
- Science Notes Posts
- Contact Science Notes
- Todd Helmenstine Biography
- Anne Helmenstine Biography
- Free Printable Periodic Tables (PDF and PNG)
- Periodic Table Wallpapers
- Interactive Periodic Table
- Periodic Table Posters
- Science Experiments for Kids
- How to Grow Crystals
- Chemistry Projects
- Fire and Flames Projects
- Holiday Science
- Chemistry Problems With Answers
- Physics Problems
- Unit Conversion Example Problems
- Chemistry Worksheets
- Biology Worksheets
- Periodic Table Worksheets
- Physical Science Worksheets
- Science Lab Worksheets
- My Amazon Books

Representative Elements on the Periodic Table

In chemistry, the representative elements are the elements with atoms filling s and p electron orbitals. Another name for the representative elements is the main group elements . The representative elements are groups 1 and 2 and group 13-17 on the periodic table . The outer electron shell is not filled for these elements, giving them a valence electron configuration of ns 1-2 ( s -block elements) and np 1-5 ( p -block elements). Some sources also include group 18.
Are the Noble Gases Representative Elements?
Whether or not the noble gases or group 18 elements are representative elements depends on who you ask. On the one hand, they are s – and p -block elements. On the other hand, they have filled valence shells and are not very reactive.
Why Are They Called Representative Elements?
Representative elements get their name because each element shares or represents the properties of other elements in its group. An element in a periodic table group shares the same number of valence electrons and electron configuration as other elements in the group. For example, lithium and sodium are both elements in group 1 (the alkali metals) and have 1 valence electron and similar properties. In contrast, valence and electron configuration gets less clear-cut with the transition metals , lanthanides , and actinides ( d – and f -block elements).
Examples of Representative Elements
Elements belonging to groups 1, 2, 13-17 are representative elements Elements belonging to group 3-16 (and 18, usually) are not representative elements. In other words, the transition metals, lanthanides, actinides, and (usually) noble gases are not representative elements.
For example, lithium, sodium, and potassium are representative of group 1. Beryllium and strontium are representative of group 2. Carbon and silicon are representative of group 14. Hydrogen, magnesium, gallium, and iodine are all representative elements. Titanium, iron, and uranium are not representative elements.
S-Block and P-Block Properties
The properties of the representative elements depends on whether they are s -block or p -block elements. Overall, the s -block elements are most like each other than the p -block elements.
S-Block Properties
- S-block elements have a single oxidation state. For group 1 (alkali metals) this is +1. For group 2 (alkaline earths) this is +2. Of course, there are exception. For example, hydrogen usually takes a +1 oxidation state, but sometimes has a -1 oxidation state.
- S-block elements are highly reactive. Helium is the exception here, but it isn’t normally considered to be a representative element.
- These elements are soft reactive metals (again, except helium), with low melting and boiling points.
P-Block Properties
- P-block elements typically display multiple oxidation states, favoring states separated by two units. For example, the oxidation states of sulfur are -2 , 0, +2 , +4, +6 (where the bold states are more common).
- This collection of elements includes nonmetals, metalloids, and metals. However, elements within a group still share some common properties.
Importance of the Representative Elements
The representative elements include the majority of the most abundant elements on Earth and in the universe. Similarly, they include many of the elements essential to organic molecules and life. Most commercial projects are rich in these elements.
- Jensen, William B. (2003). “The Place of Zinc, Cadmium, and Mercury in the Periodic Table”. Journal of Chemical Education . 80 (8): 952. doi: 10.1021/ed080p952
- Steudel, Ralf (1998). Chemie der Nichtmetalle (Chemistry of the Nonmetals) (2nd ed.). Berlin: Walter deGruyter. ISBN 3-11-012322-3.
- Yao, Benzhen; Kuznetsov, Vladimir L.; et al. (2020). “Metals and non-metals in the periodic table”. Philosophical Transactions of the Royal Society A . 378. doi: 10.1098/rsta.2020.0213
Related Posts

- History & Society
- Science & Tech
- Biographies
- Animals & Nature
- Geography & Travel
- Arts & Culture
- Games & Quizzes
- On This Day
- One Good Fact
- New Articles
- Lifestyles & Social Issues
- Philosophy & Religion
- Politics, Law & Government
- World History
- Health & Medicine
- Browse Biographies
- Birds, Reptiles & Other Vertebrates
- Bugs, Mollusks & Other Invertebrates
- Environment
- Fossils & Geologic Time
- Entertainment & Pop Culture
- Sports & Recreation
- Visual Arts
- Demystified
- Image Galleries
- Infographics
- Top Questions
- Britannica Kids
- Saving Earth
- Space Next 50
- Student Center
- Introduction & Top Questions
- The first periodic table
- Other versions of the periodic table
- Discovery of new elements
- Significance of atomic numbers
- Elucidation of the periodic law
- Classification of elements into groups
- Periodic trends in properties
- Electronic structure
- Periodicity of properties of the elements
- Other chemical and physical classifications


What is the periodic table?
Where does the periodic table come from, why does the periodic table split.
- Is mathematics a physical science?
- How is the atomic number of an atom defined?

periodic table
Our editors will review what you’ve submitted and determine whether to revise the article.
- UEN Digital Press with Pressbooks - Introductory Chemistry - The Periodic Table
- Chemistry LibreTexts - The Periodic Table
- Ohio State University - Origins - Mendeleev's Periodic Table
- National Center for Biotechnology Information - PubChem - Periodic Table of Elements
- NeoK12 - Educational Videos and Games for School Kids - Periodic Table
- LiveScience - Periodic Table of the Elements
- Khan Academy - The periodic table, electron shells, and orbitals
- Western Oregon University - A brief history of the development of Periodic Table
- Royal Society of Chemistry - Patterns in the Periodic Table
- periodic table - Children's Encyclopedia (Ages 8-11)
- periodic table - Student Encyclopedia (Ages 11 and up)
- Table Of Contents

The periodic table is a tabular array of the chemical elements organized by atomic number , from the element with the lowest atomic number, hydrogen , to the element with the highest atomic number, oganesson . The atomic number of an element is the number of protons in the nucleus of an atom of that element. Hydrogen has 1 proton, and oganesson has 118.
What do periodic table groups have in common?
The groups of the periodic table are displayed as vertical columns numbered from 1 to 18. The elements in a group have very similar chemical properties, which arise from the number of valence electrons present—that is, the number of electrons in the outermost shell of an atom .
The arrangement of the elements in the periodic table comes from the electronic configuration of the elements. Because of the Pauli exclusion principle , no more than two electrons can fill the same orbital. The first row of the periodic table consists of just two elements, hydrogen and helium . As atoms have more electrons, they have more orbits available to fill, and thus the rows contain more elements farther down in the table.
The periodic table has two rows at the bottom that are usually split out from the main body of the table. These rows contain elements in the lanthanoid and actinoid series, usually from 57 to 71 ( lanthanum to lutetium ) and 89 to 103 ( actinium to lawrencium ), respectively. There is no scientific reason for this. It is merely done to make the table more compact.
periodic table , in chemistry , the organized array of all the chemical elements in order of increasing atomic number —i.e., the total number of protons in the atomic nucleus. When the chemical elements are thus arranged, there is a recurring pattern called the “periodic law” in their properties, in which elements in the same column (group) have similar properties. The initial discovery, which was made by Dmitry I. Mendeleev in the mid-19th century, has been of inestimable value in the development of chemistry .
It was not actually recognized until the second decade of the 20th century that the order of elements in the periodic system is that of their atomic numbers, the integers of which are equal to the positive electrical charges of the atomic nuclei expressed in electronic units. In subsequent years great progress was made in explaining the periodic law in terms of the electronic structure of atoms and molecules. This clarification has increased the value of the law, which is used as much today as it was at the beginning of the 20th century, when it expressed the only known relationship among the elements.
History of the periodic law

The early years of the 19th century witnessed a rapid development in analytical chemistry—the art of distinguishing different chemical substances—and the consequent building up of a vast body of knowledge of the chemical and physical properties of both elements and compounds . This rapid expansion of chemical knowledge soon necessitated classification , for on the classification of chemical knowledge are based not only the systematized literature of chemistry but also the laboratory arts by which chemistry is passed on as a living science from one generation of chemists to another. Relationships were discerned more readily among the compounds than among the elements; it thus occurred that the classification of elements lagged many years behind that of compounds. In fact, no general agreement had been reached among chemists as to the classification of elements for nearly half a century after the systems of classification of compounds had become established in general use.

J.W. Döbereiner in 1817 showed that the combining weight, meaning atomic weight , of strontium lies midway between those of calcium and barium , and some years later he showed that other such “ triads ” exist (chlorine, bromine , and iodine [halogens] and lithium , sodium , and potassium [alkali metals]). J.-B.-A. Dumas, L. Gmelin, E. Lenssen, Max von Pettenkofer, and J.P. Cooke expanded Döbereiner’s suggestions between 1827 and 1858 by showing that similar relationships extended further than the triads of elements, fluorine being added to the halogens and magnesium to the alkaline-earth metals, while oxygen , sulfur , selenium , and tellurium were classed as one family and nitrogen , phosphorus , arsenic , antimony , and bismuth as another family of elements.
Attempts were later made to show that the atomic weights of the elements could be expressed by an arithmetic function , and in 1862 A.-E.-B. de Chancourtois proposed a classification of the elements based on the new values of atomic weights given by Stanislao Cannizzaro’s system of 1858. De Chancourtois plotted the atomic weights on the surface of a cylinder with a circumference of 16 units, corresponding to the approximate atomic weight of oxygen. The resulting helical curve brought closely related elements onto corresponding points above or below one another on the cylinder, and he suggested in consequence that “the properties of the elements are the properties of numbers,” a remarkable prediction in the light of modern knowledge.
Classification of the elements

In 1864, J.A.R. Newlands proposed classifying the elements in the order of increasing atomic weights, the elements being assigned ordinal numbers from unity upward and divided into seven groups having properties closely related to the first seven of the elements then known: hydrogen , lithium, beryllium , boron , carbon , nitrogen, and oxygen. This relationship was termed the law of octaves, by analogy with the seven intervals of the musical scale.
Then in 1869, as a result of an extensive correlation of the properties and the atomic weights of the elements, with special attention to valency (that is, the number of single bonds the element can form), Mendeleev proposed the periodic law, by which “the elements arranged according to the magnitude of atomic weights show a periodic change of properties.” Lothar Meyer had independently reached a similar conclusion, published after the appearance of Mendeleev’s paper.
Periodic Table of Elements
Enable JavaScript for a plethora of interactivity including property trend visualization, thousands of isotopes, compound mixing, and 3D orbital diagrams. Don't like ads? No problem! Ptable will always be free for everyone. Find yourself here daily? Consider either unblocking the single ad banner, donating $1 a month ( log in after donating ), or buying a poster or wallet card , order number
- Atomic number
- Property values
- Logarithmic
- Exponential
- 1 H Hydrogen 1.008
- 2 He Helium 4.0026
- 3 Li Lithium 6.94
- 4 Be Beryllium 9.0122
- 5 B Boron 10.81
- 6 C Carbon 12.011
- 7 N Nitrogen 14.007
- 8 O Oxygen 15.999
- 9 F Fluorine 18.998
- 10 Ne Neon 20.180
- 11 Na Sodium 22.990
- 12 Mg Magnesium 24.305
- 13 Al Aluminium 26.982
- 14 Si Silicon 28.085
- 15 P Phosphorus 30.974
- 16 S Sulfur 32.06
- 17 Cl Chlorine 35.45
- 18 Ar Argon 39.948
- 19 K Potassium 39.098
- 20 Ca Calcium 40.078
- 21 Sc Scandium 44.956
- 22 Ti Titanium 47.867
- 23 V Vanadium 50.942
- 24 Cr Chromium 51.996
- 25 Mn Manganese 54.938
- 26 Fe Iron 55.845
- 27 Co Cobalt 58.933
- 28 Ni Nickel 58.693
- 29 Cu Copper 63.546
- 30 Zn Zinc 65.38
- 31 Ga Gallium 69.723
- 32 Ge Germanium 72.630
- 33 As Arsenic 74.922
- 34 Se Selenium 78.971
- 35 Br Bromine 79.904
- 36 Kr Krypton 83.798
- 37 Rb Rubidium 85.468
- 38 Sr Strontium 87.62
- 39 Y Yttrium 88.906
- 40 Zr Zirconium 91.224
- 41 Nb Niobium 92.906
- 42 Mo Molybdenum 95.95
- 43 Tc Technetium (98)
- 44 Ru Ruthenium 101.07
- 45 Rh Rhodium 102.91
- 46 Pd Palladium 106.42
- 47 Ag Silver 107.87
- 48 Cd Cadmium 112.41
- 49 In Indium 114.82
- 50 Sn Tin 118.71
- 51 Sb Antimony 121.76
- 52 Te Tellurium 127.60
- 53 I Iodine 126.90
- 54 Xe Xenon 131.29
- 55 Cs Caesium 132.91
- 56 Ba Barium 137.33
- 57 La Lanthanum 138.91
- 58 Ce Cerium 140.12
- 59 Pr Praseodymium 140.91
- 60 Nd Neodymium 144.24
- 61 Pm Promethium (145)
- 62 Sm Samarium 150.36
- 63 Eu Europium 151.96
- 64 Gd Gadolinium 157.25
- 65 Tb Terbium 158.93
- 66 Dy Dysprosium 162.50
- 67 Ho Holmium 164.93
- 68 Er Erbium 167.26
- 69 Tm Thulium 168.93
- 70 Yb Ytterbium 173.05
- 71 Lu Lutetium 174.97
- 72 Hf Hafnium 178.49
- 73 Ta Tantalum 180.95
- 74 W Tungsten 183.84
- 75 Re Rhenium 186.21
- 76 Os Osmium 190.23
- 77 Ir Iridium 192.22
- 78 Pt Platinum 195.08
- 79 Au Gold 196.97
- 80 Hg Mercury 200.59
- 81 Tl Thallium 204.38
- 82 Pb Lead 207.2
- 83 Bi Bismuth 208.98
- 84 Po Polonium (209)
- 85 At Astatine (210)
- 86 Rn Radon (222)
- 87 Fr Francium (223)
- 88 Ra Radium (226)
- 89 Ac Actinium (227)
- 90 Th Thorium 232.04
- 91 Pa Protactinium 231.04
- 92 U Uranium 238.03
- 93 Np Neptunium (237)
- 94 Pu Plutonium (244)
- 95 Am Americium (243)
- 96 Cm Curium (247)
- 97 Bk Berkelium (247)
- 98 Cf Californium (251)
- 99 Es Einsteinium (252)
- 100 Fm Fermium (257)
- 101 Md Mendelevium (258)
- 102 No Nobelium (259)
- 103 Lr Lawrencium (266)
- 104 Rf Rutherfordium (267)
- 105 Db Dubnium (268)
- 106 Sg Seaborgium (269)
- 107 Bh Bohrium (270)
- 108 Hs Hassium (277)
- 109 Mt Meitnerium (278)
- 110 Ds Darmstadtium (281)
- 111 Rg Roentgenium (282)
- 112 Cn Copernicium (285)
- 113 Nh Nihonium (286)
- 114 Fl Flerovium (289)
- 115 Mc Moscovium (290)
- 116 Lv Livermorium (293)
- 117 Ts Tennessine (294)
- 118 Og Oganesson (294)

Periodic Table of Elements
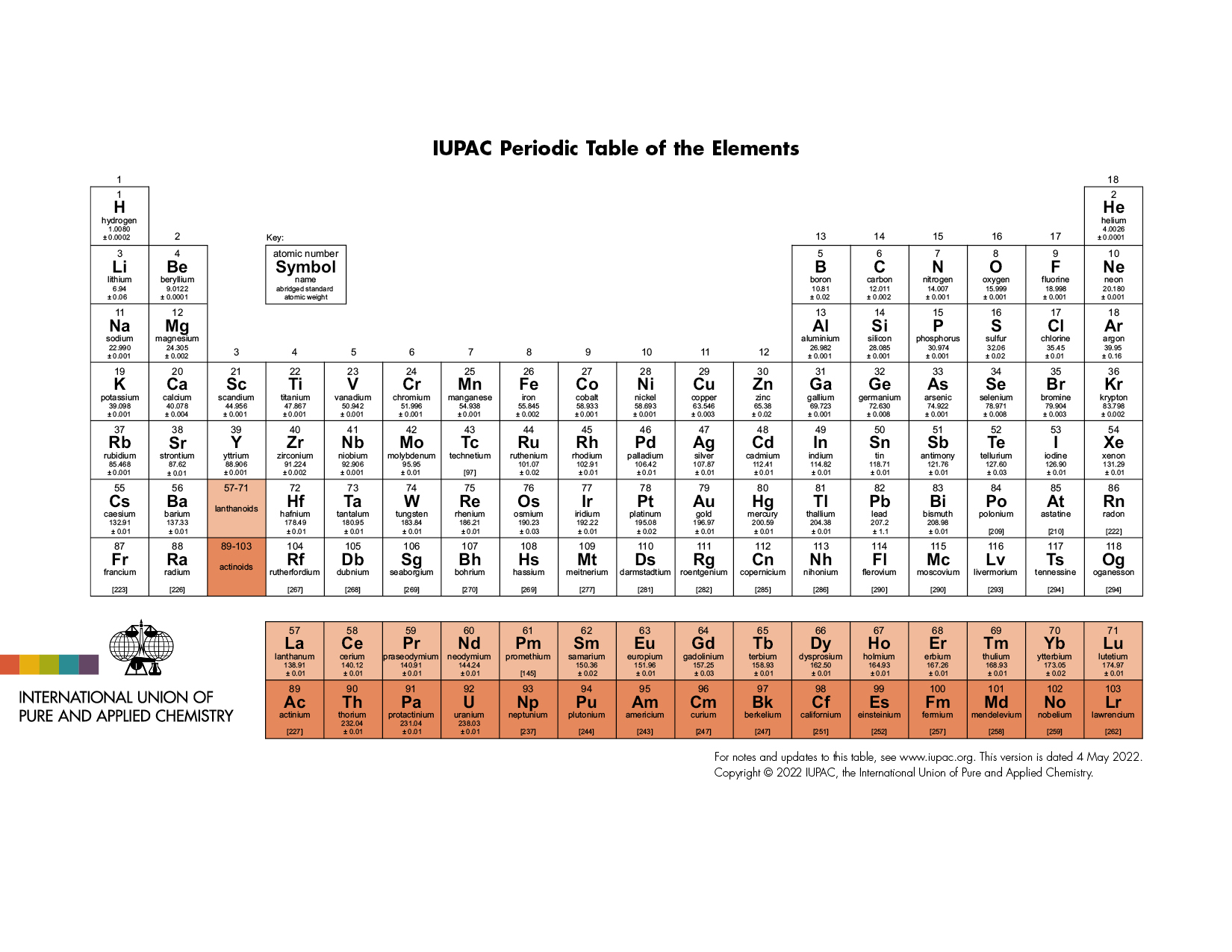
The latest release of the Periodic Table (dated 4 May 2022) includes the most recent abridged standard atomic weight values released by the IUPAC Commission on Isotopic Abundances and Atomic Weights ( CIAAW ), compiled as part of the 2021 Table of Standard Atomic Weights 2021. For elements that lack isotopes with a characteristic isotopic abundance in natural terrestrial samples, the mass number of the nuclide with the longest confirmed half-life is listed between square brackets. See PAC (AOP 4 May 2022; https://doi.org/10.1515/pac-2019-0603 ) for full details or visit Commission II.1 @ ciaaw.org
Download the PDF version (letter size or A4) or A3 (PDF) or see earlier versions
Check out SPECIAL Chem Int Jan 2019 — International Year of the Periodic Table (IYPT) — with contributions by Jan Reedijk, Natalia Tarasova, G.J. Leigh, Sigurd Hofmann, Eric Scerri, Juris Meija, Norman E. Holden, Tyler B. Coplen, Peter Mahaffy, Ian Mills, Roberto Marquardt, and more.
– Follow IUPAC project 2007-038-3-200 and follow-up project 2014-024-1-200 – Read “Atomic Weights: No Longer Constants of Nature”, Chem Int 33(2), 10–15 (2011), https://doi.org/10.1515/ci.2011.33.2.10 – Explore the interactive version at iupac.org/isotopes-matter (or see release ) – Learn more Why Isotopes Matter! https://iupac.org/100/stories/why-isotopes-matter/ – Review the latest IPTEI element-by-element review including a chart of all known stable and radioactive isotopes for each element and examples practical applications of isotopic measurements and technologies https://iupac.org/iptei/ – Access at http://ciaaw.org/periodic-table-isotopes.htm a full resolution of this Table as PDF (made available by King’s Center for Visualization in Science).
By virtue of its work in relation with the chemical elements, IUPAC can dispense a periodic table that is up-to-date. IUPAC involvement covers various aspects of the table and data that it unveils, and several reports and recommendations, some quite recent, attest of that input.
In particular, IUPAC is directly involved in the following:
- establishing the criteria for a new element discovery
- defining the structure of a temporary name and symbol
- assessing claims resulting in the validation and assignation of an element discovery
- coordinating the naming of a new element , involving the research laboratory and allowing for public comments
- setting up precise rules for how to name a new element
- defining Group 1-18 and collective names
- determining which elements belong to Group 3
- regularly reviewing standard atomic weights
The table is yours to use . Details about the latest release are provided above . Details below provide multiple references to IUPAC journal in Pure and Applied Chemistry ( PAC ) and magazine Chemistry International ( CI ).
- Criteria for a new element discovery
Assessing if an element has been “discovered” is not a simple task. While reviewing the discovery profiles of the transfermium elements in the early 90s’, IUPAC and IUPAP set up to establish a series of criteria that must be satisfied for the discovery of an element to be recognized. See details in PAC 1991, Vol. 63, No. 6, pp. 879-886 ( https://doi.org/10.1351/pac199163060879 ) and PAC 1993, Vol. 65, No. 8, pp. 1757-1814 ( https://doi.org/10.1351/pac199365081757 )
In Nov 2018, a provisional report ON THE DISCOVERY OF NEW ELEMENTS was released by IUPAC/IUPAP. Criteria and guidelines for establishing priority of discovery of potential new elements are presented. — learn more
- Temporary name and symbol
While an element can have been claimed, before the claim has been validated and before the element is formally named, the element has a temporary name and symbol. The pertinent recommendations setting-up that systematic nomenclature was published in 1978; see PAC 1979, Vol. 51, No. 2, pp. 381-384; https://doi.org/10.1351/pac197951020381
In result, that is how, in March 2016, element 113 was called ununtrium or with the symbol Uut.
The story behind the three-letter symbols is recounted in a feature prepared by Lars Öhrström and Norman Holden and published in Chem Int 2016, Vol. 38, No. 2, pp. 4-8; https://doi.org/10.1515/ci-2016-0204
- Validation and assignation of an element discovery
Claims for the discoveries of new elements appear time to time in the scientific literature. IUPAC, along with IUPAP, is involved in assessing these claims. In result, IUPAC technical reports are released that review each pertaining references and recognize the laboratory(ies) whose claims fulfill the agreed criteria.
In 2016, two such reports have been released that cover elements 113, 115, 117, and element 118; See PAC 2016, Vol. 88, No. 1-2, pp. 139–153; https://doi.org/10.1515/pac-2015-0502 and PAC 2016, Vol. 88, No. 1-2, pp. 155–160; https://doi.org/10.1515/pac-2015-0501
- Naming new element
When the discovery of a new element has been validated and the priority for its discovery has been assigned, the naming process can begin. The Laboratory to which the discovery has been assigned is invited to propose a name and symbol. IUPAC will then review the proposal, and if agreed, after an additional 5-month public review, will formalize the name. The most recent example of such recommendations were published in 2012 and for the names and symbols of the elements 114 and 116; See PAC 2012, Vol. 84, No. 7, pp. 1669-1672; https://doi.org/10.1351/PAC-REC-11-12-03
A short review of the current procedures is published in a recent feature by John Corish; See CI 2016, Vol. 38, No. 2, pp. 9-11; https://doi.org/10.1515/ci-2016-0205
On 8 June 2016, IUPAC released the provisional names for the latest 4 elements 113, 115, 117, and 118 – see release and on 28 November 2016, IUPAC announced the approved names and symbols – see release .
For a reflection on the 2016 experience of the naming of elements, see Chem Int Apr 2017, pp. 30-21, by Jan Reedijk; https://doi.org/10.1515/ci-2017-0222
see Archives with earlier compilation and references
- How to name a new element
Here again, IUPAC has a set of guidelines that outline what sort of name an element can bear. Both the root and the ending must be consistent with the agreed recommendations. The detailed recommendations were published in 2002 and a revision published in 2016 to better accommodate element in group 17 and 18. See PAC 2002, Vol. 74, No. 5, pp. 787-791; https://doi.org/10.1351/pac200274050787 and PAC 2016, Vol. 88, No. 4, pp. 401-405 https://doi.org/10.1515/pac-2015-0802 (or https://iupac.org/project/2015-031-1-200 )
- Group 1-18 and collective names
Since 1988, IUPAC recommended that the groups ( i.e . columns) be simply numbered from 1 to 18. ( PAC 1988, Vol. 60, No. 3, pp 431-436; https://doi.org/10.1351/pac198860030431 )
Lanthanoids and actinoids are collective names also recommended by IUPAC. Lanthanoids (La to Lu) is preferred over lanthanide, and although lanthanoid means ‘like lanthanum’ and so should not include lanthanum, lanthanum has become included by common usage, however. Actinoids include Ac to Lr.
The question of precisely which elements should be placed in group 3 has been debated from time to time. An IUPAC project has been recently initiated to resolve the question. Will group 3 consist of Sc, Y, Lu, and Lr or, will it consist of Sc, Y, La and Ac?
Stay tune and see https://iupac.org/project/2015-039-2-200 and CI 2016, Vol. 38, No. 2, pp. 22-23; https://doi.org/10.1515/ci-2016-0213
- Standard atomic weights
One of the tasks of the Commission on the Isotopic Abundances and Atomic Weights (CIAAW) is to periodically review atomic-weight determinations. The most recent report “Standard Atomic Weights of the Elements 2021” has been published in PAC in May 2022 (AOP 4 May 2022; https://doi.org/10.1515/pac-2019-0603 )
The Commission was established in 1899 (yes, in eighteen ninety nine) and is now operating under the Inorganic Chemistry Division of IUPAC. (see www.ciaaw.org ) It also reviews regularly Isotopic compositions of the elements; the latest compilation is also published in PAC in March 2016 ( PAC 2016, Vol. 88, No. 3, pp. 293–306; https://doi.org/10.1515/pac-2015-0503 )
Yours to use
While IUPAC has no recommendation for a specific form of the periodic table, i.e . 18-column or 32-column format, the version here presented is in the conventional long form and is yours to use.
Check out earlier versions .
Subscribe to Concentrate
IUPAC e-newsletter with short, simple, and regular updates in your inbox.
Atom Diagrams Showing Electron Shell Configurations of the Elements
- Periodic Table
- Chemical Laws
- Projects & Experiments
- Scientific Method
- Biochemistry
- Physical Chemistry
- Medical Chemistry
- Chemistry In Everyday Life
- Famous Chemists
- Activities for Kids
- Abbreviations & Acronyms
- Weather & Climate
- Ph.D., Biomedical Sciences, University of Tennessee at Knoxville
- B.A., Physics and Mathematics, Hastings College
It's easier to understand electron configuration and valence if you can actually see the electrons surrounding atoms. For that, we have electron shell diagrams .
Here are electron shell atom diagrams for the elements , ordered by increasing atomic number .
For each electron shell atom diagram, the element symbol is listed in the nucleus. The electron shells are shown, moving outward from the nucleus. The final ring or shell of electrons contains the typical number of valence electrons for an atom of that element. The element atomic number and name are listed in the upper left. The upper right side shows the number of electrons in a neutral atom. Remember, a neutral atom contains the same number of protons and electrons.
The isotope is defined by the number of neutrons in an atom, which might be equal to the number of protons—or not.
An ion of an atom is one in which the number of protons and electrons is not the same. If there are more protons than electrons, an atomic ion has a positive charge and is called a cation. If there are more electrons than protons, the ion has a negative charge and is called an anion.
Elements are shown from atomic number 1 (hydrogen) up to 94 (plutonium). However, it's easy to determine the configuration of electrons for heavier elements by making a chart .
Lithium is the first element in which an additional electron shell is added. Remember, the valence electrons are found in the outermost shell. The filling of the electron shells depends on their orbital. The first orbital (an s orbital) can contain only two electrons.
Praseodymium
Protactinium.
- Electron Configuration Chart
- The Periodic Properties of the Elements
- Here's How to Download the Periodic Table With Electron Configurations
- How to Use a Periodic Table of Elements
- Radon Chemical and Physical Properties
- Why Lanthanides and Actinides Are Separate on the Periodic Table
- Chart of Periodic Table Trends
- Clickable Periodic Table of the Elements
- Introduction to the Periodic Table
- What Is Periodicity on the Periodic Table?
- Size of the Elements on the Periodic Table
- How Is the Periodic Table Organized Today?
- Printable Periodic Table Image and Periodic Table Wallpaper
- Why Is the Periodic Table Important?
- What Is the Most Electronegative Element?
- Printable Periodic Tables - 2015 Edition

IMAGES
VIDEO
COMMENTS
In chemistry, the representative elements are the elements with atoms filling s and p electron orbitals. Another name for the representative elements is the main group elements. The representative elements are groups 1 and 2 and group 13-17 on the periodic table.
The periodic table, also known as the periodic table of the elements, is an ordered arrangement of the chemical elements into rows ("periods") and columns ("groups"). It is an icon of chemistry and is widely used in physics and other sciences.
The development of modern atomic theory revealed much about the inner structure of atoms. It was learned that an atom contains a very small nucleus composed of positively charged protons and uncharged neutrons, surrounded by a much larger volume of space containing negatively charged electrons.
A representative element is one that has incomplete outermost shells and is reactive. The representative elements in a given group show similar chemical properties. What blocks...
Periodic table, in chemistry, the organized array of all the chemical elements in order of increasing atomic number. When the elements are thus arranged, there is a recurring pattern called the ‘periodic law’ in their properties, in which elements in the same column (group) have similar properties.
Interactive periodic table showing names, electrons, and oxidation states. Visualize trends, 3D orbitals, isotopes, and mix compounds. Fully descriptive writeups.
Key Takeaway. The chemistry of the third-period element in a group is most representative of the chemistry of the group because the chemistry of the second-period elements is dominated by their small radii, energetically unavailable d orbitals, and tendency to form π bonds with other atoms.
Write and interpret symbols that depict the atomic number, mass number, and charge of an atom or ion. Define the atomic mass unit and average atomic mass. Calculate average atomic mass and isotopic abundance. The development of modern atomic theory revealed much about the inner structure of atoms.
View the latest release of the Periodic Table (dated 8 Jan 2016) includes the recently added elements 113, 115, 117, and 118 with their temporary names and symbols.
Elements are shown from atomic number 1 (hydrogen) up to 94 (plutonium). However, it's easy to determine the configuration of electrons for heavier elements by making a chart.