuscript for the important intellectual content: YM, MS. Project administration: YM and HGK. All authors have approved the final manuscript draft.
Corresponding authors
Correspondence to Hamed Gilzad Kohan or Yousef Moradi .
Ethics declarations
Ethics approval and consent to participate, consent for publication, competing interests.
The authors declare no competing interests.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Soheili, M., Khateri, S., Moradpour, F. et al. The efficacy and effectiveness of COVID-19 vaccines around the world: a mini-review and meta-analysis. Ann Clin Microbiol Antimicrob 22 , 42 (2023). https://doi.org/10.1186/s12941-023-00594-y
Download citation
Received : 04 October 2022
Accepted : 10 May 2023
Published : 19 May 2023
DOI : https://doi.org/10.1186/s12941-023-00594-y
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Effectiveness
Annals of Clinical Microbiology and Antimicrobials
ISSN: 1476-0711
- Submission enquiries: [email protected]
- U.S. Department of Health & Human Services

- Virtual Tour
- Staff Directory
- En Español
You are here
News releases.
Media Advisory
Wednesday, December 30, 2020
Peer-reviewed report on Moderna COVID-19 vaccine publishes
Data from Phase 3 clinical trial confirm vaccine is effective.

The investigational vaccine known as mRNA-1273 was 94.1% efficacious in preventing symptomatic coronavirus disease 2019 (COVID-19), according to preliminary results from a Phase 3 clinical trial reported in the New England Journal of Medicine. The vaccine also demonstrated efficacy in preventing severe COVID-19. Investigators identified no safety concerns and no evidence of vaccine-associated enhanced respiratory disease (VAERD).
The vaccine was co-developed by Moderna, Inc., a biotechnology company based in Cambridge, Massachusetts, and the National Institute of Allergy and Infectious Diseases (NIAID), part of the National Institutes of Health. Moderna and NIAID previously shared initial results from the COVE trial. On Dec. 18, 2020, the FDA issued an Emergency Use Authorization allowing Moderna to make the vaccine available for the prevention of COVID-19 in adults in the United States.
The trial was led by principal investigators Lindsey R. Baden, M.D. of Brigham and Women’s Hospital in Boston, Hana M. El-Sahly, M.D. of Baylor College of Medicine in Houston, and Brandon Essink, M.D., of Meridian Clinical Research. The trial was implemented under the U.S. government’s Operation Warp Speed program and supported by NIAID and the Biomedical Advanced Research and Development Authority (BARDA) of the U.S. Department of Health and Human Services’ Office of the Assistant Secretary for Preparedness and Response.
The trial began on July 27, 2020, and enrolled 30,420 adult volunteers at clinical research sites across the United States. Volunteers were randomly assigned 1:1 to receive either two 100 microgram (mcg) doses of the investigational vaccine or two shots of saline placebo 28 days apart. The average age of volunteers is 51 years. Approximately 47% are female, 25% are 65 years or older and 17% are under the age of 65 with medical conditions placing them at higher risk for severe COVID-19. Approximately 79% of participants are white, 10% are Black or African American, 5% are Asian, 0.8% are American Indian or Alaska Native, 0.2% are Native Hawaiian or Other Pacific Islander, 2% are multiracial, and 21% (of any race) are Hispanic or Latino.
From the start of the trial through Nov. 25, 2020, investigators recorded 196 cases of symptomatic COVID-19 occurring among participants at least 14 days after they received their second shot. One hundred and eighty-five cases (30 of which were classified as severe COVID-19) occurred in the placebo group and 11 cases (0 of which were classified as severe COVID-19) occurred in the group receiving mRNA-1273. The incidence of symptomatic COVID-19 was 94.1% lower in those participants who received mRNA-1273 as compared to those receiving placebo.
Investigators observed 236 cases of symptomatic COVID-19 among participants at least 14 days after they received their first shot, with 225 cases in the placebo group and 11 cases in the group receiving mRNA-1273. The vaccine efficacy was 95.2% for this secondary analysis.
There were no concerning safety issues with vaccination, according to the authors. Local reactions to the vaccine were generally mild. About 50% of participants receiving mRNA-1273 experienced moderate-to-severe side effects — such as fatigue, muscle aches, joint pain and headache — after the second dose, which resolved in most volunteers within two days.
Investigators also observed no evidence of VAERD among those who received mRNA-1273. This rare complication was seen in individuals vaccinated with a whole-inactivated respiratory syncytial virus (RSV) vaccine in the 1960s, before there was a capacity to define protein structures and measure immune responses with precision. VAERD can occur when a vaccine induces an immune response that is not strong enough to protect against infection.
Although mRNA-1273 is highly efficacious in preventing symptomatic COVID-19, there is not yet enough available data to draw conclusions as to whether the vaccine can impact SARS-CoV-2 transmission. Preliminary trial data suggests there may be some degree of prevention of asymptomatic infection after a single dose. Additional analyses are underway of the incidence of asymptomatic infection and viral shedding post-infection to understand the vaccine’s impact on infectiousness.
The authors concluded by discussing the unprecedented efficiency of the candidate vaccine’s development, noting, “this process demonstrates what is possible in the context of motivated collaboration among key sectors of society, including academia, government, industry, regulators and the larger community.”
LR Baden, et al . Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine. The New England Journal of Medicine . DOI: 10.1056/NEJMoa2035389.
NIAID Director Anthony S. Fauci, M.D. is available to comment on this study. John R. Mascola, M.D., director of NIAID’s Vaccine Research Center, is also available to comment.
To schedule interviews, please contact the NIAID News & Science Writing Branch, (301) 402-1663, [email protected] .
NIAID conducts and supports research — at NIH, throughout the United States, and worldwide — to study the causes of infectious and immune-mediated diseases, and to develop better means of preventing, diagnosing and treating these illnesses. News releases, fact sheets and other NIAID-related materials are available on the NIAID website .
About the National Institutes of Health (NIH): NIH, the nation's medical research agency, includes 27 Institutes and Centers and is a component of the U.S. Department of Health and Human Services. NIH is the primary federal agency conducting and supporting basic, clinical, and translational medical research, and is investigating the causes, treatments, and cures for both common and rare diseases. For more information about NIH and its programs, visit www.nih.gov .
NIH…Turning Discovery Into Health ®
Connect with Us
- More Social Media from NIH
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
- My Bibliography
- Collections
- Citation manager
Save citation to file
Email citation, add to collections.
- Create a new collection
- Add to an existing collection
Add to My Bibliography
Your saved search, create a file for external citation management software, your rss feed.
- Search in PubMed
- Search in NLM Catalog
- Add to Search
Genomic insights into mRNA COVID-19 vaccines efficacy: Linking genetic polymorphisms to waning immunity
Affiliations.
- 1 Department of Family Medicine, Taipei Veterans General Hospital, Taipei, Taiwan.
- 2 Department of Medical Research, Taipei Veterans General Hospital, Taipei, Taiwan.
- 3 Big Data Center, Taipei Veterans General Hospital, Taipei, Taiwan.
- 4 Department of Information Management, Taipei Veterans General Hospital, Taipei, Taiwan.
- 5 Department of Statistics, Tamkang University, New Taipei, Taiwan.
- 6 Department of Pathology and Laboratory Medicine, Taipei Veterans General Hospital, Taipei, Taiwan.
- 7 Department of Biotechnology and Laboratory Science in Medicine, National Yang Ming Chiao Tung University, Taipei, Taiwan.
- 8 School of medicine, National Yang Ming Chiao Tung University, Taipei, Taiwan.
- 9 Biosafety level 3 laboratory, Taipei Veterans General Hospital, Taipei, Taiwan.
- 10 Institute of Biomedical Informatics, National Yang Ming Chiao Tung University, Taipei, Taiwan.
- 11 Institute of Food Safety and Health Risk Assessment, National Yang Ming Chiao Tung University, Taipei, Taiwan.
- 12 Department of Family Medicine, Taipei Veterans General Hospital Yuli Branch, Hualien, Taiwan.
- PMID: 39254005
- DOI: 10.1080/21645515.2024.2399382
Genetic polymorphisms have been linked to the differential waning of vaccine-induced immunity against COVID-19 following vaccination. Despite this, evidence on the mechanisms behind this waning and its implications for vaccination policy remains limited. We hypothesize that specific gene variants may modulate the development of vaccine-initiated immunity, leading to impaired immune function. This study investigates genetic determinants influencing the sustainability of immunity post-mRNA vaccination through a genome-wide association study (GWAS). Utilizing a hospital-based, test negative case-control design, we enrolled 1,119 participants from the Taiwan Precision Medicine Initiative (TPMI) cohort, all of whom completed a full mRNA COVID-19 vaccination regimen and underwent PCR testing during the Omicron outbreak. Participants were classified into breakthrough and protected groups based on PCR results. Genetic samples were analyzed using SNP arrays with rigorous quality control. Cox regression identified significant single nucleotide polymorphisms (SNPs) associated with breakthrough infections, affecting 743 genes involved in processes such as antigenic protein translation, B cell activation, and T cell function. Key genes identified include CD247, TRPV1, MYH9, CCL16, and RPTOR, which are vital for immune responses. Polygenic risk score (PRS) analysis revealed that individuals with higher PRS are at greater risk of breakthrough infections post-vaccination, demonstrating a high predictability (AUC = 0.787) in validating population. This finding confirms the significant influence of genetic variations on the durability of immune responses and vaccine effectiveness. This study highlights the importance of considering genetic polymorphisms in evaluating vaccine-induced immunity and proposes potential personalized vaccination strategies by tailoring regimens to individual genetic profiles.
Keywords: COVID-19; genetic polymorphisms; long-term memory CD8+ T cells; mRNA-based vaccines; waning immunity.
PubMed Disclaimer
- Search in MeSH
Supplementary concepts
Related information, linkout - more resources, full text sources.
- Taylor & Francis
- MedlinePlus Health Information
Research Materials
- NCI CPTC Antibody Characterization Program
Miscellaneous
- NCI CPTAC Assay Portal
- Citation Manager
NCBI Literature Resources
MeSH PMC Bookshelf Disclaimer
The PubMed wordmark and PubMed logo are registered trademarks of the U.S. Department of Health and Human Services (HHS). Unauthorized use of these marks is strictly prohibited.
Newly Discovered Antibody Protects Against All COVID-19 Variants
Researchers have discovered an antibody able to neutralize all known variants of SARS-CoV-2, the virus that causes COVID-19, as well as distantly related SARS-like coronaviruses that infect other animals.
As part of a new study on hybrid immunity to the virus, the large, multi-institution research team led by The University of Texas at Austin discovered and isolated a broadly neutralizing plasma antibody, called SC27, from a single patient. Using technology developed over several years of research into antibody response , the team led by UT engineers and scientists obtained the exact molecular sequence of the antibody, opening the possibility of manufacturing it on a larger scale for future treatments.
“The discovery of SC27, and other antibodies like it in the future, will help us better protect the population against current and future COVID variants,” said Jason Lavinder, a research assistant professor in the Cockrell School of Engineering’s McKetta Department of Chemical Engineering and one of the leaders of the new research, which was recently published in Cell Reports Medicine .
During the more than four years since the discovery of COVID-19, the virus that causes it has rapidly evolved. Each new variant has displayed different characteristics, many of which made them more resistant to vaccines and other treatments.
Protective antibodies bind to a part of the virus called the spike protein that acts as an anchor point for the virus to attach to and infect the cells in the body. By blocking the spike protein, the antibodies prevent this interaction and, therefore, also prevent infection.
SC27 recognized the different characteristics of the spike proteins in the many COVID variants. Fellow UT researchers, who were the first to decode the structure of the original spike protein and paved the way for vaccines and other treatments, verified SC27’s capabilities.
The technology used to isolate the antibody, termed Ig-Seq, gives researchers a closer look at the antibody response to infection and vaccination using a combination of single-cell DNA sequencing and proteomics.
“One goal of this research, and vaccinology in general, is to work toward a universal vaccine that can generate antibodies and create an immune response with broad protection to a rapidly mutating virus,” said Will Voss, a recent Ph.D. graduate in cell and molecular biology in UT’s College of Natural Sciences, who co-led the study.
In addition to the discovery of this antibody, the research found that hybrid immunity — a combination of both infection and vaccination — offers increased antibody-based protection against future exposure compared with infection or vaccination alone.
The work comes amid another summer COVID spike . This trend shows that while the worst of the pandemic may have passed, there’s still a need for innovative solutions to help people avoid and treat the virus.
The researchers have filed a patent application for SC27. Other members of the team from UT are Jason McLellan, Patrick O. Byrne, Sean A. Knudson, Douglas R. Townsend, Jessica Kain and Yimin Huang of the Department of Molecular Biosciences; George Georgiou, Ed Satterwhite and Allison Seeger of the McKetta Department of Chemical Engineering; Jeffrey M. Marchioni of the Department of Biomedical Engineering; and Chelsea Paresi of the Department of Chemistry. Team members from other institutions include Greg Ippolito of the Texas Biomedical Research Institute; Ralph S. Baric, Michael A. Mallory, John M. Powers, Sarah R. Leist, Jennifer E. Munt and Trevor Scobey of the University of North Carolina at Chapel Hill’s Department of Epidemiology; Izabella N. Castillo, Melissa Mattocks and Premkumar Lakshmanane of UNC’s Department of Microbiology and Immunology; and Bernadeta Dadonaite and Jesse D. Bloom of Fred Hutchinson Cancer Center. The research team received funding from the National Institutes of Health and the Bill & Melinda Gates Foundation.
Explore Latest Articles
Sep 12, 2024
Architecture Students Designing With AI

Sep 11, 2024
Newly Discovered Antimicrobial Could Prevent or Treat Cholera
Sep 10, 2024
AI in Education Is Here

Our websites may use cookies to personalize and enhance your experience. By continuing without changing your cookie settings, you agree to this collection. For more information, please see our University Websites Privacy Notice .
UConn Today
September 4, 2024 | Anna Zarra Aldrich, College of Agriculture, Health and Natural Resources
New Study Provides Insight to Why Covid Vaccines Hit Some Harder than Others
From exercise to birth control, researchers found many factors contribute to vaccine side effects

Students receiving the COVID 19 vaccine at Hawley Armory on April 8, 2021. The rollout of the vaccines across the state in the spring helped set up a return to a more familiar university experience. (Sean Flynn/UConn Photo)
When you got the SARS-CoV2 vaccine to protect against COVID-19, you may have experienced severe side effects. Or maybe you didn’t.

This study was published in the Journal of Agriculture and Food Research .
Concerns about potential side effects were a major barrier for some people to getting the vaccine at all, yet little research had been done on what could make someone more vulnerable to experiencing side effects.
One of Andersen’s collaborators on the paper is Christa Palancia Esposito at Fairfield University. Esposito is a nurse practitioner and midwife who noticed in both her practice and in emerging literature that COVID-19 infection was impacting women’s health differently from men’s.
Andersen and Esposito had previously collaborated on a study looking at how women respond to certain dietary interventions based on whether they use hormonal birth control.
“We thought that was interesting because there was a lot of research coming out about sex-specific differences and COVID-19 illness severity but less about responsiveness and side effects to vaccines,” Andersen says.
Given all of this, they decided to see if sex, hormonal birth control use, diet, body mass index (BMI), or exercise impacted someone’s experience of post-vaccine symptoms.
“It just got us thinking more about personalized health and whether certain characteristics could be playing a role here,” Andersen says.
For this pilot study, the researchers surveyed 82 people who received any of the three vaccines available in 2021.
They found that stress, BMI, exercise, and use of hormonal birth control all played a role.
The researchers found a significant correlation between stress and one’s perception of the intensity of the side effects from the vaccine.
“Whether stress influences psychological perception of side effects, or whether stress responses lead to biological changes that result in side effects and impact efficacy of SARS-CoV2 vaccines, as it has been shown to do with other vaccines, is worth studying,” Andersen says.
While there were no sex-dependent differences in the experience of side effects, women did generally report higher levels of stress and less regular exercise than men in the study.
Some of the associations between individual characteristics and perceived vaccine side effects were dose-dependent for the Moderna and Pfizer vaccines, which had two initial doses. For example, people who exercise regularly reported experiencing a lower severity of side effects for their first dose than those who did not exercise regularly. But for the second dose, they experienced a greater severity of side effects.
“Exercise in itself, especially acutely, can be inflammatory and certainly can impact the immune system,” Andersen says, with growing evidence that exercise impacts vaccine efficacy.
Women who were on hormonal birth control also had an increased experience of side effects, especially those using the birth control pill. People with a higher BMI also reported greater severity of side effects.
The researchers also looked at what kinds of supplements people took and what dietary patterns they followed. But, due to the small sample size, they were not able to find any significant associations between these characteristics and perceived vaccine side effects.
Andersen says she will use data from this study to support future work in her lab which focuses on the connections between diet and lifestyle factors, metabolic health, and immune function.
“My lab will be able to immediately take these factors and use the knowledge we gained to better understand individualized responses to lifestyle and dietary interventions that are aimed at achieving specific immune outcomes,” Andersen says.
As more data from SARS-CoV2 vaccinations become available, this study will provide a foundation for more work looking at these and other factors to improve vaccine delivery and reduce side effects through an individualized approach to health.
“The long-term goal is to make vaccines more effective and at the same time minimize side effects or adverse responses that may influence acceptance of potentially lifesaving, preventative health measures.”
This work relates to CAHNR’s Strategic Vision area focused on Enhancing Health and Well-Being Locally, Nationally, and Globally.
Follow UConn CAHNR on social media
Recent Articles

September 13, 2024
Career Expo Draws Recruiters from 50 Top Corporations, Hundreds of Eager Student Job-Seekers
Read the article


Student Researcher Karen Lau Engages Youth in Asian American Labor History

Ribbon-Cutting Ceremony Held For Connecticut Hall
- Adult Vaccines
- COVID-19 Vaccine
Novavax or Nothing? For Some, It’s Their COVID Vaccine Choice
Sept. 11, 2024 – Social media platforms lit up minutes after the FDA announced its authorization of the updated Novavax COVID-19 vaccine right before Labor Day weekend. It was the last of three vaccines authorized for 2024-2025, and the news was greeted with glee by many.
“That’s great!” proclaimed one Reddit user, as others posted “Yes!!” and “Bravo!” In All About Novavax, a private Facebook group of more than 1,000 members, many reposted the FDA announcement and began to compare notes about when they expect to be able to get it. Another group that had submitted petitions to the FDA, urging the agency not to delay the Novavax authorization for this year, posted, “We did it!” and “Success!” after the announcement.
In terms of doses given, Novavax trails far behind Moderna’s and Pfizer’s COVID vaccines, placing dead last. Estimates vary, but as of Aug. 10, in the U.S., more than 400 million Pfizer doses had been given, more than 251 million Moderna, and 83,047 Novavax doses, according to data researchers.
But for many, Novavax is #1 – the one and only COVID vaccine they would consider. This enthusiasm for Novavax isn’t new. As far back as 2022, news reports described a kind of Novavax fan club – a term that irritates some – and it’s still here.
What accounts for this passionate support of Novavax? It’s complicated and personal.
It’s a ‘Traditional’ Vaccine
Some say they prefer Novavax is because it’s a more traditional vaccine, compared to the Pfizer and Moderna vaccines, which are made with messenger RNA technology, said William Schaffner, MD, an infectious disease doctor at Vanderbilt University Medical Center in Nashville and a spokesperson for the Infectious Diseases Society of America.
Some people are still skeptical about the mRNA technology, he said, which they still regard as new, even though “it isn’t new anymore because we’ve given millions and millions of doses.”
Even so, “Novavax is made in a much more traditional fashion, and it’s reassuring to those who are antsy about the mRNA vaccine,” Schaffner said. “It takes a protein part of the virus, links it to an immune system stimulant, an adjuvant, and then it’s injected and stimulates the immune system.” It’s the same way flu vaccines are made.
“The mRNA vaccine is in effect a blueprint [for the protein], which is then injected into the body; the body actually makes the protein, and then the immune system responds to it.”
One of the advantages of the mRNA vaccines over traditional ones, Schaffner said, is that they are easier and faster to make. And this can let you update mRNA vaccines somewhat faster than you can update traditional vaccines, letting you better keep up with changing variants, he said.
Tradition appeals to Sam Biller, 58, of Tampa, FL, who said he is choosing Novavax this year again after first getting a Johnson & Johnson vaccine and then a previous Novavax shot because he prefers what he calls the “proven technology” of Novavax.
If the “traditional” aspect of the Novavax vaccine persuades people to get vaccinated, Schaffner said, he’s all for it, noting the lagging interest recently in getting updated vaccines. According to the CDC, only 22.9% of U.S. adults have gotten the updated 2023-2024 COVID-19 vaccine.
Plus: Fewer Side Effects
Fewer side effects in the short term, in general, is another big draw of the Novavax shot, said Peter Chin-Hong, MD, a professor of medicine and infectious disease specialist at the University of California San Francisco School of Medicine.
"For short-term side effects, like fever, myalgia (muscle pains), headache, in the studies – [although] there aren’t head-to-head comparisons – fewer participants in the Novavax studies reported those, compared to people in the Pfizer or Moderna trials.”
Other research has also found the mRNA vaccines to be the most reactive. Some researchers compared people who received an mRNA vaccine as their first booster, then got either Novavax or an mRNA vaccine as a second booster. They found the Novavax group had fewer side effects but higher rates of infection. No differences in immune responses were found between the two groups.
The immune system stimulant used in the Novavax vaccine, Chin-Hong said, compared to others in “traditional” vaccines, helps explain why it produces less severe side effects.
“For traditional vaccines, it’s all about the adjuvant,” he said.
Novavax’s adjuvant is called Matrix-M, derived from compounds in the bark of a Soapbark tree. According to Chin-Hong, the Novavax adjuvant is “not as well-known for causing as many side effects” as some others, such as the shingles vaccine adjuvant.
Judy, 74, a Northern Virginia retiree who asked that just her first name be used, had seven mRNA vaccines before switching to Novavax in May.
“The first six were Pfizer,” she said in an email. “Each one partially disabled me for a couple of days,” with fatigue, a sore arm, a headache, body aches, and malaise over 2 to 3 days. The seventh shot was Moderna's after her pharmacy ran out of Pfizer doses.
“Moderna really kicked me in the behind,” she said.
She prepared once again for side effects when she went for the Novavax in May but was surprised.
“I had a very limited reaction; I had a mild temperature with a mild headache; no sore arm. I went to bed earlier than usual, and while I was tired the next day, I was up and out of bed and ready for my coffee,” she said.
Adam Van Bavel, 45, of Baltimore, said he has long COVID, and the mRNA vaccines left him dealing with symptoms for 2 to 3 days, including a high fever, chills, a headache, and congestion. The Novavax shot only left him sore where the needle went in. He calls it an “easy decision.”
Every time he got the mRNA vaccine, “I was down for a day,” recalled Paul Hennessy, 34, an entertainment project manager in Los Angeles. After he switched to Novavax twice last year, he was fine the next day, he said.
Rare Allergic Reactions: PEG
For some, it’s the risk of a serious allergic reaction linked with the Pfizer or Moderna vaccines. Both mRNA COVID vaccines use polyethylene glycol (PEG) as a stabilizer. PEG is also found in foods, cosmetics, and other products. Serious allergic reactions to PEG are rare but do happen.
While the cause of these reactions has been debated, it’s not proven, according to some research.
Even so, Moderna and Pfizer caution anyone who’s had a severe allergic reaction to any ingredient in their COVID vaccines not to get them.
Vaccine Scorecards
Discussion on social media is brisk about the “better” COVID vaccine for this year, in terms of preventing infection and targeting the “right” variant.
In early June, the FDA advised vaccine makers that this year’s formula should be monovalent vaccines targeting JN.1. The agency then said the preferred JN.1 lineage is the KP.2 strain.
This year’s Novavax targets JN.1, the “parent strain” of currently circulating variants, the company said, and “has shown robust cross-reactivity against JN.1 lineage viruses, including KP.2.3, KP.3, KP.3.11 and LB.1.”
The updated mRNA vaccines from Moderna and Pfizer target the KP.2 strain.
“Antibody data shows the Novavax JN.1 spike vaccine and the Moderna/Pfizer KP.2 vaccine have comparable virus neutralization levels of the currently circulating variants,” said Matthew Frieman, PhD, a professor of viral pathogen research at the University of Maryland School of Medicine in Baltimore. “There have been no differences in clinical protection between the mRNA and protein vaccines, demonstrating that they are all functioning to protect from severe disease and hospitalization.”
Novavax Advocates on a Mission
Don Ford, an activist in Los Angeles, has advocated for Novavax for 2 years, has organized letter-writing campaigns, and sent a petition urging the FDA and CDC to approve the Novavax vaccine for 2024-2025. Ford said he much prefers Novavax for himself and his family, which includes a cancer patient.
“We’ve been very aggressive,” he said of his efforts, writing to the Vaccines and Related Biological Products Advisory Committee (VRBPAC) of the FDA and calling in to make public oral comments when allowed.
Once the Novavax authorization came through, he pasted a “Success!” headline on the online materials.
Next Up: Finding the Shot
Soon after the authorization, those in search of a Novavax vaccine began posting on social media, asking where to find it this year and offering tips. They know from experience they often have to shop around to find it and there can be a lack of awareness about the vaccine.
Distribution, Schaffner reminds people, has nothing to do with the FDA or the CDC, but exclusively the company. According to Novavax, the vaccine will be available “in thousands of locations across the country, including retailers, regional grocers and independent pharmacies.”
Peter Liepmann, MD, 70, a family doctor in Pasadena, CA, and his wife belong to a health plan that doesn’t stock Novavax, so they decided to pay out of pocket last fall for their preferred vaccine. And they had to hunt. “We had to look around to find a small, independent pharmacy that had it,” he said. “Most of the big chains said they had it but didn’t.”
After calling half a dozen places, he found it.

Top doctors in ,
Find more top doctors on, related links.
- COVID-19 Vaccine News
- COVID-19 Vaccine Reference
- COVID-19 Vaccine Video
- COVID-19 Vaccine Podcast
- Find a Doctor
- Coronavirus Resource Center
- Coronavirus in Context
- COVID-19 Symptom Checker
- Children’s Vaccines
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Metabol Open
- v.12; 2021 Dec
COVID-19 vaccines: Current evidence and considerations
Alireza tavilani.
a Islamic Azad University, Hamedan Branch, Hamedan, Iran
Ebrahim Abbasi
b Department of Clinical Biochemistry, Hamadan University of Medical Sciences, Hamadan, Iran
Farhad Kian Ara
c Medical Biology Research Center, Kermanshah University of Medical Sciences, Kermanshah, Iran
d Department of Nanotechnology, Pharmaceutical Sciences Branch, Islamic Azad University (IAUPS), Tehran, Iran
Zahra Asefy
e School of Nursing and Allied Medical Sciences, Maragheh University of Medical Sciences, Maragheh, Iran
Associated Data
Data are available upon reasonable request.
The coronavirus disease 2019 (COVID-19) pandemic is a global crisis, with devastating health, business and social impacts. Vaccination is a safe, simple, and effective way of protecting a person against COVID-19. By the end of August 2021, only 24.6% of the world population has received two doses of a COVID-19 vaccine. Since the emergence of COVID-19, several COVID-19 vaccines have been developed and approved for emergency use. Current vaccines have shown efficacy with low risk of adverse effects. However, COVID-19 vaccines have been related to a relatively small number of cases of heart inflammation, anaphylaxis (allergic reactions), and blood clots formation. On the other hand, COVID-19 vaccination is not recommended for children less than 12 years of age. Furthermore, It has been proposed that some new variants (e.g., Lambda and Delta) are proficient in escaping from the antiviral immunity elicited by vaccination. Herein we present current considerations regarding the COVID-19 vaccines including: efficacy against new variants, challenges in distribution, disparities in availability, dosage gender and race difference, COVID-19 vaccine transport and storage, limitations in children and pregnant women. Long-time monitoring is essential in order to find vaccine efficacy and to rule out related side effects.
1. Introduction
Numerous medicines have been used for the treatment of coronavirus disease 2019 (COVID-19) during the past year. Although most of the medicines failed to show efficacy in treating COVID-19, researchers have encouraged herd immunity to control the current pandemic [ 1 , 2 ]. Vaccination is a safe, simple, and effective way of protecting a person against COVID-19. Although a massive number of experiments have been done since the virus was first recognized, there are still many unknowns about this COVID-19. Certain persons including pregnant women, breastfeeding individuals, autoimmune conditions and immunocompromised persons, diabetic patients, and people with respiratory and heart disease require special consideration for COVID-19 vaccination [ [3] , [4] , [5] , [6] ]. Having certain medical conditions can make a person more likely to get severely ill from COVID-19 [ 2 ].
The effects of vaccines on the COVID-19 pandemic depend on various factors, including the efficiency; how rapidly they are manufactured, approved, and delivered; the immunity against new variants and how many subjects get vaccinated. Various health organizations are working to help confirm that approved COVID-19 vaccines are as effective as possible, so that they can have the most significant effect on the COVID-19 pandemic. A vaccine is a vital tool in the battle against COVID-19 infection, and there are many lifesaving and public health benefits to using the tools we now have [ 7 , 8 ].
At present, 184 candidate vaccines were being evaluated in preclinical and 104 in clinical stages of development. Furthermore, there are 41 vaccines in phase 3 and 18 COVID-19 vaccines approved, and are currently in use worldwide. These vaccines are in four primary groups using various platforms: (1) viral vector vaccines, (2) whole virus vaccines, (3) nucleic acid vaccines, and (4) protein-based vaccines [ 9 ]. Table 1 depicts the main characteristics of the currently available vaccines.
Comparison of Pfizer/BioNTech, Moderna, Johnson & Johnson, and AstraZeneca vaccines [ 7 , 33 ].
| Name of vaccines | Pfizer-BioNTech vaccine | Moderna | Johnson & Johnson | AstraZeneca |
|---|---|---|---|---|
| mRNA vaccine | mRNA vaccine | Vector vaccine | Adenovirus vector vaccines | |
| Stored for 6 months at −70 °C. Undiluted vials can be stored at room temperature for no more than 2 h. | Stored for 30 days between 2 °C and 8 °C. | Stored for up to 3 months between 2 °C and 8 °C. | Store in a refrigerator (2–8 °C). Do not freeze. Preserve the vials from light. | |
| 95% in preventing the COVID-19 infection. | 94.5% in preventing the COVID-19 infection. | 85% in preventing the COVID-19 infection. | 70% in preventing the COVID-19 infection. | |
| 2 shots, given 21 days apart. | 2 shots, given 28 days apart. | One dose is needed. | 2 shots, given 28 days apart. | |
| People age 16 and older. | People age 18 and older. | People age 18 and older. | People age 18 and older. | |
| Quite effective against the South African, UK variant, and Latin American variants. | Quite effective against the South African, UK variant, and Latin American variants. | Less effective against the South African and Latin American strains. | Less effective against South African variant, but appears effective against Brazilian and UK variants. | |
| Swelling, pain, and redness at the site of vaccine. Fatigue, headache, fever, vomiting, chills, myalgia, urticarial, and arthralgia. Bell's palsy and facial swelling has also been reported. | Swelling, pain, and redness at the site of vaccine. Nausea, vomiting, tiredness, muscle pain, chills, headache, and fever. | Swelling, pain, warmth, itching or bruising, and redness at the site of vaccine. Fatigue, headache, fever, vomiting, diarrhea nausea, chills, joint pain, muscle ache. Vaccine induced thrombotic thrombocytopenia, which are estimated to occur in 1 in 100,000 vaccinated people. |
Although the striking amount of experiments carried out since the COVID-19 was first recognized, there are still a huge number of unknowns about this disease. Hence, there are multiple concerns about COVID-19 vaccines [ 8 ]. In the next section, we will discuss about vaccination in view of gender and race difference, new variants, efficacy and immunity, safety, dosage, transport and storage, distribution, vaccination in special groups, and virus transmission in vaccinated people.
2. Vaccination in view of gender difference
It has been shown that several factors, including the genetic, immune system, gut microbiome, and steroid hormones are varied between men and women that contribute to gender - and sex-specific vaccine responses and outcomes. Women produce more antibodies as a result of vaccination and respond more actively to infections. In women, a strong response of the immune system may increase the risk of autoimmune diseases and a good capability to fight against various infections. A higher level of COVID-19 antibody has been reported in women than in men after COVID-19 infection. Women display more strong cellular and humoral-mediated immune responses to vaccination and infection when compared to men [ 10 ]. Thus, the vaccine efficacy suggested for adults is potentially greater for women than men. Men, due to high levels of testosterone, show low levels of COVID-19 vaccine effectiveness. In this respect, males may need more doses of the COVID-19 vaccine compared with females [ 10 ].
3. Vaccination in view of race difference
Among those reported, the ethnic and racial distribution of the sample was not always stated, and methods are different, which may affect the results [ 11 ]. Asian, Hispanic, and Black people are infected with COVID-19 more than White ethnicity, with a possible relationship of higher risk of mortality and intensive care unit (ICU) admission in Asians [ 10 ]. Black females and males were about 4.2 times more likely to die from COVID-19 infection than White females and males [ 10 ]. However, in the UK, the mortality risks do not apply to Black ethnicity alone. Ethnicities of the people of Indian, Bangladeshi, Iranian, Pakistani, and Mixed had substantially increased risk of death by COVID-19 infection when compared with the White ethnicity [ 10 ].
4. COVID-19 vaccines and variants
RNA viruses such as the novel coronavirus are known for mutating and evolving quickly. RNA replication is more error-prone compared to DNA replication, so mutations happen commonly during copying. Sometimes the random mutation is beneficial for the virus, which helps it evade the host's immune system and infect new species or systems. A new variant of novel coronavirus emerged with a high number of mutations. The new variants are B.1.1.7 (Alpha), B.1.351 (Beta), P.1 (Gamma), B.1.617.2 (Delta), and C.37 (Lambda). The new variants are spread more easily, lead to severe disease, and may change the efficiency of COVID-19 vaccines [ 12 ].
These variants may be associated with a higher mortality rate. There is concern that the available COVID-19 vaccines may not provide sufficient immunity against new variants.
The vaccines are expected to protect subjects against new virus variants and effective at preventing severe respiratory disease and death. An update of vaccine composition may be necessary in order to maintain high efficacy against new variants. Furthermore, the revaccination schedule may also be essential if variants develop that are potentially different from the original coronavirus that the vaccines were produced against. Another variant, B.1.1.7, revealed in the UK, has been reported to have a high mortality rate and faster transmission speed. New variants reported in various countries can decrease the efficiency of the current COVID-19 vaccines. If the pandemic persists, the mutations of coronavirus will increase, and humanity must struggle for vaccination and worldwide distribution [ 13 ].
5. COVID-19 vaccines efficacy and immunity
No vaccine is 100% effective. There's no report so far that the COVID-19 vaccine can prevent transmission, but it can help protect against COVID-19 infection. Various countries have reported that the numbers of new cases and transmission rates of COVID-19 have reduced in many areas, probably due to the protective efficacy of vaccines and/or restrictions. However, the vaccine candidates have been evaluated in isolation, which makes it challenging to compare the efficiency of different vaccines. Therefore, it would be premature to hail the immunogenicity and safety observed in vaccine trials as a real achievement [ 14 ]. None of the approved COVID-19 vaccines contain the live virus that causes COVID-19. This means these vaccines cannot lead to COVID-19 infection. Generally a few weeks after vaccination, the body builds immunity against COVID-19 infection. Hence, it is possible for people to be infected with COVID-19 just before or after vaccination and yet get sick with COVID-19. This is because the COVID-19 vaccine has not yet had an adequate period to provide protection [ 15 ].
It has been reported that mRNA COVID-19 vaccines provide immunity for at least 6 months [ 16 ]. All COVID-19 vaccines have only been produced in the past months, It's too early to judge the duration of the immunity of these vaccines. Available findings [ 17 , 18 ] show that most patients who recover from disease develop an immune response against COVID-19 infection that provides about five to eight months of protection– although the exact immunity levels and protection period are not measured. Under normal conditions, phase 3 of vaccine studies could have continued for another few years, displaying how long protection lasts before the vaccine was distributed to the general community. The current COVID-19 vaccines are all two-dose vaccines (except for the vaccine from Johnson & Johnson). Appropriate immune response has been reported within about two weeks after the first dose. And the second dose then significantly increases the immune response and a shorter time after the second dose [ 15 ].
6. COVID-19 vaccines safety
The safety of the COVID-19 vaccine should be evaluated in participants of different ages and comorbidities a few months of follow-up after their first or second dose. We need a complete risk management and safety monitoring (pharmacovigilance) system, which determines the potential side effects. Similar to other vaccines, COVID-19 vaccines can cause mild or moderate side effects within a few days after injection. Some side effects such as headache, muscle pain, fatigue, fever, diarrhea, and chills have been reported, and most have happened during the first 48 h after vaccination. Therefore, subjects should continually monitor to distinguish adverse events [ 15 ].
WHO is aware that some people may show a severe allergic reaction to the vaccines (e.g., anaphylaxis). According to The United States Centers for Disease Control and Prevention (CDC) report, 11.1 per million cases of vaccinated people reported anaphylaxis in the USA [ 19 ]. If the subjects report a history of anaphylaxis with previous vaccines, they are advised not to take the new vaccine. Polyethylene glycol (PEG) and PEG derivatives (e.g., polysorbates) are probably responsible for anaphylaxis [ 13 ]. It has been recommended that before vaccination, people should notify the healthcare workers about any anaphylaxis they may have had previously. It has been proposed that all vaccinated cases remain at the vaccination site for 30 min to detect any serious side effects. It has been reported that the AstraZeneca and Johnson & Johnson/Janssen vaccines may have a possible link to a very rare side effect of unusual blood clots combined with low levels of platelet levels [ 7 , 20 ].
Various vaccines entered into clinical trials in a short time and were conditionally approved in less than one year. This unique speed was motivated by the timely detection of novel coronavirus genomic sequences, strong collaboration among the research centers, sufficient funding, and the urgent/huge market demand. Since the beginning of the COVID-19 pandemic, many countries are competing to develop vaccines. The development of the standard vaccine is a long process, and experiments are complete in sequential steps. However, the development of COVID-19 vaccines is being fast-tracked globally. Despite the significant progress, the safety and quality of various vaccines are the main concern. The UK, Germany, USA, and China have developed vaccines in phase 4 (post-market studies) [ 21 ].
7. COVID-19 vaccines dose
The Johnson & Johnson vaccine only requires one dose, while the Moderna, Pfizer-BioNTech, Oxford-AstraZeneca (in a 8–12 week interval), Sputnik V (in a 3 week interval), Novavax (in a 3 week interval), Coronavac (in a 1 month interval) need two doses. The CDC documented that while there's no priority for one vaccine over another, the vaccines aren't interchangeable.
Mixing two different vaccines can show long-lasting and strong immune responses when compared to the single vaccine. Scientists hope that mix-and-match COVID-19 vaccination regimens (e.g., e.g. AstraZeneca and Pfizer) can trigger stronger, more robust immune responses than two doses of a single vaccine. Mix-and-match COVID-19 vaccination is recognized by high levels of both T cells and antibodies, which kill infected cells and support other antiviral responses [ 22 , 23 ].
According to the CDC report the second dose should be injected as close to the suggested interval as possible. It may be injected up to 42 days after the first dose when a delay is inevitable. If the second dose is injected after the suitable interval, the series does not need to be restarted. Furthermore, the vaccine team should not inject second doses before the proposed interval or save or hold doses for cases who have not returned more than 42 days after their first dose [ 24 ]. The second dose of vaccine may be missed due to personal reasons or a fluctuating vaccine supply. If more than 3 weeks have passed since the first dose was received, the next dose can be injected as soon as possible [ 13 ].
8. COVID-19 vaccines transport and storage
Most of the available vaccines should be stored and transported in refrigeration to freezing temperatures (e.g., the Pfizer vaccine at −70 °C and Oxford-AstraZeneca 2–8 °C). Therefore, the storage and transport of mRNA vaccines is challenging. Some new vaccines can be stored at −15 to −25 °C for up to 14 days12 [ 25 ]. On the other hand, some other vaccines need ultra-cold storage (below −80 °C). That means they will be really challenging to administer effectively in poor countries or remote areas of the globe as they are far away from the central transport system. It can cause low COVID-19 immunization in these areas and, consequently, increase the endemicity of infections [ 25 ]. Care is necessary after transferring these vaccines to refrigerating to freezing temperatures or the following thawing to protect their quality. A regular schedule for temperature is vital for the preservation of stability, potency, and efficacy of COVID-19 vaccines [ 25 ]. Distribution and transportation of COVID-19 vaccines are difficult and complicated particularly in hot climate and low-income countries [ 26 ].
Stable and effective storage and transport of vaccines mean they need them at cold temperatures and transfer them quickly from the manufacturer to the medical centers. A previous report showed that 2.8 million vaccines were missed in 5 countries due to cold chain failures, and less than 10% of countries met WHO protocol for effective vaccine management [ 27 ]. Interestingly, nearly 80% of vaccine costs are related to the cold chain programme. Henceforth, the lyophilized vaccine has good stability compared with liquid form. Providing a cold chain for poor countries is the main concern. Proper preparation of lyophilized form is necessary, and powder should not be prepared until the administration. Liquid form loses its efficacy when kept at freezing temperatures because slow freezing leads to great stress to the colloids and increased aggregations [ 28 ]. Cold chain technology is needed for the liquid form, which can be challenging for use in poor countries. Appropriate cold chain infrastructure can prevent up to 25% vaccine loss in poor countries [ 8 ].
9. COVID-19 vaccine distribution
Many people in poor and middle-income countries may not be receiving vaccines; therefore, equitable COVID-19 vaccine distribution is essential. More than 700 million COVID-19 vaccines have been injected globally; low-income countries received only 0.2%, while wealthy countries have received more than 87%. On average, 1 in more than 500 people in poor countries has received COVID-19 vaccines, compared with 1 in 4 people in wealthy countries [ 13 ].
As of May 11, 2021, about 1.32 billion people had received the COVID-19 vaccine worldwide, equal to 17 doses for every 100 people. Some countries (e.g., Gibraltar and Israel) had vaccinated 78% of people, while Mauritius, Pakistan, Guyana, Cambodia, Albania, Bolivia, and Ecuador had less than 0.1 doses administered per 100 people. It is a disappointment that healthcare workers are dying in various countries, showing a global moral failure in these regions. Researchers believe that this uneven administration pattern can also cause virus mutations and new vaccine-resistant variants [ 25 ].
Many poor countries have low socioeconomic status (SES) with low income, high unemployment rates and poor education. These conditions may potentially influence the vaccine-accepting and purchasing processes of their people. The geographical landscape of some poor countries poses a substantial challenge to COVID-19 vaccine distribution. High altitude areas within Hindu-Kush Himalayan regions, such as Pakistan, Bhutan, Nepal, and Afghanistan, make it very difficult for health workers to distribute COVID-19 vaccines. The problematic condition may be aggravated in the desert, and remote areas participated in the war, conflict, and instability. In this respect, more than 160 million subjects have been expected to be at risk of COVID-19 vaccine inaccessibility in Syria, Yemen, Ethiopia, and South Sudan [ 25 ].
10. COVID-19 vaccine for children and pregnant women
COVID-19 infection has been a more dangerous and severe disease among older people. Most of the vaccines are commonly offered to adults first to avoid exposing children who are still growing and developing. Because of the high risk of severe disease in the children, elderly, immunocompromised subjects, and pregnant women, the vaccination programme should be conducted with care [ 10 ]. COVID-19 vaccine teams need to follow-up pregnancies long-term to recognize effects on infants and pregnancy.
The mRNA vaccines (Pfizer-BioNTech and Moderna) do not have the live coronavirus that leads to COVID-19 and, consequently, cannot infect. Moreover, the mRNA vaccines do not interact with an individual's DNA or lead to genetic alterations since the mRNA does not enter the cell's nucleus. The viral vector vaccines (J&J/Janssen vaccine) can be administered to pregnant women in all trimesters of pregnancy (like the Ebola vaccine). However, there are various types of COVID-19 vaccines, and our direct knowledge is currently limited about their effects during pregnancy. The efficacy and safety of COVID-19 vaccines in lactating women, the impact of COVID-19 vaccination on the breastfed infant, and effects on milk excretion or production have not been determined. However, non-replicating COVID-19 vaccines pose no risk for lactating women or their babies; hence lactating women may safely be vaccinated [ 29 ].
11. COVID-19 infection transmission in vaccinated people
The risks of COVID-19 in vaccinated subjects cannot be entirely eliminated as long as there is continued public transmission of the virus. Vaccinated subjects can still get COVID-19 and spread it to other people. Hence, the COVID-19 test and self-quarantine are required for travellers. Some vaccinated subjects later exposed to the coronavirus still get COVID-19. In this context, a fully vaccinated person should continue to wear a face mask, maintain social distance, and follow health care recommendations. Preliminary data from some countries showed that the viral load was 4–fold lower among those fully vaccinated with an effective vaccine. This finding suggests that viral transmission from fully vaccinated people is lower, as viral load has been recognized as the main factor for virus transmission [ 30 ]. So far, SARS-CoV-2 has not been detected in breast milk, and there are no recognized cases of transmission of virus to the infant through breast milk. However, infected women may select to breastfeed with protections to prevent transmission of the virus through respiratory droplets. Some newborns have shown COVID-19 shortly after birth. It is unknown if these newborns got the virus after, during, or before birth [ 31 ].
12. Low intends to take COVID-19 vaccine
It has been reported that about 15–20% of adults do not intend to take the COVID-19 vaccine. People who don't intend to get the COVID-19 vaccine are at higher risk of transmitting and contracting the virus. They can also enormously increase the pandemic period, contributing to spikes in COVID-19 cases and facilitating viral replication and the emergence of new viral variants. Common concerns among the people, who do not intend to get the COVID-19 vaccine, include the efficacy, safety, and the perceived hasty timeline for vaccine production. African American race, younger age, people with lower education, and conservative political ideology has lower intention to get COVID-19 vaccine. Receiving health care recommendations and having more fear of severe disease were both accompanied with more intention to vaccinate [ 11 , 32 ].
13. Conclusion
In conclusion, there are various types of vaccines worldwide. However, additional studies are necessary to determine the effectiveness of the COVID-19 vaccine against variants of concern. COVID-19 vaccines have obtained emergency use and there are various limitations such as vaccine distribution, variants of concern, vaccination willingness, herd immunity, vaccine efficacy, vaccine safety, and vaccine dose. To combat the current pandemic, manufacturers and healthcare authorities should work together to provide appropriate and adequate vaccinations for the prevention of COVID-19. Healthcare authorities should constantly update COVID-19-related information. Furthermore, vaccine booster doses may be required for several reasons; inadequate protection, reduced protection against new variants, and waning protection against disease or infection. However, the rationale for COVID-19 vaccine booster doses may vary by vaccine product, risk group epidemiological setting, and vaccine coverage rates.
This research did not receive any specific grant from funding agencies in the public, commercial, or Non-Profit sectors.
Ethics approval
Not applicable.
Availability of data and material
Credit authorship contribution statement.
Alireza Tavilani: Conceptualization, Visualization, Data curation, Validation, Writing – original draft. Ebrahim Abbasi: Project administration, Visualization, Validation, Supervision, Data curation, Writing – review & editing. Farhad Kian Ara: Software, Writing – review & editing. Ali Darini: Validation, Writing – original draft, Writing – review & editing. Zahra Asefy: Validation, Visualization, Writing – original draft, Writing – review & editing.
Declaration of competing interest
None to be declared.
Acknowledgement
We would like to thank Hamadan University of Medical Sciences.
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Open access
- Published: 13 September 2024
Risk benefit analysis to evaluate risk of thromboembolic events after mRNA COVID-19 vaccination and COVID-19
- Huong N. Q. Tran ORCID: orcid.org/0000-0003-3828-3862 1 ,
- Malcolm Risk 2 ,
- Girish B. Nair 3 &
- Lili Zhao 4
npj Vaccines volume 9 , Article number: 166 ( 2024 ) Cite this article
Metrics details
- Epidemiology
- Thromboembolism
- Viral infection
We compared the risks and benefits of COVID-19 vaccines using a causal pathway analysis to weigh up possible risk factors of thromboembolic events post-vaccination. The self-controlled case series (SCCS) method examined the association between thromboembolic events and vaccination while a case-control study assessed the association between thromboembolic events and COVID-19, addressing under-reported infection data issues. The net vaccine effect was estimated using results from SCCS and case-control studies. We used electronic health record data from Corewell Health (16,640 subjects in SCCS and 106,143 in case-control). We found increased risks of thromboembolic events post-vaccination (incidence rate ratio: 1.19, 95% CI: [1.08, 1.31] after the first dose; 1.22, 95% CI: [1.11, 1.34] after the second dose). Vaccination attenuated infection-associated thromboembolic risks (odds ratio: 4.65, 95% CI: [4.18, 5.17] in unvaccinated vs 2.77, 95% CI: [2.40, 3.24] in vaccinated). After accounting for vaccine efficacy and protection against infection-associated thromboembolic events, vaccination decreases thromboembolic event risk, especially during high infection rate periods.
Introduction
The Coronavirus Disease 2019 (COVID-19) pandemic prompted a race to develop and distribute effective vaccines. Approximately 81.4% of the US population have been vaccinated with at least one dose, and 69.5% have completed the primary series of COVID-19 vaccination 1 . While the benefits of vaccination are widely acknowledged, concerns have emerged regarding the development of thromboembolic events after vaccination 2 . Phase 3 clinical trials were not statistically powered to identify rare adverse events 3 . The risks of new vaccines were not fully known during regulatory approval, particularly for mRNA-based vaccines (mRNA-1273 or BNT162b2), which were under authorized emergency use. Therefore, it is important to conduct post-marketing safety surveillance of the vaccines. More specifically, cases of venous thromboembolism following a mRNA-based vaccination were reported in 2022 after COVID-19 vaccines were administered in the US and some other countries 4 , 5 , 6 , 7 , drawing attention to the potential risk of thromboembolic events after the first vaccination dose. One study confirmed an increased risk of thromboembolism, ischemic stroke, and cerebral venous sinus thrombosis after the first dose of BNT162b2 8 , and another retrospective cohort study found an increased risk of cerebral venous thrombosis and portal vein thrombosis after any mRNA-based vaccination 9 . Moreover, a recent systematic review 10 has shown that thromboembolism is the most frequent cardiovascular complication following a mRNA-based vaccination. Despite those findings, vaccination is still recommended to reduce the likelihood of COVID-19, hospitalization, and mortality 8 , 11 . Furthermore, COVID-19 itself substantially increases the risk of thromboembolic events 12 , 13 , 14 , 15 , 16 , 17 , 18 , with a more prolonged and significant threat compared to vaccine-associated risks 8 . Therefore, studying the risk of thromboembolic events after COVID-19 vaccination should incorporate the protective effect of vaccines against COVID-19 severity and hence COVID-19-associated thromboembolic events.
Several studies have reported a positive correlation between thromboembolic events and mRNA-based vaccines, with reported incidence rate ratios (IRRs) between 1.04 and 1.22 8 , 19 , 20 , 21 , 22 . These studies used the self-controlled case series 23 (SCCS) design, which is a standard approach to studying adverse events of vaccines. The same design was used to evaluate the risk of thromboembolic events after COVID-19, with reported IRRs between 6.18 and 63.52 8 , 11 , 14 . However, since a thromboembolic event typically requires a hospital visit (emergency visit or hospital admission), subjects with a thromboembolic event are subject to a higher rate of COVID-19 testing, and so at a lower likelihood of misclassification as uninfected compared to subjects without an event. Hence, the SCCS design is subject to some risks of bias 24 , which we would expect to inflate the SCCS estimated relative risk (RR) of thromboembolic events after COVID-19.
The objective of this study is to evaluate whether the overall effect of the COVID-19 vaccination is to increase or decrease the risk of thromboembolic events. To do so, we first quantified the risk of thromboembolic events after mRNA-based vaccination using the SCCS method. Secondly, we evaluated the association between thromboembolic events and COVID-19 using a case-control study, avoiding the misclassification bias associated with the SCCS method. Finally, we conducted a risk-benefit analysis by comparing the magnitude of the increased risk through the direct effect of the COVID-19 vaccination with the reduced risk through the indirect pathway via protection against infection-associated thromboembolic events.
Our studies used electronic health record (EHR) data from the Corewell Health East (CHE, formerly known as Beaumont Health) and Corewell Health West (CHW, formerly known as Spectrum Health) healthcare systems, which includes demographics, mortality, hospital admissions, and COVID-19 testing. We obtained accurate COVID-19 vaccination records (vaccine types, dates, and doses) by linking EHR data at Corewell Health with the Michigan Care Improvement Registry (MCIR), giving more complete data for individuals who received the COVID-19 vaccines outside the healthcare system. We included all patients aged ≥ 18-years-old and were registered with a primary care physician within 18 months before Jan 1st, 2021.
We identified thromboembolic events based on ICD-10 (International Classification of Diseases version 10) codes from a hospital visit (emergency visit or hospital admission). These ICD-10 codes represent diagnoses for venous thromboembolism, arterial thrombosis, cerebral venous sinus thrombosis, ischemic stroke, and myocardial infarction (Supplementary Table 1 ). We also used patients with physical injury at a hospital visit (list of ICD-10 codes in Supplementary Table 2 ) to identify potential bias related to the misclassification and further leveraged them as a control group to estimate the effect of COVID-19 on thromboembolic events.
Estimate effect of mRNA-vaccination on thromboembolic events
We used the SCCS design to examine the association of thromboembolic events and the first two doses of mRNA-based COVID-19 vaccines (mRNA-1273 or BNT162b2) from December 1st, 2020, to August 31st, 2022. The SCCS method compares the incidence rate of thromboembolic events before and after vaccination. In this method, subjects are under their own control, and comparisons are made within subjects, thus avoiding any time-invariant confounding. We included subjects who had a thromboembolic event and received at least one dose of the primary series of mRNA-based vaccines in the study period. The control period was defined from December 1st, 2020, to 28 days before the first dose of vaccination, excluding the period of 28 days prior to vaccination to avoid bias due to contra-indications 25 . Two separate risk periods for the first and second doses were defined until 28 days after vaccination, death, or August 31st, 2022, whichever occurred first (Supplementary Fig. 1 ). We also excluded subjects who had COVID-19 within 90 days before a thromboembolic event to remove the confounding effect of infection on that event. We used a conditional Poisson regression 22 with an offset for the length of each period to estimate the IRRs of dose one and dose two simultaneously. Specifically, the model has an independent variable of the period with three categories (control periods, and two risk periods after the first and second dose). Using the control period as the reference, we derived the IRRs for the two doses. As Poisson regression assumes the independence between recurrent events, therefore, we considered only events that occurred at least one year after the previous events.
Estimate effect of COVID-19 on thromboembolic events
In an initial analysis of the association between thromboembolic events and COVID-19, we used the SCCS design and included patients who had at least one positive COVID-19 test (PCR or antigen) and a thromboembolic event at a hospital visit during the same period as in the previous study of vaccination. However, due to the missing infection data in patients who did not have any hospital visits for thromboembolic events or other reasons, the SCCS design resulted in a biased estimate of the association between thromboembolic events and COVID-19. Patients visiting the hospital, almost always received a COVID-19 (PCR or antigen) test, especially early in the pandemic, while patients who did not visit the hospital were subject to underreporting infection data. This underreporting (or misclassification of infected as uninfected) led to an inflated IRR of thromboembolic events after COVID-19.
We proposed a simple and efficient method to quantify the association between thromboembolic events and COVID-19 while dealing with the misclassification issue. The main idea is to select a subset of control (i.e., subjects without thromboembolic events) who had a hospital visit for reasons independent of COVID-19 and therefore had complete infection data. To this end, we used patients who had a diagnosis code for physical injury (see Supplementary Table 2 ) at a hospital visit as the control group, since we would not expect any causal association between physical injury and COVID-19. We used a case-control design, in which patients with a thromboembolic event are considered as cases, and patients with a physical injury are considered as controls. If an individual had multiple hospital visits for thromboembolic events or physical injuries, we considered only the first visit. As physical injuries can be risk factors for thromboembolic events 26 , 27 , we therefore excluded patients who experienced both events at the same visit. We determined the COVID-19 status based on the COVID-19 test results during the 28 days prior to the date of the event (Supplementary Fig. 2 ). If an individual had a positive test result, this subject was classified as exposed to COVID-19, otherwise, unexposed. We compared the odds of infection (exposed) vs no infection (unexposed) in the cases (with thromboembolic events) vs controls (with physical injury) using a logistic regression model adjusted for age, race, gender, Charlson comorbidity index (CCI), number of visits, and prior vaccination status (yes/no). Patients who had any COVID-19 vaccine between the date of the positive COVID-19 test and the date of the event were removed. The number of visits was fit with a natural spline with three degrees of freedom. The CCI was obtained using the R package comorbidity and categorized into four categories, ‘0’, ‘1–2’, ‘3–4’, and ‘ ≥ 5’ 28 , 29 . Analyses were done after excluding patients with incomplete covariate data.
Estimate the net effect of mRNA-vaccination on thromboembolic events: a risk-benefit analysis
COVID-19 vaccines are protective against COVID-19 and COVID-19 severity 30 , 31 , 32 , and so can indirectly decrease the likelihood of experiencing a thromboembolic event. Hence, we conducted a risk-benefit analysis to estimate the net RR of thromboembolic events after vaccination by considering the role of vaccination in preventing infection-associated thromboembolic events. Figure 1 illustrates the direct and indirect effect of the COVID-19 vaccination on the occurrence of thromboembolic events while considering vaccine efficacy (VE). As presented in the diagram, the association between thromboembolic events and COVID-19 vaccination is described by two paths, the direct association between thromboembolic events and vaccination, and the indirect association between thromboembolic events and vaccination via potential reduction in the risk of thromboembolic events through decreasing the risk of COVID-19. We estimated the overall influence of vaccination on the occurrence of thromboembolic events by considering both direct and indirect paths.
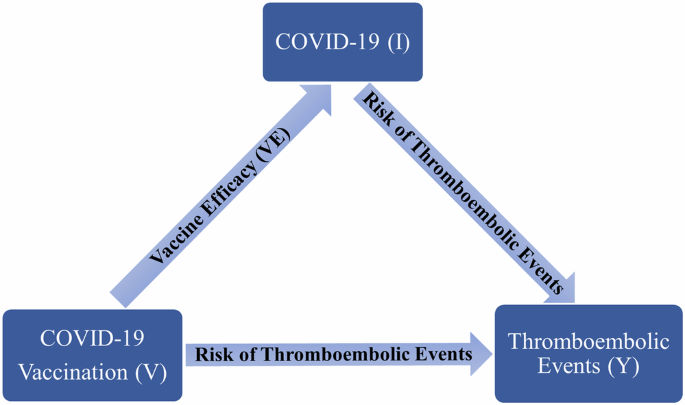
COVID-19 (I), individuals with COVID-19. COVID-19 vaccination (V), individuals with COVID-19 vaccines. Thromboembolic events (Y), individuals with thromboembolic events. V → I indicates vaccine effect (VE) in preventing COVID-19, V → Y indicates the risk of thromboembolic events after COVID-19 vaccination, I → Y indicates the risk of thromboembolic events after COVID-19, V → Y (via I) indicates the risk of thromboembolic events after vaccination accounting for vaccine effect in reducing infection-associated thromboembolic events.
Let \({\rm{P}}\left({\rm{I}}|{\rm{V}}\right)\) and \({\rm{P}}\left({\rm{I}}|\bar{{\rm{V}}}\right)\) be the probability of COVID-19 ( \({\rm{I}})\) in vaccinated ( \({\rm{V}}\) ) and unvaccinated ( \(\bar{{\rm{V}}}\) ) subjects, respectively. Let \({\rm{P}}\left({\rm{Y}}|\bar{{\rm{V}}},\bar{{\rm{I}}}\right),{\rm{P}}\left({\rm{Y}}|{\rm{V}},\bar{{\rm{I}}}\right),{\rm{P}}\left({\rm{Y}}|{\rm{I}},\bar{{\rm{V}}}\right),\) and \({\rm{P}}\left({\rm{Y}}|{\rm{I}},{\rm{V}}\right)\) be the probability (or risk) of thromboembolic events ( \({\rm{Y}})\) in unvaccinated and uninfected, vaccinated and uninfected, unvaccinated and infected, and vaccinated and infected subjects, respectively.
With the above notations, for a vaccinated subject, the total risk of thromboembolic events is \({\rm{P}}\left({\rm{Y}}|{\rm{V}},\bar{{\rm{I}}}\right)+{\rm{P}}\left({\rm{I}}|{\rm{V}}\right)\times {\rm{P}}\left({\rm{Y}}|{\rm{I}},{\rm{V}}\right)\) , where the product \({\rm{P}}\left({\rm{I}}|{\rm{V}}\right)\times {\rm{P}}\left({\rm{Y}}|{\rm{I}},{\rm{V}}\right)\) is the indirect risk calculated by multiplying the risk of COVID-19 of a vaccinated subject and the risk of thromboembolic events given a COVID-19 in the vaccinated group. Similarly, the overall risk of thromboembolic events for an unvaccinated subject is given by \({\rm{P}}\left({\rm{Y}}|\bar{{\rm{V}}},\bar{{\rm{I}}}\right)+{\rm{P}}\left({\rm{I}}|\bar{{\rm{V}}}\right)\times {\rm{P}}\left({\rm{Y}}|{\rm{I}},\bar{{\rm{V}}}\right)\) . Hence the net RR ( \({{\rm{RR}}}_{{\rm{Net}}}\) ) of thromboembolic events for a vaccinated subject compared to an unvaccinated subject is
The terms \({{\rm{RR}}}_{{\rm{V}}}\) is the RR of thromboembolic events comparing vaccinated versus unvaccinated in subjects without COVID-19, and \({{\rm{RR}}}_{{\rm{I|}}\bar{{\rm{V}}}}\) is the RR of thromboembolic events comparing subjects with and without COVID-19 in the unvaccinated group. The term \({{\rm{RR}}}_{{\rm{IV}}}\) is the RR of thromboembolic events in subjects who have both vaccination and infection, compared to the group of subjects who do not have any exposures.
We further defined VE as \({\rm{VE}}=1-{\rm{P}}({\rm{I|V}})/{\rm{P}}({\rm{I|}}\bar{{\rm{V}}})\) , then plugged VE into Eq. (1) to obtain
If \({{\rm{RR}}}_{{\rm{Net}}}\) is smaller than one, COVID-19 vaccination offers protection against thromboembolic events, with a lower \({{\rm{RR}}}_{{\rm{Net}}}\) implying a stronger protection.
Statistical analyses were performed in R 4.3.0. We reported odds ratio (OR) and IRR with 95% CIs and p -values from the two-sided test. We generated a figure for \({{\rm{RR}}}_{{\rm{Net}}}\) over a range of VE values based on the estimates of ORs and IRRs.
We used de-identified EHR data, the use of which was approved by the Institutional Review Board of Corewell Health.
Study population
During the study period from December 1st, 2020, to August 31st, 2022, there were 747,070 subjects at Corewell Health who received mRNA-based vaccines, among which 279,229 (37.38%) had the primary series of mRNA-1273 and 467,841 (62.62%) took BNT162b2. Overall, the number of fully vaccinated patients was 711,460 (95.23%), and 35,610 (4.77%) patients received only one dose. The median age was 57 (with interquartile range [IQR]: 40–69), and 59.81% of patients were female. There were 367,105 patients taking at least one COVID-19 test (antigen or PCR), among which 78,568 (21.4%) patients received positive results. The median age was 52 (with interquartile range [IQR]: 34–67), and 61.44% of patients were female.
In the study cohort of vaccination exposure, there were 16,640 patients who had at least one thromboembolic event and had the first dose of either mRNA-1273 or BNT162b2 vaccine. Patient demographics are presented in Table 1 . We identified 2724 events in the control period, 722 events within 28 days after the first dose, and 786 events within 28 days after the second dose.
In the study cohort of COVID-19 exposure, there were 18,004 patients who had a thromboembolic event (cases) and 88,139 patients who had a physical injury (controls) at a hospital visit. 16.96% of cases and 1.48% of controls had COVID-19 within 28 days before the event. Demographics of patients are presented in Table 2 .
Based on the SCCS analysis, we found an increased risk of thromboembolic events 28 days after the first dose (IRR = 1.19, 95% confidence interval (CI): [1.08, 1.31], p -value < 0.001), and after the second dose (IRR = 1.22, 95% CI: [1.11, 1.34], p -value < 0.001) of the mRNA-based vaccines.
We studied the risk of thromboembolic events in a 28-day window after vaccination based on prior research 8 . An event that occurs in a short period (such as 28 days) is more likely to be attributable to the vaccines. We also conducted a sensitivity analysis using a 60-day window after vaccination. The conclusions remained the same with slightly lower IRRs (IRR = 1.13, 95% CI: [1.03, 1.24] after the first dose, and IRR = 1.14, 95% CI: [1.05, 1.3] after the second dose).
Supplementary Figs. 3 and 4 show the IRRs for subgroup analyses by age (“18–31”, “31–50”, and “≥51”) and gender (female/male). We found that the effects of vaccination on thromboembolic events were similar between age groups and gender groups.
Naïve SCCS analysis showed a very large increased risk of thromboembolic events associated with COVID-19 (IRR = 19.36, 95% CI: [17.64, 21.26], p -value < 0.001). However, a similar analysis using the physical injury as an event also derived a large increased risk (IRR = 3.31, 95% CI: [3.10, 3.54], p -value < 0.001), indicating misclassification bias as COVID-19 should not substantially increase the risk of physical injury. In the case-control analysis with controls having a physical injury, we found that COVID-19 increased the risk of thromboembolic events but with a much smaller magnitude than the risk in the SCCS analysis (although it is still larger than the vaccination exposure). Moreover, the degree of the increased risks was modified by vaccination status (Fig. 2 ). The reported OR for the unvaccinated group was 4.65 (95% CI: [4.18, 5.17], p -value < 0.001) compared to 2.77 (95% CI: [2.40, 3.24], p -value < 0.001) for the vaccinated group. We observed the increased risks of thromboembolic events after COVID-19 in both groups, but vaccination appears to confer some protection against infection-associated thromboembolic events, given the lower OR. Alternatively, we divided the vaccinated group into four categories based on the time to the last vaccination (“≥365 days”, “180–365 days”, “90–180 days”, and “<90 days”). The effects of COVID-19 on thromboembolic events were similar across the four vaccinated groups. The results are in Supplementary Fig. 5 .
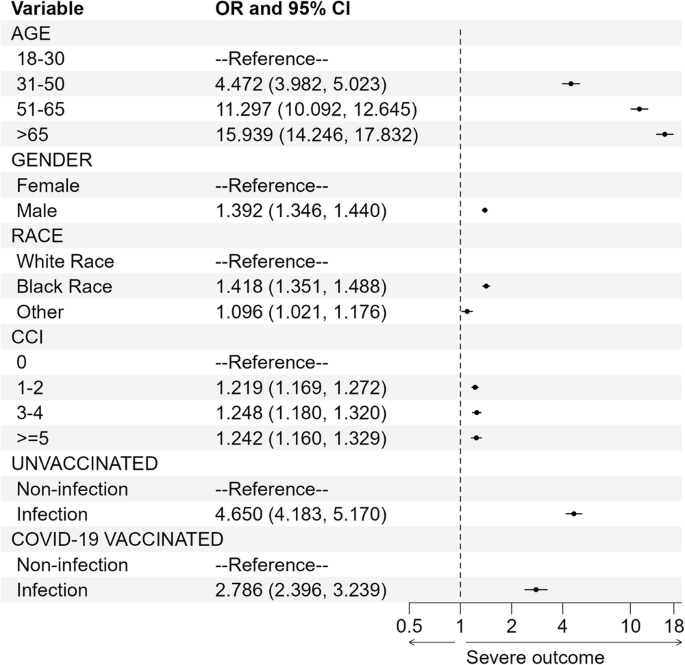
OR is denoted by a solid circle and a 95% CI is represented by a line. The x -axis is plotted on the natural log scale. CCI Charlson comorbidity index. Infection or non-infection refers to COVID-19.
We also conducted two sensitivity analyses. In the first analysis, rather than adjusting for the CCI, we adjusted individual risk factors that might be related to a thromboembolic event. These are congestive heart failure, peripheral vascular disease, cerebrovascular disease, chronic pulmonary disease, diabetes with complications, cancer, moderate or severe liver disease, and metastatic solid tumors. We included the above eight risk factors (present or absent) in the logistic regression model. The effect of COVID-19 on the outcome of thromboembolic events was similar to the analysis with CCI. Results can be found in Supplementary Fig. 6 .
We assumed that patients who visited hospitals were routinely tested for COVID-19, especially during the early pandemic. Based on Corewell Health’s policy, patients who visited the healthcare system before March 1st, 2022, were tested for COVID-19. In our study cohort, 74.05% of participants had a hospital visit before March 1st, 2022. We conducted a sensitivity analysis using only these patients and the conclusions remained the same. See results in Supplementary Fig. 7 .
Our analysis in the previous sections gave an IRR of 1.22 as the measure of the association between thromboembolic events and the second dose of COVID-19 vaccination, therefore, we set \({{\rm{RR}}}_{{\rm{V}}}\) = 1.22. We also obtained odd ratios \({{\rm{OR}}}_{{\rm{I|}}\bar{{\rm{V}}}}\) = 4.65 and \({{\rm{OR}}}_{{\rm{IV}}}\) = 2.82 from the analysis using the case-control design. Since the RR is very close to the OR when the event is rare, we therefore set \({{\rm{RR}}}_{{\rm{I|}}\bar{{\rm{V}}}}\) = 4.65 and \({{\rm{RR}}}_{{\rm{IV}}}\) = 2.82, as the thromboembolic events are rare 33 . Hence, plugging these estimators into Eq. (2), the \({{\rm{RR}}}_{{\rm{Net}}}\) becomes
Figure 3 illustrates the \({{\rm{RR}}}_{{\rm{Net}}}\) of thromboembolic events after COVID-19 vaccination as a function of VE. As VE increases from 0 to 1, \({{\rm{RR}}}_{{\rm{Net}}}\) decreases and reaches a point where vaccine benefits outweigh the harms. Specifically, vaccines with higher VE offer higher protection against thromboembolic events. For example, the effectiveness of mRNA-based COVID-19 vaccines against infection was 61% during the Delta period and 46% during the Omicron period 34 , 35 , 36 . Given an infection rate of 0.08 among unvaccinated subjects, the risk of thromboembolic events was decreased by 4.62% in the Delta period, which is higher than 2.07% in the Omicron period. Moreover, vaccines offer stronger protection during periods with higher infection rates. For example, with the infection rate of 0.1 in unvaccinated subjects, the reduction of the risk of thromboembolic events was higher (by 9.19% in Delta and 6.23% in the Omicron period), compared to the scenario when the infection rate was 0.08.
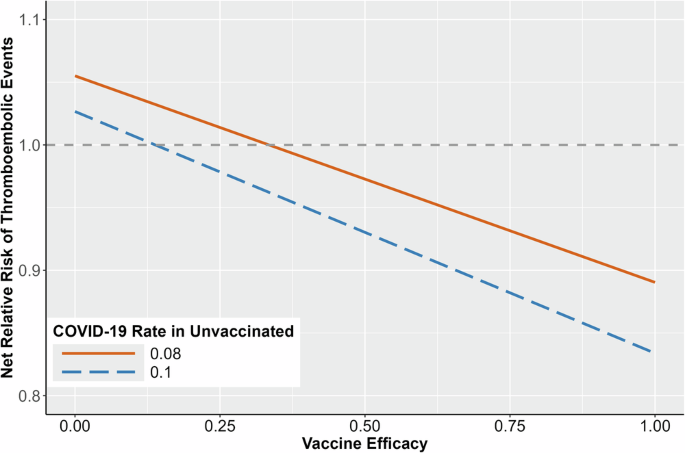
The x -axis is VE, and the y -axis is the net RR of thromboembolic events.
The list of ICD-10 codes for thromboembolic events is based on a previous publication 8 , including old myocardial infarction (I252). Old myocardial infarction (I252) reports for any myocardial infarction described as older than four weeks. However, our study cohort removed subjects with an I252 code who had any thromboembolic event with ICD-10 codes listed in Table S1 in the prior year. Therefore, we can consider observing I252 in the study period as a new incidence. There were 20,002 (18.84%) patients with a hospital visit associated with the I252 code. We conducted a sensitivity analysis by excluding these patients and the conclusions did not change. The estimated IRRs of thromboembolic events are 1.16 and 1.17 after vaccine dose 1 and dose 2, respectively, which are slightly smaller than the original results including the I252 code (IRRs were 1.19 and 1.22 after the first and second dose). The association between COVID-19 and thromboembolic events is higher in the unvaccinated group (OR = 5.77 without I252 and OR = 4.65 with I252) and similar in the vaccinated group (OR = 2.80 without I252 and OR = 2.77 with I252). Hence, given the same infection rate and VE, vaccination offered a stronger protection, compared to the analysis with the I252 codes. For example, given an infection rate in the unvaccinated population of 0.08 and a VE of 0.8, vaccination lowers the risk of thromboembolic events by 17.14% without I252, compared to 6.67% in the analysis with I252. Detailed results are in Supplementary Figs. 8 and 9 . We considered the analysis that includes the I252 code as the main analysis to represent more conservative results.
We found that both COVID-19 vaccination and COVID-19 increase the risk of thromboembolic events. However, evidence implies that the likelihood of experiencing a thromboembolic event after COVID-19 is much higher than after vaccination. Our analysis agrees with previous research, indicating that COVID-19 is a more dangerous risk factor for thromboembolic events than vaccination 8 , 11 , 12 , 13 , 14 .
Different from existing work, we evaluated the association between thromboembolic events and COVID-19 using a case-control study, avoiding the misclassification issue associated with the SCCS design. We also studied the effect of prior vaccination on reducing infection-associated thromboembolic events. Moreover, we included both COVID-19 vaccination and COVID-19 in the analysis of the risk of thromboembolic events and conducted a risk-benefit analysis by comparing the magnitude of the increased risk through the direct effect of COVID-19 vaccination with the reduced risk through the indirect pathway via protection against severe diseases. Our analysis provides evidence that COVID-19 vaccination directly increases the risk of thromboembolic events, but indirectly reduces the risk of infection-associated events. Results show that the indirect benefit of preventing infection-associated thromboembolic events outweighs the direct harm if the VE and infection rate reaches certain levels. Moreover, COVID-19 vaccination may have additional benefits in preventing thromboembolic events associated with COVID-19, as a higher rate of vaccination increases the overall level of immunity in the population, reducing the spread of the virus and conferring collective protection against infection-associated thromboembolic events and other health risks associated with COVID-19.
There are several limitations to this study. First, using ICD-10 codes to identify thromboembolic events may be subject to phenotype errors. Second, Corewell Health has 22 hospitals, and the catchment area for these hospitals is across many counties, hence patients may seek care at other facilities outside the Corewell Health system, leading to missing data such as infection data. To deal with the missing infection data, we used the case-control study. Moreover, the use of a prior number of hospital visits as covariates in the regression model mitigates the bias due to differing degrees of interaction with the Corewell Health system between infected and control subjects. However, patients with a hospital visit due to injuries may not be the perfect control group, but it is clearly better than a control group of patients without thromboembolic events. Therefore, we may not totally correct the bias, but we reduce it. Finally, the study population for vaccine doses 1 and 2 are different. If a subject had a thromboembolic event after the first vaccine dose, this subject is unlikely to receive the second dose, therefore, the population who received the second dose only includes subjects who did not have a thromboembolic event after the first dose.
Despite these limitations, our study makes a critical contribution to quantifying the net risk of thromboembolic events associated with COVID-19 vaccination. It accounts for both the direct effects of vaccination and the indirect effects of protection against COVID-19 and severe diseases. The dual consideration is vital for a comprehensive understanding of the risk-benefit profile. The mechanism of vaccination is to simulate the immune response the body has against infection using a dead/attenuated virus or mRNA, which can lead to side effects similar to those of the virus, albeit in a less severe form (e.g., thromboembolic events, myocarditis 37 , acute kidney injury 38 , 39 ). Our finding highlights the necessity of evaluating both the indirect benefits and direct harms of vaccination to provide a complete and accurate assessment of vaccine safety. This comprehensive approach ensures a balanced understanding of the risks and the benefits, reinforcing the overall safety and efficacy of vaccination programs.
Our risk-benefit analysis was conducted on the population level. This analysis can also be stratified by patient groups of interest. For example, the risk-benefit of vaccination might be different between older and younger populations. Moreover, our findings are for a broad range of thromboembolic conditions, so more research is needed on the specific biological mechanisms connecting COVID-19 and mRNA vaccination to these events, both to establish causality and help identify a more specific set of conditions or risk factors.
Data availability
The datasets analyzed during the current study are not publicly available due to privacy or ethical restrictions.
Code availability
Code for this study is available from the corresponding author on request.
Centers for Disease Control and Prevention. COVID Data Tracker. Atlanta, GA: U.S. Department of Health and Human Services, CDC ; https://covid.cdc.gov/covid-data-tracker (2023).
Avinash, M. & Vineeta, O. Thromboembolism after COVID-19 vaccination: a systematic review of such events in 286 patients. Ann. Vasc. Surg. 84 , 12–20 (2022).
Article Google Scholar
Fan, B. E. et al. COVID-19 mRNA vaccine-associated cerebral venous thrombosis: rare adverse event or coincidence? Am. J. Hematol . 98 , E4–E7 (2023).
Leonor, D. et al. Cerebral venous thrombosis after BNT162b2 mRNA SARS-CoV-2 vaccine. J. Stroke Cerebrovasc. Dis . 30 , 105906 (2022).
Elizabeth, A. A., Rohan, K., Mirnal, C. & Ulka, S. Three cases of acute venous thromboembolism in females after vaccination for coronavirus disease 2019. J. Vasc. Surg. Venous Lymphat. Disord. 10 , 14–17 (2021).
Google Scholar
Giuseppe, C., Ilaria, N., Marco, R., Salvatore, B., & Alberto, T. Deep vein thrombosis (DVT) occurring shortly after the second dose of mRNA SARS-CoV-2 vaccine. Intern. Emerg. Med . 16 , 803–804 (2021).
Zaitun, Z., Nur, A. S. & Abdul, R. I. G. Cerebral venous sinus thrombosis 2 weeks after the first dose of mRNA SARS-CoV-2 vaccine. Acta Neurochir. 163 , 2359–2362 (2021).
Julia, H. et al. Risk of thrombocytopenia and thromboembolism after COVID-19 vaccination and SARS-CoV-2 positive testing: self-controlled case series study. BMJ . 374 , n1931 (2021).
Maxime, T., Masud, H., John, R. G., Sierra, L., & Paul, J. H. Cerebral venous thrombosis and portal vein thrombosis: a retrospective cohort study of 537,913 COVID-19 cases. EClinicalMedicine . 39 , 101061 (2021).
Farah, Y. et al. Adverse events following COVID‐19 mRNA vaccines: a systematic review of cardiovascular complication, thrombosis, and thrombocytopenia. Immun. Inflamm. Dis . 11 , e807 (2023).
Frederick, K. H. et al. Thromboembolic risk in hospitalized and nonhospitalized COVID-19 patients: a self-controlled case series analysis of a nationwide cohort. Mayo Clin. Proc . 96 , 2587–2597 (2021).
Xiaoming, X., Jianhua, C., & Qinglei, G. Prevalence and risk factors of thrombotic events on patients with COVID-19: a systematic review and meta‐analysis. Thromb. J . 19 , 32 (2021).
Sam, S., Yu, H., & Stavros, K. Venous thromboembolism in COVID-19. Thromb. Haemost . 120 , 1642–1653 (2020).
Ioannis, K., Osvaldo, F., Paddy, F., Krister, L., & Anne-Marie, F. C. Risk of acute myocardial infarction and ischaemic stroke following COVID-19 in Sweden: a self-controlled case series and matched cohort study. Lancet . 398 , 599–607 (2021).
Mahmoud, B. M. et al. Thromboembolism risk of COVID-19 is high and associated with a higher risk of mortality: a systematic review and meta-analysis. EClinicalMedicine . 29 , 100639 (2020).
Ahuja, N. et al. Venous thromboembolism in patients with COVID-19 infection: risk factors, prevention, and management. Semin. Vasc. Surg . 34 , 101–116 (2021).
Yu, Y. et al. Incidence and risk factors of deep vein thrombosis in hospitalized COVID-19 patients. Clin. Appl. Thromb. Hemost . 26 , 1076029620953217 (2020).
Klok, F. A. et al. Confirmation of the high cumulative incidence of thrombotic complications in critically ill ICU patients with COVID-19: an updated analysis. Thromb. Res. 191 , 148–150 (2020).
Article CAS PubMed PubMed Central Google Scholar
Simpson, C. R. et al. First-dose ChAdOx1 and BNT162b2 COVID-19 vaccines and thrombocytopenic, thromboembolic and hemorrhagic events in Scotland. Nat. Med. 29 , 1290–1297 (2021).
Marie, J. J. et al. Myocardial infarction, stroke, and pulmonary embolism after BNT162b2 mRNA COVID-19 vaccine in people aged 75 years or older. JAMA 327 , 80–82 (2022).
Jacob, D. B. et al. Analysis of thromboembolic and thrombocytopenic events after the AZD1222, BNT162b2, and MRNA-1273 COVID-19 vaccines in 3 Nordic Countries. JAMA Netw. Open . 5 , e2217375 (2022).
Chui, C. S. L. et al. Thromboembolic events and hemorrhagic stroke after mRNA (BNT162b2) and inactivated (CoronaVac) covid-19 vaccination: a self-controlled case series study. EClinicalMedicine 50 , 101504 (2020).
Whitaker, H. J., Farrington, C. P., Spiessens, B., & Musonda P. Tutorial in biostatistics: the self-controlled case series method. Stat. Med . 25 , 1768–1797 (2006).
Gareth, J. G. et al. Collider bias undermines our understanding of COVID-19 disease risk and severity. Nat. Commun . 11 , 5749 (2020).
Bu, F. et al. Bayesian safety surveillance with adaptive bias correction. Stat. Med. 43 (2), 395–418 (2023).
Article PubMed Google Scholar
Knudson, M. M., Ikossi, D. G., Khaw, L., Morabito, D. & Speetzen, L. S. Thromboembolism after trauma: an analysis of 1602 episodes from the American College of Surgeons National Trauma Data Bank. Ann. Surg. 240 , 490–498 (2004).
Article PubMed PubMed Central Google Scholar
Van, S. K. J., Rosendaal, F. R., & Doggen, C. J. Minor injuries as a risk factor for venous thrombosis. Arch. Intern. Med . 168 , 21–26 (2008).
Charlson, M. E., Pompei, P., Ales, K. L. & MacKenzie, C. R. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J. Chronic Dis. 40 , 373–383 (1987).
Article CAS PubMed Google Scholar
Gasparini, A. An R package for computing comorbidity scores. J. Open Source Softw. 3 , 648 (2018).
Fleming-Dutra, K. E. et al. Association of prior BNT162b2 COVID-19 vaccination with symptomatic SARS-CoV-2 infection in children and adolescents during omicron predominance. JAMA 327 , 2210–2219 (2022).
Katherine, E. F. et al. Preliminary estimates of effectiveness of monovalent mRNA vaccines in preventing symptomatic SARS-CoV-2 infection among children aged 3–5 years—increasing community access to testing program, United States, July 2022–February 2023. MMWR Morb. Mortal. Wkly. Rep. 72 , 177–182 (2023).
Kelly, M. H. et al. Effectiveness of coronavirus disease 2019 (COVID-19) vaccination against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection among residents of US nursing homes before and during the delta variant predominance. Clin. Infect. Dis . 75 , S147–S154 (2022).
Silverstein, M. D., Heit, J. A., Mohr, D. N., Petterson, T. M., O’Fallon, W. M., & Melton. L. J. Trends in the incidence of deep vein thrombosis and pulmonary embolism: a 25-year population-based study. Arch. Intern. Med . 158 , 585–593 (1999).
Chen, S. et al. Efficacy of COVID-19 vaccines in patients taking immunosuppressants. Ann. Rheum. Dis. 81 , 875–880 (2022).
Malcolm, R. et al. Comparative effectiveness of coronavirus disease 2019 (COVID-19) vaccines against the delta variant. Clin. Infect. Dis . 75 , e623–e629 (2022).
Malcolm, R. et al. COVID-19 vaccine effectiveness against omicron (B.1.1.529) variant infection and hospitalisation in patients taking immunosuppressive medications: a retrospective cohort study. Lancet Rheumatol. 4 , e775–e784 (2022).
Martian, P. et al. Risk of myocarditis after sequential doses of COVID-19 vaccine and SARS-CoV-2 infection by age and sex. Circulation . 146 , 743–754 (2022).
Huiting, L. et al. Acute kidney injury after COVID-19 vaccines: a real-world study. Ren. Fail . 44 , 958–965 (2022).
Fabrizi, F. et al. COVID-19 and acute kidney injury: a systematic review and meta-analysis. Pathogens 9 , 1052 (2020).
Download references
Acknowledgements
We thank Kevin Heinrich at Quire and Martin Witteveen-Lane for querying the data from the Corewell Health Epic system. This study was funded by the National Institute of Allergy and Infectious Diseases of the National Institutes of Health under award number R01AI158543. The funder played no role in the study design, data collection, analysis, and interpretation of data, or the writing of this manuscript.
Author information
Authors and affiliations.
Division of Biostatistics & Health Informatics, Corewell Health Research Institute, Royal Oak, MI, USA
Huong N. Q. Tran
Department of Biostatistics, University of Michigan, Ann Arbor, MI, USA
Malcolm Risk
William Beaumont University Hospital, Corewell Health East, Royal Oak, MI, USA
Girish B. Nair
Division of Biostatistics, Department of Preventive Medicine, Northwestern University, Chicago, IL, USA
You can also search for this author in PubMed Google Scholar
Contributions
H.N.Q.T.: manuscript writing, study design, statistical analysis, and data preparation. M.R.: manuscript writing and study design. G.B.: clinical advice and study design. L.Z.: manuscript writing, method development, study design, and statistical analysis.
Corresponding author
Correspondence to Lili Zhao .
Ethics declarations
Competing interests.
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary information, rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/ .
Reprints and permissions
About this article
Cite this article.
Tran, H.N.Q., Risk, M., Nair, G.B. et al. Risk benefit analysis to evaluate risk of thromboembolic events after mRNA COVID-19 vaccination and COVID-19. npj Vaccines 9 , 166 (2024). https://doi.org/10.1038/s41541-024-00960-7
Download citation
Received : 06 May 2024
Accepted : 29 August 2024
Published : 13 September 2024
DOI : https://doi.org/10.1038/s41541-024-00960-7
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily.
Moderna slashes research budget as vaccine sales disappoint, but no layoffs planned

Moderna aims to reduce its research and development budget by about 20 percent over the next three years as the biotech tries to find a path to profitability following disappointing vaccine sales.
The Cambridge-based company is discontinuing five programs in its pipeline and slowing some late-stage studies of treatments for latent and rare diseases to achieve the $1.1 billion cut from its annual R&D budget by 2027, according to a statement Thursday.
The plan may appease investors who want to see the company avoid further losses. In a recent note, Jefferies analyst Michael Yee said Moderna needed to cut as much as $1 billion in expenses “to regain any investor confidence or credibility on profitability.”
Shares dropped by 7 percent in premarket trading on Thursday and were down 17 percent by mid-morning. Through Wednesday’s close, Moderna’s shares have dropped about 20 percent this year.
Advertisement
Moderna spokesman Chris Ridley said the company, which has about 6,000 employees worldwide, has no plans for layoffs.
The company is slowing its pace of studying new treatments in part because of recent commercial challenges. On Thursday, it also pushed back its target to break even from 2026 to 2028.
Last month, it cut sales expectations for the year after seeing low COVID vaccine revenue in Europe and increased competition in the US, leading to a disappointing launch of its new RSV vaccine.
The company sells two products — a vaccine for COVID and another for RSV. It projected sales of between $2.5 billion and $3.5 billion next year. It has said this year’s sales will range between $3 billion and $3.5 billion, down from its previous outlook of about $4 billion.
“We are still dealing with a market of uncertainty,” Moderna Chief Financial Officer Jamey Mock said in an interview. “We hope that will settle out this year but we have to brace ourselves just in case vaccination rates continue to go down.”
Mock said the R&D cuts are a sign that the company “is exercising financial discipline.”
Moderna needed to reduce its R&D budget in part because its clinical trials have succeeded and later-stage studies require more funding, he said. Moderna expects 10 products to get approved over the next three years.
“We do recognize the need to pace ourselves because there is now this huge bolus of important medicines to get approved,” Moderna President Stephen Hoge said in an interview.
The company is known for spending heavily on R&D, often more than its peers as a percentage of sales. The spending has been fueled by the belief that its mRNA technology could effectively treat and prevent a range of illnesses, from flu to cancer. But the sharp decline of its COVID vaccine business is forcing the company to rein in some of its ambitions.
One of its biggest hopes is its cancer program. Late last year, Moderna and its partner, Merck & Co., said its melanoma vaccine helped prevent the recurrence of severe skin cancer for three years.
Moderna executives have hoped to file for faster approval based on data from that mid-stage study. Moderna said initial feedback from US regulators “has not been supportive” of that, and the company and Merck are focused on its late-stage trial.
The company said it plans to increase its research and development investments in oncology.
It’s also no longer pursuing an accelerated approval for its standalone flu vaccine. Instead, it will focus on filing for approval this year for its vaccine that combines flu and Covid protection.
“We just think that has the chance to be a bigger product and have a larger impact,” Mock said.
Jonathan Saltzman of the Globe staff contributed to this report.

IMAGES
COMMENTS
In this rapid living systematic evidence synthesis and meta-analysis, we searched EMBASE and the US National Institutes of Health's iSearch COVID-19 Portfolio, supplemented by manual searches of COVID-19-specific sources, until Dec 1, 2022, for studies that reported vaccine effectiveness immediately and at least 112 days after a primary vaccine series or at least 84 days after a booster dose.
Discussion. A two-dose regimen of BNT162b2 (30 μg per dose, given 21 days apart) was found to be safe and 95% effective against Covid-19. The vaccine met both primary efficacy end points, with ...
Community‐based studies in five countries show consistent strong benefits from early rollouts of COVID‐19 vaccines. By the beginning of June 2021, almost 11% of the world's population had received at least one dose of a coronavirus disease 2019 (COVID‐19) vaccine. 1 This represents an extraordinary scientific and logistic achievement — in 18 months, researchers, manufacturers and ...
Despite questions remain about the impact of virus variants and the duration of the immune response, messenger RNA (mRNA)-based and adenoviral vectored vaccines have demonstrated an overall efficacy from 70 to 95% in both phase III trials and real life. In addition, all these vaccines also reduce the severe forms of the disease and might ...
Safety and adverse effects of current COVID-19 vaccines. As shown in Table I, current vaccines have demonstrated considerable efficacy in diminishing mild, moderate and severe cases with a low risk of adverse events 21.For some of these vaccines [such as Convidicea (AD5-nCoV), Janssen (Ad26.COV2.S), Sinopharm (BBIBP-CorV), Covaxin (BBV152) and Sinovac (CoronaVac)], there is the information ...
The protective effects of vaccination and prior infection against severe Covid-19 are reviewed, with proposed directions for future research, including mucosal immunity and intermittent vaccine boo...
The persisting risk of long-term health consequences of SARS-CoV-2 infection and the protection against such risk conferred by COVID-19 vaccination remains unclear. Here we conducted a ...
No vaccine was statistically significantly associated with a decreased risk for severe COVID-19 than other vaccines, although mRNA-1273 and Gam-COVID-Vac have the highest P-scores (0.899 and 0.816 ...
Although Covid-19 vaccines have been recommended for adults with chronic medical conditions ... The activity reported in this article was deemed not to be research as defined in 45 Code of Federal ...
Objectives This meta-analysis evaluated the Efficacy and Effectiveness of several COVID-19 vaccines, including AstraZeneca, Pfizer, Moderna, Bharat, and Johnson & Johnson, to better estimate their immunogenicity, benefits, or side effects. Methods Studies reporting the Efficacy and Effectiveness of COVID-19 vaccines from November 2020 to April 2022 were included. The pooled Effectiveness ...
There is no question that the current vaccines are effective and safe. The risk of severe reaction to a COVID-19 jab, say researchers, is outweighed by the protection it offers against the deadly ...
The effectiveness of the mRNA vaccines in preventing COVID-19 disease progression in 2021 set new expectations about the role of prevention interventions for the disease. Efficacy observed in the trials was more than 90%.1,2 The efficacy of other vaccines evaluated in large randomised trials, such as the Oxford-AstraZeneca (70%) and Sputnik V (91%) vaccines, have been criticised for elements ...
The first COVID-19 vaccine was delivered outside of a clinical trial setting on Dec 8, 2020. 1 By Dec 8, 2021, 55·9% of the global population was estimated to have received at least one dose of a COVID-19 vaccine, 45·5% estimated to have received two doses, and 4·3% estimated to have received a booster dose. 2 Despite the incredible speed ...
The investigational vaccine known as mRNA-1273 was 94.1% efficacious in preventing symptomatic coronavirus disease 2019 (COVID-19), according to preliminary results from a Phase 3 clinical trial reported in the New England Journal of Medicine. The vaccine also demonstrated efficacy in preventing severe COVID-19.
The duration of protection afforded by coronavirus disease 2019 (Covid-19) vaccines in the United States is unclear. Whether the increase in postvaccination infections during the summer of 2021 was...
Implications for research Future research should evaluate the long-term effect of vaccines, compare different vaccines and vaccine schedules, assess vaccine efficacy and safety in specific populations, and include outcomes such as preventing long COVID-19. Ongoing evaluation of vaccine efficacy and effectiveness against emerging variants of ...
2. Vaccination strategies. Many efforts have been directed towards the development of the vaccines against COVID-19, to avert the pandemic and most of the developing vaccine candidates have been using the S-protein of SARS-CoV-2 (Dhama et al., 2020).As of July 2, 2020, the worldwide SARS-CoV-2 vaccine landscape includes 158 vaccine candidates, out of which 135 are in the preclinical or the ...
Study design. This is a test negative case-control study combined with genome-wide association study (GWAS) analysis. We included participants who completed the full mRNA COVID-19 vaccination regimen and had COVID-19 testing due to encountering COVID-like symptoms during the outbreak of the Omicron variant in Taiwan, aiming to assess the genetic variants that may influence the sustainability ...
Genetic polymorphisms have been linked to the differential waning of vaccine-induced immunity against COVID-19 following vaccination. Despite this, evidence on the mechanisms behind this waning and its implications for vaccination policy remains limited. We hypothesize that specific gene variants ma …
Vaccine-induced Th1 cell response. Some COVID-19 vaccines would induce Th1 cell responses. After recognition of the AP-MHC class II complex and T-cell receptor (TCR), CD4 + T cells distributed in ...
In addition to the discovery of this antibody, the research found that hybrid immunity — a combination of both infection and vaccination — offers increased antibody-based protection against future exposure compared with infection or vaccination alone. The work comes amid another summer COVID spike. This trend shows that while the worst of ...
Students receiving the COVID 19 vaccine at Hawley Armory on April 8, 2021. The rollout of the vaccines across the state in the spring helped set up a return to a more familiar university experience. ... "We thought that was interesting because there was a lot of research coming out about sex-specific differences and COVID-19 illness severity ...
Introduction. The coronavirus disease 2019 (COVID-19) pandemic caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has resulted in over 192 million cases and 4.1 million deaths as of July 22, 2021. 1 This pandemic has brought along a massive burden in morbidity and mortality in the healthcare systems. Despite the implementation of stringent public health measures, there ...
The Coronavirus Efficacy (COVE) phase 3 trial was launched in late July 2020 to assess the safety and efficacy of the mRNA-1273 vaccine in preventing SARS-CoV-2 infection. An independent data and ...
Sept. 11, 2024 - Social media platforms lit up minutes after the FDA announced its authorization of the updated Novavax COVID-19 vaccine right before Labor Day weekend. It was the last of three ...
1. Introduction. Numerous medicines have been used for the treatment of coronavirus disease 2019 (COVID-19) during the past year. Although most of the medicines failed to show efficacy in treating COVID-19, researchers have encouraged herd immunity to control the current pandemic [1,2].Vaccination is a safe, simple, and effective way of protecting a person against COVID-19.
Specifically, the pharma and biotech company plans to slash its research and development budget by 20% over the next three years to $16 billion from a prior budget outlay of $20 billion.In ...
BNT162b2 Vaccine against Covid-19 in 5-to-11-Year-Olds. E.B. Walter and OthersN Engl J Med 2022;386:35-46. After a dose for further testing was determined in a phase 1 study, a phase 2-3 trial ...
The Coronavirus Disease 2019 (COVID-19) pandemic prompted a race to develop and distribute effective vaccines. Approximately 81.4% of the US population have been vaccinated with at least one dose ...
Moderna aims to reduce its research and development budget by about 20 percent over the next three years as the biotech tries to find a path to profitability following disappointing vaccine sales.